Abstract
Candida albicans is the most prevalent fungal pathogen in humans. In this study, flavonoids from unprocessed multifloral honey were extracted and investigated their anticandidal activity in vitro. These results indicated that honey flavonoids inhibited Candida growth; however, they did not kill the yeasts and did not directly affect the cytoplasmic membrane. The viability tests of cells yeast with the fluorescent probe Sybr green I in combination with propidium iodide suggested that antifungal activity of honey flavonoids depended on their relative lipophilic properties and that they may reach a possible intracellular site of action without compromising membrane-associated functions.
INTRODUCTION
Raw honey is a treasure chest of nutritional constituents and medicinal remedies. It contains sugars, as well as vitamins, minerals, enzymes, and plant-derived flavonoids, many of which are biologically active.[Citation1, Citation2] The authors have previously demonstrated that honey flavonoids act as antioxidants, scavenging and eliminating free radicals.[Citation3, Citation4] The effectiveness of honey in many of its medical uses is probably due to its antibacterial activity.[Citation5, Citation6] In addition to the glucose-oxidase enzyme, the ability of honey to reduce inflammation could be also due to the presence of the flavonoids, which may function as antibacterial agents. They appear to be effective against a wide range of microorganisms, probably because of their ability to complex with extracellular and soluble proteins and with the bacterial cell wall.[Citation7] More lipophilic flavonoids may also disrupt the microbial membrane.[Citation7] There are many studies about bactericidal and bacteriostatic activity of honey,[Citation8] as well as reports about its antifungal activity.[Citation9]
Among the fungal pathogens, Candida albicans, an opportunistic polymorphic fungus, is considered to be the major yeast pathogen.[Citation10, Citation11] Under normal circumstances, it lives in 80% of the human population with no harmful effects; in fact, uncontrolled multiplication resulting in disease symptoms is kept under control by other naturally occurring microorganisms and by the human immune system. Conventional treatment with antibiotics can lead to elimination of the yeast's natural competitors, and increase the severity of the condition. A better, more long-term beneficial remedy for Candida infections is to practice a natural cure that focuses on treating the infection before it causes irritation. This is done by focusing on how to reduce the overgrowth of yeasts found in the body. Granted that conventional antifungals will not disappear any time soon, preservation of commensalism could make smaller doses of conventional antifungals given over shorter periods of time more effective and less harmful. Therefore, there is a growing need for the development of combined therapeutics that couple immunomodulation with antifungal therapy.
Italian honeys are recognized throughout the world because of their quality. However, there is little scientific research published about the microbiological properties of Italy's honeys. Thus, this study was carried out (i) to determine the polyphenol composition of Italian honey by reverse phase high-performance liquid chromatography (HPLC) and (ii) to assess the effectiveness of honey extract and some of its important polyphenols as antifungal agents against C. albicans by virtue of their hydrophobic structure and synergistic effect.
MATERIALS AND METHODS
Honey Sample and Chemicals
Unprocessed multifloral honey was locally obtained from Associazione Marchigiana Apicoltori (A.M.A., Marche, Italy) with the guarantee of genuiness and known history. The sample was harvested in 2008 and stored in the darkness at room temperature to minimize any alterations. Quercetin, kaempferol, apigenin, chrysin, and galangin were purchased from Sigma (St. Louis, MO, USA).
Preparation and HPLC/MS Analysis of Phenolic Extract of the Honey
Phenolic compounds were extracted from the whole honey by use of a nonionic polystyrene resin (Amberlite XAD-2; Sigma, St. Louis, MO, USA) and more hydrophobic flavonoids were separated by diethyl ether and analyzed by HPLC/MS, as reported by Fiorani et al.[Citation3] Ether extracts (EtE) were stored at −80°C until further analysis. Just before use, an aliquot of EtE was taken and diluted with dimethyl sulfoxide (DMSO) to reach the concentrations reported. DMSO at used concentration did not interfere with Candida growth.
Yeast Strain
C. albicans ATCC 10123 strain was used. The strain was stored in slant of Sabouraud dextrose agar (SDA) (Oxoid, Basingstoke, UK) at 4°C. For the experiments, the strain was grown in yeast nitrogen base (YNB) medium (Difco, Franklin Lakes, NJ, USA), supplemented with 5% (w/v) glucose, up to the early exponential phase at 37°C.
Susceptibility Testing
Antibacterial susceptibility testing is used to determine if microorganisms tested are likely to be killed or inhibited by a particular product. The results from susceptibility testing generally categorize the microorganisms as “susceptible,” “intermediate,” or “resistant” to each product tested. The antimicrobial tests were carried out by the agar diffusion method[Citation12] using a C. albicans suspension containing about 1.5 × 106 colony forming unit (CFU)/ml and 100 μl were dispended on plates of SDA. The concentration of the suspension was standardized by adjusting the optical density to 0.1 at 600 nm (PerkinElmer-Lambda 25 UV-VIS spectrophotometer, Waltham, MA, USA). Serial 2-fold dilutions of EtE (to maintain the same DMSO concentration in the sample), ranging from 6 to 384 μg/ml, were prepared, and 2 μl were put on the agar surface and, after 30 min to allow the compound absorption, the plates were incubated at 37°C for 24 h. DMSO served as a negative control.
MIC Determination
The minimum inhibitory concentration (MIC) values were evaluated by the broth microdilution method[Citation13] at a final inoculum of 1.5 × 106 CFU ml using YNB medium. Serial 2-fold dilutions of EtE and commercial flavonoids ranging from 6 to 192 μg/ml, were made in 96-well microtiter trays and incubated at 37°C for 24 h, in a dark chamber. The readings were performed by optical density (O.D.) determination using a microtiter plate reader at 600 nm The MIC50 was defined as the lowest extract concentration that resulted in a 50% decrease in absorbance compared to the control (no extract). In addition, fluconazole (Pfizer, Sao Paulo, Brazil) was used as a standard antifungal drug. At least three independent antimicrobial assays were performed.
Plate Count Test
C. albicans was diluted with fresh medium to achieve an approximate density of 1 × 107 CFU/ml. This was inoculated in four tubes containing 3 ml of growth medium added with 48, 96, 192, and 384 μg/ml of EtE, respectively, and incubated at 37°C +/−1°C for 6 h. Positive controls for growth of yeast in the absence of EtE were performed. To confirm the initial fungal counts, serially diluted cultures were plated on the appropriate agar plates and enumerated. After incubation, mixtures were subjected to successive 10-fold serial dilutions in the appropriate medium, mixed with a vortex shaker to ensure dispersion, and quantitatively cultured in duplicate onto agar plates to determine the number of viable yeast cells. The number of colonies that appeared on the agar plates was taken as the measure of the number of viable (surviving) cells. Each colony was regarded as a single CFU. Thus, CFU/ml (viable count) = Number of colonies × Dilution factor × 10. Viable cell counts are expressed as log10 CFU/ml. Each test was performed in triplicate.
Life/Dead Stain
The live-dead protocol is based on the simultaneous staining of microbial nucleic acids by a permeant (SYBR Green I; Molecular Probes, Eugene, OR, USA) and an impermeant [Propidium Iodide (PI); Sigma, St. Louis, MO, USA] fluorescent probe and the interpretation of the green versus the red fluorescence cytograms. Membrane-compromised microorganism cells are expected to fluoresce in the red-wavelength range, whereas the fluorescence emitted by membrane intact cells will be restricted to the green wavelengths. Cells with a partially damaged membrane will enable various amounts of PI to bind some nucleic acids that will result in a corresponding increase of the red fluorescence and a decrease of the green fluorescence. Consequently orange-red, green, and green plus orange-red cells were identified as dead, live, and damaged cells, respectively.
A 1:30 dilution of SYBR Green I commercial stock solution was made in DMSO and a 1 mg/ml PI work solution was made in MilliQ water. For the assessment of cell viability, 4 μl of SYBR Green I and 4 μl of PI were added simultaneously to 100 μl of Candida cells grown in the presence of EtE and incubated for 15 min in the dark at room temperature. A coverslip with 10 μl of C. albicans was mounted on a clear glass slide and examined under a fluorescence microscope (Zeiss Axioplane, Oberkochen, Germany) using filterset 09 (Exitation BP 450/490, Emission 520, Zeiss, Oberkochen, Germany). C. albicans grown in absence of EtE and in the presence of fluconazole was used as the control.
Flavonoid Uptake by C. albicans Cells
Candida suspensions (1.5 × 106 CFU/ml) were incubated at 37°C for 6 h in the absence and in the presence of 48 μg/ml EtE. After incubation, the samples were centrifuged at 3000 rpm for 10 min and the supernatants were discarded. The pellets were then extensively washed, suspended in 8 ml of solution saline standard (NaCl 0.85%, w/v), and lysed (400 watt × 3 min at 10%) by EmulsiFlex®-C5 High Pressure Homogenizer (Avestin, Mannheim, Germany). The samples were extracted three times with 500 μl of ethyl acetate and taken to dryness by rotary evaporation. All the samples were dissolved in DMSO (10 μl) and diluted with 2-propanol (90 μl) just before HPLC analysis. The HPLC system (Waters Alliance 2795, Fort Lauderdale, FL, USA) was coupled with a photodiode array detector (Waters 2996 PDA, Fort Lauderdale, FL, USA), followed by an electro spray mass spectrometer detector (ESI-MS) (Waters-Micromass ZQ, Fort Lauderdale, FL, USA) worked by Mass Lynx 4.0 SP4 software (Waters, Fort Lauderdale, FL, USA). The ESI-S analyses were performed in a positive mode by using the following conditions: source and desolvation temperature 120 and 240°C; capillary and cone voltage 3.5 KV and 30 V; and cone and desolvation flow (nitrogen gas) 40 and 400 l/h. HPLC analysis was performed by using a 250 × 4 mm Purospher Star RP18 endcapped (5 μm) column (Merck, Darmstadt, Germany) equipped with a LiCrosphere 100 RP18 guard column (4 × 4 mm, 5 μm) (Merck, Darmstadt, Germany). A modified version of the analytical HPLC method from Day et al.[Citation14] was used. Solvents A (0.1% formic acid) and B (acetonitrile) were run at a flow rate of 1 ml/min. The running gradient was adjusted to 17% B (2 min), increasing to 25% B (5 min), 35% B (8 min), 50% B (5 min), and then 100% B (5 min), followed by a re-equilibration at 17% B (3 min). All solvents were HPLC grade (Aldrich-Sigma, St. Louis, MO, USA) and water was purified via a Milex Q-plus system (Millipore, Billerica, MA, USA). Calibration curves of the main flavonoids analyzed were linear within the range investigated (0.5–100 M) and the method was reproducible and precise (within-day and between-day coefficient of variation below 10%).
RESULTS AND DISCUSSION
HPLC Analysis and Identification of Major Peaks for Honey Extract by MS
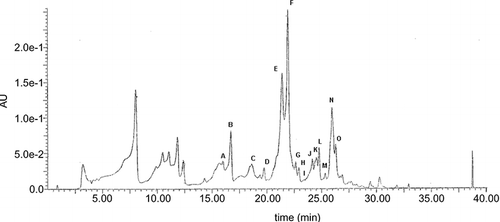
By the partition of raw Italian multifloral honey, EtE containing hydrophobic flavonoids was obtained as determined by HPLC/MS (). The main flavonoids identified were luteolin (A), quercetin (B), apigenin (E), kaempferol (F), isorhamnetin (G), acacetin (J), tamarixetin (K), chrysin (N), and galangin (O). The nature of these compounds was confirmed by UV and MS response as well as by chromatographic comparison with the authentic standards. Peak C corresponds to C16H12O7. While more information is needed for its identification, it should correspond to a methoxy derivative of kaempferol, such as 8-methoxykaempferol. This would be consistent with the analytical results collected, that is, retention time, molecular weight and formula, and lipophilic properties. In addition, the presence of 8-methoxykaempferol in other honey samples was documented in numerous studies.[Citation15, Citation16] Peaks H, I, L, and M were not identified and likely correspond to compounds with molecular characteristics different from flavonoids.
Figure 1 Typical HPLC chromatogram of honey flavonoids. EtE obtained as described under Materials and Methods was dissolved in DMSO, diluted in methanol, and analyzed by HPLC/MS. Peaks: luteolin (A), quercetin (B), 8-methoxykaempferol (C), apigenin (E), kaempferol (F), isorhamnetin (G), acacetin (J), tamarixetin (K), chrysin (N), and galangin (O). Peaks D, H, I, L, and M are unidentified compounds.
Antimicrobial Activity
The antimicrobial activity of EtE was evaluated in vitro against the standard strain of C. albicans in terms of the diameter of the inhibition zone: no antimicrobial activity or inhibition, inhibition zone <1 mm; slight antimicrobial activity, inhibition zone 2–3 mm; moderate antimicrobial activity, inhibition zone 4–5 mm; high antimicrobial activity, inhibition zone 6–9 mm; and strong antimicrobial activity, inhibition zone >9 mm. The results reported in show that EtE possess a pronounced antimicrobial activity. In fact, it inhibited the growth of C. albicans in a concentration-dependent manner producing a halo diameter of inhibition from 2 to 10 mm. DMSO at used concentrations did not interfere with Candida growth. No significant difference was observed when the amount of EtE was increased from 6 to 24 μg/ml. On the contrary, higher EtE concentrations showed an inhibition zone more than 4 mm in diameter.
Table 1 In vitro antimicrobial activity of different concentrations of honey EtE tested against C. albicans using agar-well diffusion and broth microdilution methods
The MIC values of EtE were obtained using serial 2-fold dilutions ranging from 6 to 192 μg/ml in 96-well microtiter trays and incubation at 37°C for 24 h, in a dark chamber. The readings were performed by O.D. determination using a microtiter plate reader at 600 nm and the data were reported in . It was observed that the 48 μg/ml value corresponded to MIC50 value.
Effect of Honey Flavonoids on the Viability of C. albicans
To assess the microbiostatic or microbicidal effect of the most active EtE concentrations on the C. albicans growth, a plate count test was performed. Initial yeast cultures (107 CFU/ml) were incubated with serial concentrations of EtE (from 48 to 384 μg/ml) at 37°C for 6 h. After incubation, the yeast was plated on SDA and incubated for 24 h al 37°C to determine the number of viable cells. As shown in , the lower EtE concentrations appear to have a microbiostatic effect on the C. albicans strain since, compared to the initial C. albicans CFU, viability of yeast cells after 6 h did not significantly reduce. Indeed, the highest honey EtE concentrations (192 and 384 μg/ml) showed a low microbicidal effect, in fact the number of CFU decreased about 10-fold. The positive control using fluconazole showed a great cell decrease.
Table 2 Mean viable counts of C. albican s treated with honey EtE
The staining tests with PI in combination with SYBR green allowed to confirm the viability of C. albicans on the basis of their distinct fluorescence emissions (). SYBR green I is a small molecule that relatively easily penetrates cell walls and stains the cells green regardless of their viability, whereas the yellow–orange PI cannot cross intact cell membranes and only labels cells with damaged membranes. In the absence of EtE, Candida cells were alive (only green cells) (); a 6-h incubation with 48 μg/ml of EtE (MIC50 value) did not make the cells permeable to PI and did not lead to statistically significant changes of cell viability (very few yellow-orange cells) (). shows cells of C. albicans treated with 4 μg/ml of fluconazole; the red fluorescence demonstrates the cell death.
Figure 2 Fluorescence micrographs of Candida albicans cells showing their viability after 24-h incubation in YNB, in the absence (a) or in the presence (b) of EtE. (c) negative control.

EtE treatment was found to cause cell membrane damage, as on its exposure C. albicans cells became permeable to PI. This fluorescent dye has already been used widely to evaluate membrane permeability changes, and it is considered to be a good marker for cell death associated with membrane alterations.[Citation17] The molecular targets for food-derived components have different locations in the cells of the human body. Generally they can act at the level of different biological membranes as well as inside the compartments that are limited by these membranes. In any case, the molecule must interact with the membrane to reach the target. For this reason, the lipophilicity of biologically active compounds is usually one of the most important pharmacological features, and interactions with membranes play an essential role in their biological activity.
Uptake of Honey Flavonoids to C. albicans Cells
Candida uptake of EtE flavonoids was evaluated by using an approach previously described[Citation18] that involves ethyl acetate extractions of cell lysate and medium, solvent evaporation, and sample dissolution in DMSO/methanol just prior to HPLC analysis. It should be noted that this procedure, as shown by HPLC/MS analyses of the honey EtE before and after ethyl acetate extraction and solvent evaporation, does not affect the concentration of the different flavonoids in the sample.
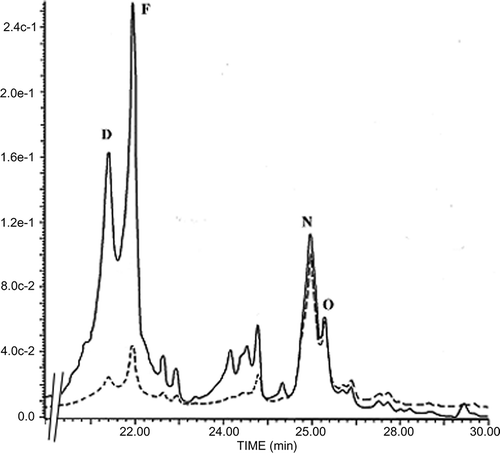
After a 6-h incubation of Candida cells with MIC50 value of EtE, 69.8% of chrysin and 74.2% of galangin were taken up by the cells, and only 9.8% of apigenin and 12.4% of kaempferol were available for the cells as judged by peak height (). Quercetin did not cross Candida membrane, presumably because of the presence of five hydroxyl groups that could make the molecule less hydrophobic (). As expected, the relative hydrophobicity of flavonoids depended on the number of hydroxyl groups on ring B of the flavonoid moiety. It is interesting to note that the active flavonoids are lipophilic compounds that are especially suitable as protective agents against microorganisms because of their ease in penetrating membranes.[Citation19]
Figure 3 Typical HPLC chromatogram for flavonoid uptake by Candida albicans cells. Flavonoids were extracted from either the whole cell suspension lysate (—) or cell pellet lysate (- - -) after 6-h incubation in the presence of EtE. Apigenin (E), kaempferol (F), chrysin (N), galangin (O).
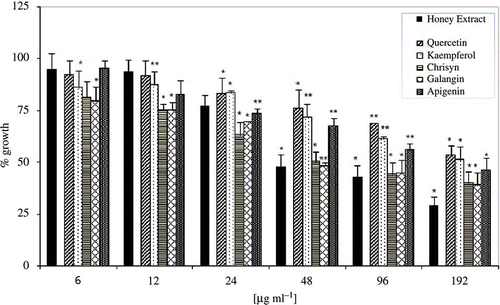
For comparative purposes, the antifungal activities of the commercial pure flavonoids, main constituents of EtE, were analyzed (). Among the standard flavonoids diluted in DMSO and used at the same concentrations of the honey extract (from 6 to 192 μg/ml), chrysin and galangin exhibited the highest antifungal activity. Chrysin, as well as galangin, because of their lower number of hydroxyl groups are much less polar than the corresponding flavone (apigenin) and flavonols (quercetin and kaempferol). This feature makes these molecules more hydrophobic, being the only structural difference. Interestingly, pure standard phenolics showed lower antifungal activities than honey extract (). It seems that the individual components of EtE may act synergically in exhibiting the anticandidal activity. Indeed, some studies have pointed to such synergism. For example, Amoros et al.[Citation20] and Cushine and Lamb[Citation7] mention the synergistic effect of apigenin and kaempferol on HSV, which would explain why honey and propolis present greater antiviral activity than their individual components.
Figure 5 Antimicrobial activities of EtE (black bar) and commercial pure phenolic compounds. The data are expressed assuming 100% growth for untreated control. Bars represent the average for four separate experiments and the error bars show the standard deviation. The distinction between the average values obtained was statistically significant at P < 0.05 (*) or P < 0.001 (**) according to Student's t test.
The present work could highlight the positive effect that honey may have on human health. Data from this study indicate that honey extract and not its constituent compounds, may be a useful adjuvant agent for the treatment of fungal infections. Moreover, the data support the hypothesis that anticandidal activity depends on their relative lipophilic properties, suggesting that they may reach a possible intracellular site of action without compromising membrane-associated functions. Understanding how these natural bioactive molecules inhibit the growth of microorganisms can lead to new technologies for the development of food products with particular nutritional functionalities.
CONCLUSION
Raw honey is important not only for his nutritional value but also for its functional and biological properties. Antioxidant, anti-inflammatory, and antibacterial activities are some of these important properties. These are mainly attributed to the phenolic compounds, such as flavonoids. According to the results of this study, raw honey may be suggested as a potential source of natural antimicrobial agents against Candida albicans, the most common fungal pathogen in humans.
REFERENCES
- Blasa , M. , Candiracci , M. , Accorsi , A. , Piacentini , M.P. , Albertini , M.C. and Piatti , E. 2006 . Raw Millefiori honey is packed full of antioxidants . Food Chemistry , 97 : 217 – 222 .
- Herken , E. , Erel , O. , Guzel , S. , Celik , H. and Ibanoglu , S. 2009 . Total antioxidant, phenolic compounds, and total oxidant status of certified and uncertified Turkey's honeys . International Journal of Food Properties , 12 : 461 – 468 .
- Fiorani , M. , Accorsi , A. , Blasa , M. , Diamantini , G. and Piatti , E. 2006 . Flavonoids from italian multifloral honey reduce the extracellular ferrycianide in human red blood cells . Journal of Agricultural and Food Chemistry , 54 : 8328 – 8334 .
- Blasa , M. , Candiracci , M. , Accorsi , A. , Piacentini , M.P. and Piatti , E. 2007 . Honey flavonoids as protection agents against oxidative damage to human red blood cells . Food Chemistry , 104 : 1635 – 1640 .
- Wahdan , H.A. 1998 . Causes of the antimicrobial activity of honey . Infection , 26 : 26 – 31 .
- Pérez , M.R.A. , Vela , H.L. , Paloma , L.L. , Rojo , C.M.D. and De Lorenzo , C.C. 2008 . in vitro antioxidant and antimicrobial activities of Spanish Honeys . International Journal of Food Properties , 11 : 727 – 737 .
- Cushinie , T. and Lamb , A. 2005 . Antimicrobial activity of flavonoids . International Journal of Antimicrobial Agents , 26 ( 5 ) : 343 – 356 .
- Molan , P.C. 1992 . The antibacterial activity of honey 1. The nature of the antibacterial activity . Bee World , 73 : 5 – 28 .
- Theunissen , F. , Grobler , S. and Gedalia , I. 2001 . The antifungal action of three South African honeys on Candida albicans . Apidologie , 32 : 371 – 379 .
- Budtz-Jørgensen , E. 1996 . Antifungal therapy in the oral cavity . Periodontology 2000 , 10 : 89 – 106 .
- Arisawa , T. , Arisawa , S. , Yokoi , T. , Kuroda , M. , Hirata , I. and Nakano , H. 2007 . Endoscopic and histological features of the large intestine in patients with atopic dermatitis . Journal of Clinical Biochemistry and Nutrition , 40 : 24 – 30 .
- Tereschuk , M.L. , Riera , M.V. , Castro , G.R. and Abdala , L.R. 1997 . Antimicrobial activity of flavonoids from leaves of Tagetes minuta . Journal of Ethnopharmacology , 56 : 227 – 232 .
- Lee , D.G. , Shin , S.Y. , Maeng , C.Y. , Jin , Z.Z. , Kim , K.L. and Hahm , K.S. 1999 . Isolation and characterization of a novel antifungal peptide from Aspergillus niger . Biochemical and Biophysical Research Communications , 263 : 646 – 651 .
- Day , A.J. , Bao , Y. , Morgan , M.R.A. and Williamson , G. 2000 . Conjugation position of quercetin glucuronides and effect on biological activity . Free Radical Biology & Medicine , 29 : 1234 – 1243 .
- Martos , I. , Cossentini , M. , Ferreres , F. and Tomas-Barberan , F.A. 1997 . Flavonoid composition of Tunisian honeys and propolis . Journal of Agricultural and Food Chemistry , 45 : 2824 – 2829 .
- Gil , M.I. , Ferreres , F. , Ortiz , A. , Subra , E. and Tomas-Barberan , F.A. 1995 . Plant phenolic metabolites and floral origin of rosemary honey . Journal of Agricultural and Food Chemistry , 43 : 2833 – 2838 .
- Pore , S. 1990 . Antibiotic susceptibility testing of Candida albicans by flow cytometry . Current Microbiology , 20 : 323 – 328 .
- Fiorani , M. and Accorsi , A. 2005 . Dietary flavonoids as intracellular substrates for an erythrocyte trans-plasma membrane oxidoreductase activity . British Journal of Nutrition , 94 : 1 – 9 .
- Wollenweber , E. and Dietz , V. 1981 . Occurrence and distribution of free flavonoids aglycones in plants . Phytochemistry , 20 : 869 – 932 .
- Amoros , M. , Simoes , C.M.O. and Girre , L. 1992 . Synergistic effect of flavones and flavonols against herpes simplex virus type l in cell culture . Comparison with the antiviral activity of propolis. Journal of Natural Products , 55 : 1732 – 1740 .