Abstract
The pH value is one of the most important process parameters during thermal treatments, regardless if the medium is a simple buffer solution or a complex food matrix. When the temperature increases after an initial measurement of the pH at ambient temperature (25°C), a significant pH shift could occur, which could produce incomparable results in different buffer solutions or lead to side reactions during food preservation. Consequently, a measurement cell was constructed to record online the pH-value and temperature up to 130°C. By applying the Nernst equation, it was possible to exclude the temperature-dependent influence of the pH glass electrode on the total pH value. The pH shift was measured over a wide temperature range (ΔT 20–130°C) in the most commonly used buffer solutions and some selected food matrices. The ΔpH of certain buffer solutions, namely TRIS and ACES, showed a significant pH decrease of −2.01 ± 0.08 (ΔT 20–130°C) and −1.27 ± 0.1 (ΔT 20–130°C), respectively, whereas the pH of PBS buffer solution was nearly independent of temperature. The ΔpH decrease recorded in milk (−0.89 ± 0.6, ΔT 20–130°C) as well as commercial and self-made baby food (−0.56 ± 0.05, ΔT 20–130°C) is of special interest for the food industry to get a deeper insight in occurring reactions during thermal preservation processes.
INTRODUCTION
Microorganisms, such as bacteria, bacterial endospores, yeast, and molds, are sensitive to pH shifts in their environment. During thermal processes, such as pasteurization, sterilization, or freezing, the dissociation equilibrium of water and buffer solutions varies with pressure and temperature.[Citation1–7] This change of the pKa-value (decadic logarithm of acid dissociation constant Ka) may play an important role in different pH-sensitive reactions, but its behavior has rarely been investigated.
For basic inactivation studies of microorganisms, buffer solutions are commonly used to achieve constant pH-values and medium properties. In view of the dissociation equilibrium shift during heating, a biased error in the results of inactivation experiments occurs, which leads to incomparable results if they are not designed correctly. Several authors have shown that the thermal inactivation of bacterial spores,[Citation8–12] vegetative microorganisms,[Citation13] or protein and enzyme denaturation[Citation14–16] strongly depends on the pH of the suspending media. According to Goldberg,[Citation17] the known temperature dependence of the acid dissociation constant Ka (T) for different buffer systems could be interpolated up to 60°C.
Furthermore, the pH-value plays an important role in determining the amount of heat required to ensure a safe product during industrial food processing. Food could, therefore, be divided by its initial pH value into different classes. Foods with very low or high pH value will avert microbial growth and could be stored over long periods of time without undergoing further preservation processes. The majority of food, however, has a pH value that only offers some protection against microbial growth, or may even provide microorganisms with optimal conditions for reproduction. Few foods, like egg white, some bakery products, and sweet corn, have an alkaline pH value. Most foods have a pH value less than 7 and are, therefore, naturally acidic. Nevertheless, the shift of the pH value of a single food ingredient or additive may have a strong influence on the behavior during thermal processing of the whole product.
Common ways to reduce the risk of food spoilage include refrigerated or frozen storage, as well as drying or thermal processes. Aside from the process temperature, pressure, and time, the pH value of the treated food plays a key role in determining the extent of heat required during pasteurization or sterilization in food industry.[Citation15, Citation18] Extensive data about the pH value of various kinds of foods at ambient temperature are available,[Citation19–21] and some data covers pH values for buffer solutions up to 80°C, or even 90°C for some foods,[Citation15] however, there are limited data available about the shift of the pH values up to 130°C. In contrast to simple buffer solutions, these pH shifts are not calculable and need to be measured in complex matrices like foods. Currently, this measurement is only possible with a few pH glass electrodes.
The aim of this research was to develop a pressure-resistant measurement system that could record the pH value in dependence of the temperature up to 130°C. Furthermore, the pH shift of commonly used buffer solutions and selected foods was measured to extend the available data base for the pH shift from 90°C up to 130°C. These data are of key interest for different research and industrial applications, such as the inactivation of microorganisms (e.g., up to 130°C) and the sterilization of food during canning (e.g., up to 121.1°C). Hence, these data could give a deeper insight how chemical, biochemical, and biological reactions are influenced by the actual pH value under the applied temperature.
MATERIALS AND METHODS
pH Measurement System
In order to measure the pH shift during heating, it was necessary to construct a pressure resistant cell with an adequate sample volume. The developed cell had a maximum filling volume of 92 ml and a maximum working pressure of 10 bar (the limit of the pH electrode). All measurements were performed with a Jumo Tecline pH glass electrode (Jumo GmbH & Co. KG, Fulda, Germany), equipped with a high temperature pH glass (max. 130°C), high temperature gel electrolyte, and a double hole diaphragm. The glass electrode was plugged in to a pH meter (Digital-Labor-pH-Meter CG 811, Schott AG, Hofheim, Germany). The sample temperature was measured with a Pt100 temperature element integrated into the pH electrode. All pH values and temperatures were acquired with a USB data acquisition system (OMB-DAQ-56, Omega Engineering, Inc., Stamford, CT, USA) (1 Hz acquisition rate) and the data were documented with self written software created in LabVIEW 6.5.1 (National Instruments, Austin, TX, USA). The cell was equipped with a magnetic stirring device to guarantee accuracy and homogenous temperature distribution inside the measurement cell. For heating up, the measurement system was immersed into preheated silicon oil (SilOil M40.165.10, Huber GmbH, Offenburg, Germany) with a fixed temperature of 140°C.
The pH electrode was calibrated every experimental day at 20°C by a two-point calibration procedure with pH reference buffer solutions at pH 7 (Certipur buffer solution pH 7, Merck KGaA, Darmstadt, Germany) and pH 4 (Certipur buffer solution pH 4, Merck KGaA, Darmstadt, Germany). During the measurements the automatic temperature compensation of the pH meter was switched off and adjusted to 20°C.
Buffer Solutions
Standard commercial pH buffer (Tampon pH 7.413, Fisher Bioblock Scientific, Illkirch, France) was used with certified data (in accordance with U.S. National Institute of Standards and Technology SRM 186-I-g potassium dihydrogen phosphate and SRM 186-II-g disodium hydrogen phosphate) for the pH shift up to 90°C (uncertainty of measurement ±0.010 pH at p = 0.05).
N-(2-Acetamido)-2-aminoethanesulfonic acid buffer (ACES) (A-9758, Sigma-Aldrich-Chemie GmbH, Steinheim, Germany) pH 7, phosphate buffer system (PBS) pH 7, tris(hydroxymethyl)aminomethane buffer (TRIS also kwon as THAM) pH 8, acetate buffer pH 4.1 and citrate buffer pH 5.6 were used. All buffers had a molarity of 0.05 M. The pH was adjusted with 1 M HCl solution or 1 M NaOH solution, respectively.
Selected Food Systems
The shift of the pH value for raw milk (Demeter e.V., Darmstadt, Germany) and ultra heat treated milk (UHT-milk, EUCO GmbH, Hamburg, Germany) with a fat content of 3.5% was measured. Furthermore, the pH change in the different milk fractions was investigated. To obtain these fractions, whey proteins were isolated by adding rennet to the raw milk (32°C). After 30 min, the coagulated casein were cut and the curd was removed. Isolation of the casein fraction from raw milk was accomplished first via a centrifugation step (20 min, 2500 g, Megafuge 1.0 R, Thermo Fisher Scientific Inc., Waltham, MA, USA) at 5°C to remove the fat phase, and afterwards the residue was ultra-centrifuged (80,000 g; 60 min; Beckmann Optima CE-80K, Beckmann Coulter Inc., Fullerton, CA, USA). The supernatant was removed and the casein pellet was again suspended in a salt solution that simulates the milk ultrafiltrate (SMUF solution).[Citation22]
To measure the pH variation in the lactose fraction of milk, lactose (Oxoid, Hampshire, GB) with a final concentration of 4.5% was suspended in a SMUF solution. The solvation of lactose in the SMUF solution was necessary to reach a conductivity in the solution >100 μS cm-1, and to, therefore, get a stable pH value in a batch measurement cell.[Citation20] The pH was measured in accordance with pH measurements in fresh milk and iso-standards.[Citation20, Citation23] As a possible canning product, the in-situ pH shift in baby mashed carrots with potatoes was investigated. The pH was measured in a commercial baby food (Carrots with Potatoes, HIPP, Pfaffenhofen, Germany) and in fresh cooked baby food (78% carrots, 10% potatoes, 0.9% rape oil, and 11% water).
All measurements were performed at least in triplicates. The functional relationship between pressure, temperature, and filling volume was solved with MathCAD 2001i Professional (MathSoft Engineering & Education, Inc., USA). The presentation of the calculated and measured data was arranged by the Origin 7SR1 software (OriginLab Corporation, USA).
Thermal Inactivation of Bacterial Endospores
Especially the inactivation kinetics of bacterial endospores, which have to be fully inactivated during a sterilization process, could be strongly affected if the thermal treatment is carried out in a buffer solution where the pH value is temperature dependent or not. To proof the impact of the pH shift on the inactivation of bacterial spores, Bacillus subtils spore suspension (PS832) was centrifuged for 5 min at 3424 g (Biofuge pico, Kendro Laboratory Products GmbH, Langenselbold, Germany) and the spore pellet was resuspended in phosphate buffer system (PBS) or in acid buffer (ACES). Both buffers had a molarity of 0.05 M and a pH of 7. The spore suspension was filled into thin glass capillaries with a volume of 60 μL and hermetically sealed to prevent evaporation of the suspension due to temperatures above 100°C. The spores were inactivated in a thermostatic bath, filled with silicon oil at 100°C, which corresponds to a pH shift of –1 ± 0.1 in ACES and 0.02 ± 0.03 in PBS. After thermal treatment the glass capillaries were immediately stored at 4°C, the treated spore solution was serially diluted with 1/4 Ringer solution (TP887925 712, Merck KGaA, Darmstadt, Germany) on micro plates (Carl Roth GmbH, Karlsruhe, Germany) and two 50-μl samples of every dilution were drop-plated in petri dishes on nutrient agar (CM 003, Oxoid Ltd., Hampshire, England). The dishes were incubated at 37°C for 2 days and the colonies were counted.
RESULTS AND DISCUSSION
During the heat-up phase, the volume of the sample increases, which results in a pressure increase. The pressure has to remain below 10 bar, to avoid a leakage of the measurement cell or a rupture of the pH electrode. To calculate the pressure increase during heating, EquationEq. (1) was used[Citation24]:
where Δp denotes the total pressure, ΔpS the partials vapor pressure, and ΔpA the air pressure. A temperature increase will result in an increase of the saturated vapor pressure and air pressure inside the measurement cell. Heating a vessel with the volume Vv , a headspace volume VH 1 and pressure pL 1 from temperature T 1 to T 2, will lead to the final pressure pL 2 Citation[24]:
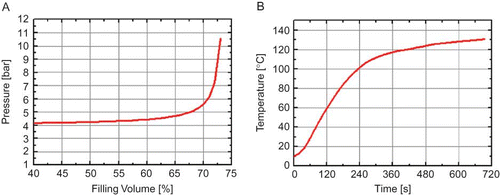
The volume of the headspace VH 2 at the temperature T 2 leads to the final pressure pL 2, which is largely determined by the heat expansion of the processed fluid. To calculate the expansion of the measured fluids, data for water was assumed, taken from the NIST Database.Citation[25] The results are presented in . Based on the calculation for water, a maximum filling volume of 70% (66 ml) was used in all experiments to avoid disrupting the pH electrode. illustrates the temperature profile during the pH measurement, which is of special interest for the pH measurement in milk and milk fractions.
Figure 1 (a) Calculated increase of pressure inside the measurement cell as filling volume increases for a temperature increase of ΔT 20–130°C. (b) Temperature profile for UHT-Milk measured inside the treatment chamber (fixed silicon oil temperature of 140°C) (color figure available online).
The response of a pH glass electrode is on an interfacial equilibrium between functional glass surface groups and oxonium and/or alkali ions (M+) in the contacting solution. Depending on the relative oxonium (pH) and alkali (pM) ion concentration, the pH glass surface may be covered, which would result in a negative charge density at the glass surface. This would generate a difference of potential between the inner and outer surface of the glass membrane. Due to the known pH of the inner electrolyte, the pH of the unknown solution could be measured as a voltage shift. If both solutions have the same pH (normally pH 7), the voltage difference is 0 (isotherms intersection in ).Citation[20, Citation26, Citation27] The response of the pH glass membrane to media with different pH values is a linear shift of the membrane potential, which could be calculated with the Nernst equation.Citation[28] The slope (m) of this straight line is dependent on the temperature and has the theoretical value of -59 mV at 25°C. Kratz Citation[29] and BauckeCitation[30] found a deviation of 0.3% from this ideal slope and traced this unique effect of glass electrodes back to the thermodynamic effects at the glass's surface.
Figure 2 (a) Measured slope shift in a certified temperature-stable standard buffer solution versus 99.7% of Nernst-slope up to 100°C[20], data up to 130°C were extrapolated. (b) Linear function of the electrode response from 20°C to 120°C in correlation with pH (color figure available online).
![Figure 2 (a) Measured slope shift in a certified temperature-stable standard buffer solution versus 99.7% of Nernst-slope up to 100°C[20], data up to 130°C were extrapolated. (b) Linear function of the electrode response from 20°C to 120°C in correlation with pH (color figure available online).](/cms/asset/5ecf635d-e6fd-4681-8fc8-4a05cd86a893/ljfp_a_446058_o_f0002g.jpg)
To evaluate the developed measurement system up to 90°C, certified standard buffer solutions were used to determine the temperature-dependant slope shift of the used glass electrode. The automatic temperature compensation of the pH-meter (feasible up to 100°C) was switched off and manually adjusted to 20°C. The measured data up to 90°C () were in excellent agreement with literature data, and thus enabled the possibility to calculate the proportion in the pH shift caused by the pH electrode at higher temperatures. illustrates the glass electrode response under different isothermal conditions (ΔT 20–120°C). The slope of each graph is determined by the construction of the glass electrode. Whereas the temperature influence on the offset of the pH glass electrode increases under alkaline or acid conditions, it has a marginal influence under neutral conditions (pH 7 = 0 mV), which is pictured as the isotherms intersection in . These basic and essential relations as well as calculations enabled the determination and measurement of the real change of the pH value in the processed fluid.
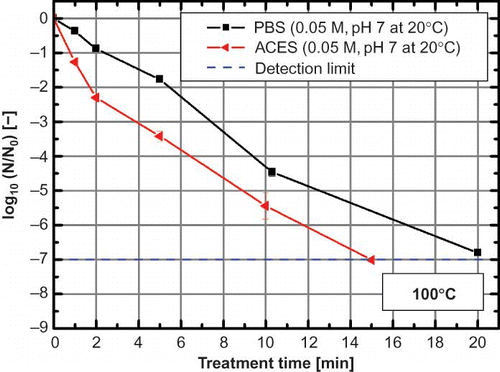
Consequently, this measurement system provided the possibility to measure the pH shift in different commonly used buffer solutions. The measured buffer solution could be divided into three categories: (i) temperature stable buffer solutions (), (ii) buffer solutions with decreasing pH during heating (), and (iii) buffer solutions with increasing pH during heating (). TRIS and ACES buffer solution had the highest pH shift [-2.01 ± 0.08, (TRIS); -1.36 ± 0.1 (ACES); ΔT 20–130°C] of all tested buffer solutions, whereas PBS buffer solution showed almost no pH change (-0.03 ± 0.03, ΔT 20–130°C) during heating. The data measured up to 90°C are in accordance with the pH shifts published elsewhere.[Citation20], [Citation31–33] The pH shifts in these buffer systems are of particular interest because they are commonly used for thermal inactivation experiments with microorganisms or enzymes up to 130°C. If this pH change is not considered during the experimental design, it could produce a much stronger inactivation of the used microorganism or enzyme in the TRIS or ACES buffer solution,[Citation12] which, in turn, would produce incomparable results to for example the same inactivation in PBS buffer solution. Thermal treatments with bacterial spores were carried out in PBS and ACES buffer at 100°C due to their similar pKa values and, hence, equal buffer capacity,[12] to proof the influence of the pH shift. The initial pH of both buffer solutions was 7 (at 20°C) and remains stable for PBS (pH 7.01 ± 0.02 at 100°C) and decreased to 6.01 ± 0.05 (at 100°C) for ACES buffer. This shift of 1 pH unit had a strong effect on the thermal inactivation of B. subtilis PS832 (). During isothermal conditions, the B. subtilis spores showed a -1 to -1.5 log10 stronger inactivation in ACES buffer solution compared to PBS, which confirms the high impact of the pH shift during thermal treatments on biological and biochemical reactions.
Figure 3 (a) pH shift in PBS buffer solution (0.05 M) and certified reference buffer-solution; (b) pH shift in ACES- (0.05 M) and TRIS buffer solution (0.05 M); C) pH shift in acetate- (0.05 M) and citrate buffer solution (0.05 M). Error bars indicate the standard error (color figure available online).
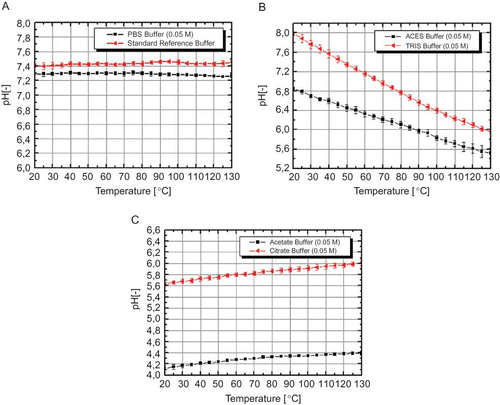
Figure 4 Thermal inactivation of Bacillus subtilis spores at 100°C in PBS and ACES buffer with an initial pH of 7 (0.05 M) at 20°C. Error bars indicate the standard error (color figure available online).
The various behaviors of the different buffer solutions at the same temperature result from the different dissociation reactions and, consequently, the different molar enthalpies (Δ r H 0). In , the standard molar enthalpies with its pKa values at 25°C are plotted, which are important for the dissociation equilibrium shift. While increasing the temperatures, the dissociation equilibrium shifts. Indeed, this relationship reflects the principle of Le Chatelier and Braun, which states that if a chemical system at equilibrium experiences a change, the equilibrium will shift in order to minimize that change. As a consequence, endothermic reactions dominate as temperatures increase. This leads to a 13 times more temperature-dependant dissociation of TRIS buffer in comparison to PBS buffer, which explains the high pH shift.
Table 1 pKA values and standard molar enthalpies (Δ r H 0) at 25°C[17]
Buffer solutions with a stable or even an increasing pH value during heating exhibited a marginal positive or even negative molar enthalpy (Δ r H 0). These are, for example, PBS, acetate, or citrate buffer solution, which has a measured pH change of +0.367 ± 0.05 (ΔT 20–130°C) for citrate and +0.277 ± 0.04 (ΔT 20–130°C) for acetate buffer, respectively. Such buffer solutions are of special interest for different kinds of chemical and biochemical reactions, where nearly stable pH conditions have to be achieved at different temperature levels. In contrast to the relatively simple composition of buffer solutions, the change of the pH value in complex matrices (like food) during heating is hardly to predict and therefore a direct measurement of the pH has to be performed. These data are of industrial relevance for food pasteurization or sterilization to increase the knowledge about side reactions and interactions of food components or added ingredients like enzymes, food dyes, or antioxidants that are often pH sensitive and are not singularly inactivated by the applied temperatures. Furthermore, estimations about the impact of the pH shift on the total microbial or enzymatic inactivation could be possible, if inactivation data in buffer solutions with an analogue pH shift to the thermal treated food are compared with inactivation data observed in the thermal treated food.
As an example for a liquid food, the pH shift for the thermal treatment of milk and its components during pasteurization (62–85°C) or sterilization (109–120°C) is presented in and . The observed pH shift of –0.9 ± 0.06 (ΔT 20–130°C ) in raw milk is in agreement with the literature (up to 90°C).[Citation15, [Citation34–36] The reduction of the pH value during thermal treatment is due to the release of calcium phosphate from the casein micelles, hence, the generation of oxonium ions that decrease the pH.[15,34] Up to 50% of the pH decrease is caused by the formation of organic acids (mainly formic and levulinc acid) from the lactose fraction of the milk.Citation[35] These reactions dominated the pH shift in milk, whereas the release of carbon dioxide, which would result in a pH increase, had a minor influence. The hydrolysis-reactions in the protein fractions of milk proceeded much slower[16] than the decomposition of lactose, which could explain the greater pH decrease above 100°C in the lactose solved in SMUF solution.
Figure 5 (a) pH shift in raw milk and ultra heat treated milk (UHT-Milk) as well as; (b) pH shift in raw milk and its components (whey protein, casein and lactose [4.5%]) in dependence of temperature. Error bars indicate the standard error (color figure available online).
![Figure 5 (a) pH shift in raw milk and ultra heat treated milk (UHT-Milk) as well as; (b) pH shift in raw milk and its components (whey protein, casein and lactose [4.5%]) in dependence of temperature. Error bars indicate the standard error (color figure available online).](/cms/asset/1eec8a0f-8ed9-4bc7-ae94-72d395bdbc1b/ljfp_a_446058_o_f0005g.jpg)
Contrary to the measured pH shift in buffer solutions, a hysteresis of the pH value measured in milk could be observed. Despite using a pH electrode, where the reference-element was equipped with a cartridge filled with sliver chloride to realize an adequate electrolyte outflow. Hence the observed hysteresis in milk could be induced by a partial or complete blockage of both diaphragms of the electrode, caused by protein aggregates. However, since the thermal treated milk reaches its initial pH-value (-0.07 ± 0.03) after 3 h storage at 20°C, the observed hysteresis is probably caused by a retarded uptake of Ca2+ ions into the casein micelles.
In contrast to the different behavior of the single milk fractions, the direct comparison of the total pH shift in raw milk versus a short time high temperature treated milk (UHT-milk) showed only marginal deviation in the pH value over the measured temperature range. This leads to the assumption, that a short time (2–8 s) high temperature (135–150°C) preservation process has only a minor influence on the single milk components and, hence, should be preferred for milk sterilization.
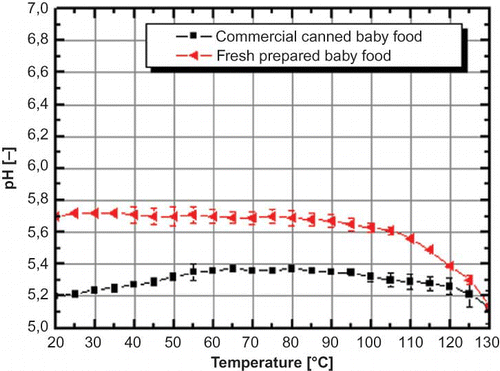
Due to the higher energy input into the treated product during sterilization (110–121°C, >10 min) a lot of temperature instable substances are decomposed or converted, which results in a different behavior concerning the pH value if a thermal untreated product is compared with a commercial sterilized product. Such a typical canning product is baby food, which is presented in . The initial pH value of the fresh prepared baby food was 5.7 in contrast to 5.2 of the canned one. While heating up both products, the pH value equalized continuously and reached nearly the same pH value at 120°C. This indicated that all temperatures in labile components are degraded at the first heat up and a second or a further extended heating has only a minor influence on the total pH change (-0.07 ± 0.05, ΔT 20–130°C for commercial canned baby food during the second heat up, deviation for the second heat up of self made baby food was marginal). The slight decrease of the pH value (-0.56 ± 0.02) for the fresh prepared baby food could originate by various substances with buffer capability, like proteins in the product, or by matrix effects caused by nonpolar substances. These substances, like sugars, could induce a fine structure in aqueous solutions and, hence, influence the pH value.Citation[37] Furthermore, the decrease of pH value could be induced by dispersed particles in the mash, which are polarizing the surrounding water molecules and, thus, increase the activity of oxonium and hydroxide ions.Citation[38]
Figure 6 pH shift in commercial canned baby food versus pH shift in fresh prepared baby food by increase in temperature. Error bars indicate the standard error (color figure available online).
Changes in the pH values of foods play an import role during the preservation process because it influences every substance in the thermal-treated food matrix. This influence is not only on desired effects, like the unwanted inactivation of microorganisms, but also on unwanted denaturation of enzymes and proteins. Furthermore, the enlargement of available pH data at temperatures above 90°C for some commonly sterilized products enables a deviation between temperature effects and the influence of the actual pH under pasteurization or sterilization conditions on chemical and biochemical reactions. Thus, it could lead to an optimization of the whole hurdle concept.
CONCLUSION
The shift of pH values in different media over a wide temperature range (20–130°C) was measured due to its important influence during thermal treatments on microorganisms, enzymes and pH-sensitive additives. A measurement system was constructed to acquire the pH value and temperature up to 130°C with an acquisition rate of 1 Hz. The system was validated with a certified buffer reference solution up to 90°C, and the temperature-dependent influence of the pH glass electrode was excluded by using the Nernst equation. All measured data up to 90°C were in excellent agreement with literature data. The acquired data for the pH shift between 90°C and 130°C represents an important process window for thermal processing of different media. With the pH shifts measured in different buffer solutions, the results of microbial and enzymatic inactivation experiments can be compared more effectively and the influence of the temperature-dependent pH shift could be controlled by changing the buffer solution. The presented pH decreases in some food systems can be of special interest for the food industry to increase the understanding of occurring microbiological, biochemical, and chemical reactions in the whole food matrix or for single ingredients in the thermal-treated food. This knowledge would help in designing an optimized hurdle concept for thermal preservation processes or for a controlled modification of food properties (e.g., gelation properties of selected protein fractions or preservation of pH instable healthful food ingredients) during thermal preservation processes. In conclusion, the shift of pH value during thermal treatment (regardless if the temperature is increased or decreased) could not be neglected because of its strong influence on all kind of biological and chemical reactions.
REFERENCES
- Distèche , A. 1959 . pH measurement with a glass electrode withstanding 1500 kg/cm2 hydrostatic pressure . Review of Scientific Instruments , 30 ( 6 ) : 474 – 478 .
- North , N.A. 1973 . Dependance of equilibrium constants in aqueous solutions . The Journal of Physical Chemistry , 77 ( 7 ) : 931 – 934 .
- Marshall , W.L. and Franck , E.U. 1981 . Ion product of water substance, 0-1000°C, 1-10,000 bars. New International Formulation and its background . Journal of Physical and Chemical Reference Data , 10 ( 2 ) : 295 – 304 .
- Hamann , S.D. 1982 . The influence of pressure on ionization equilibria in aqueous solutions . Journal of Solution Chemistry , 11 ( 1 ) : 63 – 68 .
- Kitamura , Y. and Itoh , T. 1987 . Reaction volume of protonic ionization for buffering agents. Prediction of pressure dependence of pH and pOH . Journal of Solution Chemistry , 16 ( 9 ) : 715 – 725 .
- Quinlan , R.J. and Reinhart , G.D. 2005 . Baroresistant buffer mixtures for biochemical analyses . Analytical Biochemistry , 341 ( 1 ) : 69 – 76 .
- Bruins , M.E. , Master , A.M. , Janssen , A.E.M. and Boom , R.M. 2007 . Buffer selection for HP treatments of biomaterials and its consequences for enzyme inactivation studies . High Pressure Research , 27 ( 1 ) : 101 – 107 .
- Sognefest , Hays, G.L. , Wheaton , E. and Benjamin , H.A. 1948 . Effect of pH on the thermal process requirements of canned foods . Food Research , 13 : 400 – 416 .
- Loewick , J.A.M. and Anema , P.J. 1972 . Effect of pH on the heat resistance of Clostridium sporogenes in minced meat . Journal of Applied Bacteriology , 56 : 193 – 199 .
- Alderton , G. , Ito , K.A. and Chen , J.K. 1976 . Chemical manipulation of the heat resistance of Clostridium botulinum spores . Applied and Environment Microbiology , 31 ( 4 ) : 492 – 498 .
- Hutton , M.T. , Koskinen , M.A. and Hanlin , J.H. 1991 . Interacting effects of pH and NaCl on heat resistance of bacterial spores . Journal of Food Science , 56 ( 3 ) : 821 – 822 .
- Mathys , A. , Kallmeyer , R. , Heinz , V. and Knorr , D. 2008 . Impact of dissociation equilibrium shift on bacterial spore inactivation by heat and pressure . Food Control , 19 ( 12 ) : 1165 – 1173 .
- Deng , Y. , Ryu , J.-H. and Beuchat , L.R. 1998 . Influence of temperature and pH on survival of Escherichia coli O157:H7 in dry food and growth in reconstituted infant rice cereal . International Journal of Food Microbiology , 45 ( 3 ) : 173 – 184 .
- Sieracki , N.A. , Hwang , H.J. , Lee , M.K. , Garner , D.K. and Lu , Y. 2008 . A temperature independent pH (TIP) buffer for biomedical biophysical applications at low temperatures . Chemical Communications , : 823 – 825 .
- Kessler , H.G. 2002 . Food and Bio Process Engineering—Dairy Technology , 5th , Edited by: Verlag , A. Munich, , Germany : Kessler .
- de Wit , J.N. 2009 . Thermal behaviour of bovine -lactoglobulin a temperatures up to 150°C. A review . Trends in Food Science & Technology , 20 : 27 – 34 .
- Goldberg , R.N. , Kishore , N. and Lennen , R.M. 2002 . Thermodynamic quantities for the ionization reaction of buffers . Journal of Physical and Chemical Reference Data , 31 ( 2 ) : 231 – 370 .
- Heiss , R. 2004 . Food proteirs (Lebensmitteltechnologie) , Vol. 6 , Berlin : Springer-Verlag .
- McGlynn, W. …; The Importance of Food pH in Commercial Canning Operations. Food Technology Fact Sheet Food and Agricultural Products Research and Technology Center, Oklahoma State University 2009. http://pods.dasnr.okstate.edu/docushare/dsweb/Get/Document-2442/FAPC-118pod.pdf (http://pods.dasnr.okstate.edu/docushare/dsweb/Get/Document-2442/FAPC-118pod.pdf)
- Degner , R. 2009 . pH-Messung , Germany : Wiley-VCH: Weinheim .
- U.S. Food & Drug Administration Center . 2004 . pH values of various foods. In Foodborne Pathogenic Microorganisms and Natural Toxin , 352 Mclean, , VA, USA : International Medical Publishing .
- Jenness , R. and Koops , J. 1962 . Preparation and properties of a salt solution which simulates milk ultrafiltrate . The Netherlands Milk and Dairy Journal , 16 ( 3 ) : 153 – 164 .
- 5546 , ISO- . 1979 . Caseins and caseinates—Determination of pH (Reference method) , Geneva, , Switzerland : International Organization for Standardization .
- Heiss , R. and Eichner , K. 1994 . Haltbarmachen von Lebensmitteln , Vol. 3 , Germany : Springer-Verlag: Berlin .
- National Institute of Standards and Technology (NIST) . 2002 . NIST Standard Reference Database 10 , Steam Properties Database Version 2.21 .
- Baucke , F.G.K. 2001 . Function of glass electrodes. A discussion of interfacial equilibria . Physics and Chemistry of Glasses , 42 ( 3 ) : 220 – 225 .
- Galster , H. 1990 . pH-Messung; VCH Verlagsgesellschaft , Germany : Weinheim .
- Nernst , W. 1889 . The electromotive activity of ions (Die elektromotorische Wirksamkeit der Ionen) . Journal of Physical Chemistry (Zeitschrift für Physikalische Chemie) , 4 ( 2 ) : 129 – 181 . (in German)
- Kratz , L. 1950 . The glass-electrode and its applications (Die Glasselektrode und ihre Anwendungen) , 59 in German : Scientific research reports in natural scientific series Wissenschaftliche Forschungsberichte, (Naturwissenschaftliche Reihe) .
- Baucke , F.G.K. 1994 . Thermodynamic origin of the sub-nernstian response of glass electrodes . Analytical Chemistry , 66 : 4519 – 4524 .
- Mussini , T. , Cicognini , M. , Longhi , P. and Rondinini , S. 1984 . Standard pH values for potassiumum hydrogenphthalate reference buffer solutions in 10, 30 and 50% (w/w) 1,4-dioxane/water mixed solvents at temperatures from 288.15 to 318.15 K . Analytica Chimica Acta , 162 : 103 – 111 .
- Reagecon . 2008 . Certificate of Analysis—DIN Buffer Standard pH 7.413 , Clare, , Ireland : Reagecon Diagnostics Limited .
- van den Bergen , H. 2009 . pH Buffer Solution; Monash Scientific Glass Blowing Service Pty. Ltd.: Dandedong, Victoria, Australia
- Cheftel , J.C. , Cuq , J.L. and Lorient , D. 1992 . Food Proteins (Lebensmittelproteine) , Germany : Behrs Verlag GmbH & Co.: Hamburg .
- Ternes , W. 1994 . Scientific Background of Food Processing (Naturwissenschaftliche Grundlagen der Lebensmittelzubereitung) , Germany : Behrs Verlag GmbH & Co.: Hamburg .
- Dalgleish , D.G. 1983 . Coagulation of renneted bovine casein micelles: Dependence on temperature, calcium ion concentration and ionic strength . Journal of Dairy Research , 50 : 331
- Clarke , M.A. Oct 12–13 1970 . The effect of solution structure on electrode processes in sugar solutions , Oct 12–13 , 179 – 188 . Boston, MA : Proceedings of the 1970; Technical Session on Cane Sugar Refining Research .
- Schwabe , K. 1976 . pH-Messung, Vol. WTB-Band 247 , Berlin, , Germany : Akademie-Verlag .