Abstract
This study provides a basis and principle for developing an integrated antioxidant assay of mulberry. Five genotypes (Miaoli No. 1, 73C020, 46C019, 74H3023, and 68H22024) and three maturity stages (unripe, medium ripe and fully ripe) of mulberry (Morus sp.) fruit were analyzed for their total phenolic contents (TPC), 1,1-diphenyl-2-picrylhydrazyl–scavenging (DPPH-SC) ability, ferric reducing antioxidant power (FRAP), and oxygen radical antioxidant capacity (ORAC). Significant correlations were obtained for the four assays used (r ranging from 0.390 to 0.787, all P < 0.01). Two-way ANOVA revealed that there were significant effects of genotype, maturity stage, and the interaction of genotypes and maturity stages for TPC, FRAP and ORAC, whereas only maturity stage and the interaction term were significant for DPPH-SC activity. Principal component analysis showed that Miaoli No. 1 and 74H3023 possessed the highest antioxidant capacity, while genotype 68H22024 possessed the lowest. Principal component analysis could group and separate mulberry genotypes based on their different antioxidant activities. Overall, the present results provided basic data for choosing mulberry fruits with higher antioxidant activity for direct consumption or for production of fruit juice.
INTRODUCTION
Growing evidence suggests that there is a positive correlation between the consumption of diets rich in fruits and vegetables and the reduction of risks for chronic diseases, such as cardiovascular diseases, arthritis, chronic inflammation, and cancers.[Citation1] It is generally believed that physiological function of fruits may be partly attributed to their antioxidant activity.[Citation2] Various berry fruits have been shown to have a high antioxidant potential.[Citation3–5] In vitro studies indicated that berries possessed a remarkably high scavenging activity toward chemically generated reactive oxygen species (ROS).[Citation3,Citation6] Mulberries are widely grown in Asia and are becoming an emerging fruit crop in Taiwan.[Citation7] In traditional Chinese medicine, many parts of the mulberry are used for medicinal purposes; for example, fruit is used to reduce inflammation and to stop bleeding, bark is used for toothache, and leaves are used for diabetic patients.[Citation4,Citation8]
Several methods are commonly used to determine “antioxidant activity,” including oxygen radical absorbance capacity (ORAC),[Citation9–10] ferric ion reducing antioxidant parameter (FRAP),[Citation11–12] and 1,1-diphenyl-2-picrylhydrazyl-scavenging activity (DPPH-SC).[Citation13–14] Different methods for determination of antioxidant activities are based on different reaction mechanisms, so they often give inconsistent results.[Citation15] No single method can give a comprehensive prediction of antioxidant activity, and it is not surprising that different antioxidant assay methods could produce inconsistent results.[Citation15] For example, the FRAP assay determines the reducing capability based on ferric ion, and ORAC only determines antioxidant activity against peroxyl radicals.[Citation16] Therefore, the use of more than one method has been recommended.[Citation1]
Mulberries have a highly complex mixture of phytochemicals, but there is no systematic method to integrate different antioxidant characteristics. Therefore, the aim of this research was to set up a reliable and simple procedure to estimate antioxidant activities by integrating multiple assays in mulberry fruits of different genotypes and maturity stages. A combination of different antioxidant assays was used, which included total phenolic contents (TPC), FRAP, DPPH-SC, and ORAC. Principal components analysis (PCA), a multivariate statistical technique, was performed in order to integrate the patterns of data, to reduce the dimension of data set, and to highlight the similarities and differences in the data.[Citation17]
MATERIALS AND METHODS
Chemicals
AAPH (2,2-azobis(2-amidinopropane) dihydrochloride) was purchased from Wako Chemicals (Richmond, VA, USA). B-PE (B-phycoerythrin from Porphyridium cruentum), Trolox (6-hydroxy-2,5,7,8-tetramethyl-2-carboxylic acid), DPPH (1,1-diphenyl-2-picrylhydrazyl), TPTZ (2,4,6-trypiridyls-triazine), Folin-Ciocalteu's reagent, FeCl3·6H2O, FeSO4·7H2O, and butylated hydroxytoluene (BHT) were obtained from Sigma (Sigma-Aldrich GmbH, Sternheim, Germany). All other chemicals and organic solvents are analytical grade and obtained from Merck (Merck KGaA, Darmstadt, Germany).
Sample Preparation
Miaoli No. 1 (Morus atropurpurea Roxb.), 46C019 (M. atropurpurea Roxb.) 73C020, 74H3023, and 68H22024 (unidentified genotype) were grown under natural photoperiod and identical condition in the orchard of Miaoli District Agricultural Research and Extension Station (MDARES), Kung-Kuan, Miaoli, Taiwan. All genotypes are deciduous, vigorous, and of abundant yield. All fruits start developing from February and can be harvested between late March and April of 2006. At least five different plants for each genotype, and samples of berries were taken from the same five plants throughout the experiment. The unripe stage referred to green fruit, which were harvested when the fresh weight reached approximately 2 g. The medium ripe stage refered to red fruits, which were harvested when the fresh weight reached approximately 3 g. The fully ripe stage referred to black-purple fruit, which were harvested when the fresh weight reached approximately 7 g. Undamaged berries were selected; 30–40 fruits were cut into small slices, mixed, and stored at −20°C until analyzed. To prepare the fruit extracts, samples weighing at least five grams from four replicates of each mulberry fruit ripeness stage were extracted twice with 10 mL of 80% acetone containing 0.2% formic acid using a Polytron (Brinkmann Instruments, Inc., Westbury, NY) for five minutes and then centrifuged at 13000 g for 10 min at 4°C. The supernatants were combined and transferred to vials, stored at −80°C, and then used for analyses of FRAP, ORAC, DPPH-SC and TPC.
Folin-Ciocalteu's Assay for Total Phenolic Contents (TPC)
TPC in mulberry fruit were determined according to a modified Folin-Ciocalteu's method.[Citation15] Gallic acid was used as the standard compound. Samples and standards were dissolved in 5 mL of 0.3% HCl in methanol/water (60:40, v/v). The solution (100 μL) was added to 2% Na2CO3 (2 mL). After 2 min, 50% Folin-Ciocalteu's reagent (100 μL) was added to the mixture, and set aside for 30 minutes. Absorbance was measured at 750 nm by a microplate multimode detector (Anthos Zenyth 3100, Anthos Labtec Instruments) and results were expressed as mg of gallic acid equivalent/100 g of fresh weight (mg GAE/ 100 g FW).
DPPH-Scavenging Activity Assay
DPPH, a stable radical, has been widely used for the determination of primary antioxidant activity, that is, the free radical scavenging activities of antioxidants, which produced a decrease in absorbance. DPPH-SC in mulberry fruit was determined according to a modified DPPH-SC assay.[Citation13] Briefly, aliquots (160 μL) of samples, controls, or the standard, BHT, were mixed with 40 μL of 10 mM DPPH in ethanol, respectively. The mixture was shaken vigorously and left to stand for 30 min at room temperature in the dark. Absorbance of the testing solution was measured at 525 nm by a microplate multimode detector and results were expressed as μM BHT equivalent/g FW.
Ferric Reducing Antioxidant Power (FRAP) Assay
The FRAP assay method was based on the reduction of Fe(III)(TPTZ)2Cl3 complex (as an oxidant) by antioxidants to form colored Fe(II)(TPTZ)2. FRAP in mulberry fruit was determined according to a modified FRAP assay.[Citation12] Briefly, 200 μL of the freshly prepared FRAP reagent, containing Fe(III)(TPTZ)2Cl3 and acetate buffer, was mixed with 30 μL of distilled water and 10 μL of the test sample or the blank (solvent control), pH 3.6 and was prepared freshly and warmed to 37°C. Absorbance at 595 nm was measured after incubation at 37°C for 5 min by a microplate multimode detector. FeSO4 was used as the standard. Results were expressed as mM ascorbic acid equivalent/g FW.
Oxygen Radical Absorbance Capacity (ORAC) Assay
All samples were measured using the ORAC assay, which uses B-phycoerythrin (B-PE) as an oxidizable protein substrate and AAPH as a peroxyl radical generator.[Citation18] Fifty micro-liters of samples, controls, or standard (Trolox at five different concentrations for construction of a standard curve) were mixed with 50 μL of 2.5 mg/L B-PE solution and 50 μL of PBS (pH 7.4, 75 mM). They were incubated at constant temperature (37°C, 15 min) and then 50 μL of 16 mM AAPH solution added to initiate the reaction. The fluorescence intensity [545 nm (Ex)/575 nm (Em)] was measured every five minutes for 70 minutes at 37°C by a microplate multimode detector. The ORAC value of a sample was calculated on the basis of the Trolox standard curve, with ORAC value assigned 1 μM Trolox.
Statistical Analyses
Data (mean ± SD) were analyzed using two-way ANOVA to determine the overall effect of genotype (G), maturity stage (S), and the interaction (G × S) on each of the antioxidant measurements. We performed the least significant differences (LSD) test using SAS-EG analyses software (SAS Institute Inc., Cary, NC, USA). A P value < 0.05 is considered statistically significant. The results from the four different assays were also subjected to principal component analysis (PCA), which are presented in terms of loading and score plots. Data pretreatment was conducted using the Z score transformation (mean = 0; standard deviation = 1) in order to eliminate the bias due to different units of the results from the four different antioxidant assays.
RESULTS AND DISCUSSION
Antioxidant Activity Analyses
The antioxidant values of the five genotypes of mulberry fruit obtained by the four assays (TPC, DPPH-SC, FRAP, and ORAC) varied widely; and the ranking of antioxidant values were inconsistent even for the same genotype and maturity stage. For example, TPC ranged from 475 ± 22 mg GAE/100g FW in the genotype 73C020 (medium ripe) to 2392 ± 103 mg GAE/100g FW in the genotype 46C019 (fully ripe), which is comparable to those (181 to 2570 mg GAE/100g FW) reported by others[Citation19–22] in mulberry fruits. The variation of TPC depends on many factors, such as the degree of genotype, maturity stage, and environmental conditions during fruit development.[Citation22]
Two-way ANOVA revealed significant effects of genotype (G) (P < 0.001), maturity stage (S) (P < 0.001), and interaction of genotype and maturity stage (G × S) (P < 0.001) for TPC, FRAP, OPRAC, whereas only S and G × S were significant for DPPH-SC (). In general, TPC, FRAP, OPRAC and DPPH-SC values increased with increasing maturity (i.e., from unripe to fully ripe), although TPC in genotypes 73C020, 46C019 and 74H3023 were somewhat lower at the medium ripe stage than at the unripe stage. Interestingly, TPC in raspberry fruits has also been shown to decrease to some extent from the unripe stage to the medium ripe stage, then to increase substantially at the ripe stage.[Citation9] For DPPH-SC, two-way ANOVA revealed significant effects of maturity stage (P < 0.001) and the interaction (G × S), but not of genotype (P = 0.81) (), indicating that pattern of changes in DPPH-SC value with fruit maturity were not the same for all cultivars.
Table 1 Two-way ANOVA for TPC, DPPH-SC, FRAP, and ORAC of five genotypes and three maturity stages of mulberry fruit.Footnote a
It is not surprising that antioxidant activities of mulberry fruits were affected by genotypes and maturity stages, since different antioxidant activities were also reported for various species and genotypes as well as for maturity stages of blueberries[Citation23]. Interestingly, the ORAC values of fully ripe mulberries reported here are higher than or similar to those reported for other common fruits, including tomato, raspberry, and strawberry.[Citation9,Citation13]
Correlations of Antioxidant Assays
Significant correlations existed for the results from the four different antioxidant assays used in this study (r ranging from 0.390 to 0.787, all P < 0.01). The highest correlation coefficient was found between ORAC and FRAP (r = 0.787, P < 0.001, N = 60) and the lowest one was between DPPH-SC and TPC (r = 0.390, P < 0.01, N = 60). In addition, TPC gave significantly positive correlations with FRAP and ORAC (r = 0.636 and 0.545, both P < 0.001, N = 60), suggesting that phenolic compounds were the major contributor to of FRAP and ORAC in mulberry. Significant correlations were also found between TPC and FRAP in other genotypes of mulberry (M. nigra and M. rubra)[Citation24] as well as between FRAP and ORAC in blackberry and strawberry.[Citation25–26] The relatively low correlation between TPC and DPPH-SC may be due to the fact that the Folin-Ciocalteu's reagent can only roughly estimate the amount of phenolic compounds.[Citation27] A possible explanation for the observation that high TPC did not correspond to a high DPPH-SC could be due to the antagonistic and synergistic reactions between phenolics and other phytochemicals in mulberry fruit. These results suggested that the antioxidant activity of mulberry determined by one method may only partially reflect its overall antioxidant potential.
Principle Component Analysis (PCA)
PCA was used in the classification of antioxidant activities of pomegranate juices[Citation17] and selected plant extracts.[Citation27] In the present study, PCA was performed to group and separate four antioxidant assays of the five mulberry genotypes and three maturity stages. The loadings plot (A) was used to gain an overview of the importance among the four antioxidant assays. The loadings of first and second principal components (PC1 and PC2) accounted for 63 and 18% of the variance, respectively. In PC1, all four assays had positive loadings, whereas in PC2 only DPPH-SC showed a positive loading. The results suggest that DPPH-SC may be more useful for assaying the antioxidant activity in mulberry fruits than FRAP, ORAC and TPC, because DPPH-SC only require the transfer of a hydrogen atom (i.e., an electron transfer).
Figure 1 Loadings and scores plots of principle components analysis. (A) Loadings plot for results of four different antioxidant assays. TPC: total phenolic contents; DPPH-SC: DPPH-scavenging activity; FRAP: ferric reducing antioxidant power; ORAC: oxygen radical absorbance capacity. (B) Scores plot for the five genotypes of mulberry. PC1: first principle components; and PC2: second principle components.
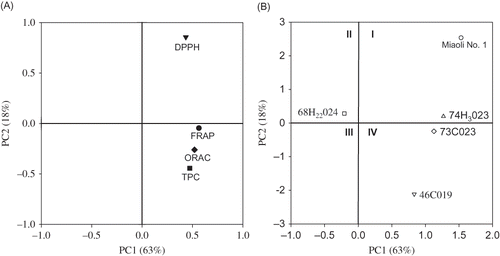
The scores plot (B) was used to gain an overview of the similarities or differences among the mulberry genotypes. The analysis demonstrated that among the five mulberry genotypes, Miaoli No. 1 and 74H3023 were located at quadrant I, which represents the highest antioxidant activity. The genotype 68H22024 was located at quadrant II, and this genotype had the lowest antioxidant activity among the five genotypes used here. The distance between the locations of any two samples on the scores plot increased as the degree of difference increased between them. For instance, the two genotypes, Miaoli No. 1 and 68H22024, were located on the opposite side of PC1, and a possible explanation for the difference is that the results of FRAP and ORAC are widely different among different genotypes. Thus, PCA could be used to discriminate the genotypes of mulberry on the basis of their antioxidant activity.
CONCLUSIONS
It is important to provide an optimum method to assess mulberry antioxidant activity so that more reliable and useful information can be used by other researchers as well as the mulberry food industry. In this study, we demonstrate significant correlations among the four antioxidant assays used. Two-way ANOVA revealed that there were significant effects of genotype, maturity stage, and the interaction of genotypes and maturity stages for TPC, FRAP, and ORAC, whereas only maturity stage and the interaction were significant for DPPH-SC activity. In addition, PCA showed that Miaoli No. 1 and 74H3023 possess the highest antioxidant capacity, while genotype 68H22024 possessed the lowest. Thus, the present results provided the basic data for choosing mulberry fruit with higher antioxidant activity for direct consumption or for production of fruit juice.
ACKNOWLEDGEMENTS
This research was financially supported by a grant from the Council of Agriculture, Executive Yuan, ROC. The authors thank Dr. Shiow Y. Wang for her valuable suggestions on the ORAC assay and critical review of the manuscript. LC Li, ZF Chiou and JC Chang are gratefully acknowledged for their technical assistance on this work.
REFERENCES
- Aruoma , O.I. 2003 . Methodological Considerations for Characterizing Potential Antioxidant Actions of Bioactive Components in Plant Foods . Mutat. Res. , 523–524 : 9 – 20 .
- Wang , H. , Cao , G. and Prior , R.L. 1996 . Total Antioxidant Capacity of Fruits . J. Agric. Food Chem. , 44 ( 3 ) : 701 – 705 .
- Zafra-Stone , S. , Yasmin , T. , Bagchi , M. , Chatterjee , A. , Vinson , J.A. and Bagchi , D. 2007 . Berry Anthocyanins as Novel Antioxidants in Human Health and Disease Prevention . Mol. Nutr. Food Res. , 51 ( 6 ) : 675 – 683 .
- Naderi , G. , Asgary , S. , Sarraf-Zadegan , N. , Oroojy , H. and Afshin-Nia , F. 2004 . Antioxidant Activity of Three Extracts of Morus nigra . Phytother. Res. , 18 ( 5 ) : 365 – 369 .
- Heinonen , I.M. , Meyer , A.S. and Frankel , E.N. 1998 . Antioxidant Activity of Berry Phenolics on Human Low-density Lipoprotein and Liposome Oxidation . J. Agric. Food Chem. , 46 ( 10 ) : 4107 – 4112 .
- Ogawa , K. , Sakakibara , H. , Iwata , R. , Ishii , T. , Sato , T. , Goda , T. , Shimoi , K. and Kumazawa , S. 2008 . Anthocyanin Composition and Antioxidant Activity of the Crowberry (Empetrum nigrum) and Other Berries . J. Agric. Food Chem. , 56 ( 12 ) : 4457 – 4462 .
- Chang , J.C. 2008 . ’Miaoli No. 1’ Mulberry: a New Cultivar for Berry Production . HortScience , 43 ( 5 ) : 1594 – 1595 .
- Andallu , B. , Suryakantham , V. , Lakshmi Srikanthi , B. and Reddy , G.K. 2001 . Effect of Mulberry (Morus indica L.) Therapy on Plasma and Erythrocyte Membrane Lipids in Patients with Type 2 Diabetes . Clin. Chim. Acta , 314 ( 1–2 ) : 47 – 53 .
- Wang , S.Y. and Lin , H.S. 2000 . Antioxidant Activity in Fruits and Leaves of Blackberry, Raspberry, and Strawberry Varies with Cultivar and Developmental Stage . J. Agric. Food Chem. , 48 ( 2 ) : 140 – 146 .
- Zheng , W. and Wang , S.Y. 2003 . Oxygen Radical Absorbing Capacity of Phenolics in Blueberries, Cranberries, Chokeberries, and Lingonberries . J. Agric. Food Chem. , 51 ( 2 ) : 502 – 509 .
- Huang , R. , Xia , R. , Hu , L. , Lu , Y. and Wang , M. 2007 . Antioxidant Activity and Oxygen-scavenging System in Orange Pulp during Fruit Ripening and Maturation . Sci. Hortic. , 113 ( 2 ) : 166 – 172 .
- Guo , C. , Yang , J. , Wei , J. , Li , Y. , Xu , J. and Jiang , Y. 2003 . Antioxidant Activities of Peel, Pulp and Seed Fractions of Common Fruits as Determined by FRAP Assay . Nutr. Res. , 23 ( 12 ) : 1719 – 1726 .
- Mahattanatawee , K. , Manthey , J.A. , Luzio , G. , Talcott , S.T. , Goodner , K. and Baldwin , E.A. 2006 . Total Antioxidant Activity and Fiber Content of Select Florida-grown Tropical Fruits . J. Agric. Food Chem. , 54 ( 19 ) : 7355 – 7363 .
- Huang , D. , Ou , B. and Prior , R.L. 2005 . The Chemistry behind Antioxidant Capacity Assays . J. Agric. Food Chem. , 53 ( 6 ) : 1841 – 1856 .
- Prior , R.L. , Wu , X. and Schaich , K. 2005 . Standardized Methods for the Determination of Antioxidant Capacity and Phenolics in Foods and Dietary Supplements . J. Agric. Food Chem. , 53 ( 10 ) : 4290 – 4302 .
- Ou , B. , Huang , D. , Hampsch-Woodill , M. , Flanagan , J.A. and Deemer , E.K. 2002 . Analysis of Antioxidant Activities of Common Vegetables Employing Oxygen Radical Absorbance Capacity (ORAC) and Ferric Reducing Antioxidant Power (FRAP) Assays: a Comparative Study . J. Agric. Food Chem. , 50 ( 11 ) : 3122 – 3128 .
- Çam , M. , HisIl , Y. and Durmaz , G. 2009 . Classification of Eight Pomegranate Juices Based on Antioxidant Capacity Measured by Four Methods . Food Chemistry , 112 ( 3 ) : 721 – 726 .
- Cao , G. , Alessio , H.M. and Cutler , R.G. 1993 . Oxygen-radical Absorbance Capacity Assay for Antioxidants . Free Radic. Biol. Med. , 14 ( 3 ) : 303 – 311 .
- Bae , S.H. and Suh , H.J. 2007 . Antioxidant Activities of Five Different Mulberry Cultivars in Korea . LWT-Food Sci. Technol. , 40 ( 6 ) : 955 – 962 .
- Ercisli , S. and Orhan , E. 2008 . Some Physico-chemical Characteristics of Black Mulberry (Morus nigra L.) Genotypes from Northeast Anatolia region of Turkey . Sci. Hortic. , 116 ( 1 ) : 41 – 46 .
- Lin , J.Y. and Tang , C.Y. 2007 . Determination of Total Phenolic and Flavonoid Contents in Selected Fruits and Vegetables, as well as Their Stimulatory Effects on Mouse Splenocyte Proliferation . Food Chem. , 101 ( 1 ) : 140 – 147 .
- Gungor , N. and Sengul , M. 2008 . Antioxidant Activity, Total Phenolic Content and Selected Physicochemical Properties of White Mulberry (Morus Alba L.) Fruits . Int. J. Food Prop. , 11 ( 1 ) : 44 – 52 .
- Prior , R.L. , Cao , G. , Martin , A. , Sofic , E. , McEwen , J. , O'Brien , C. , Lischner , N. , Ehlenfeldt , M. , Kalt , W. , Krewer , G. and Mainland , C.M. 1998 . Antioxidant Capacity as Influenced by Total Phenolic and Anthocyanin Content, Maturity, and Variety of Vaccinium Species . J. Agric. Food Chem. , 46 ( 7 ) : 2686 – 2693 .
- Özgen , M. , Serçe , S. and Kaya , C. 2009 . Phytochemical and Antioxidant Properties of Anthocyanin-rich Morus nigra and Morus rubra Fruits . Sci. Hortic. , 119 ( 3 ) : 275 – 279 .
- Reyes-Carmona , J. , Yousef , G.G. , Martinez-Peniche , R.A. and Lila , M.A. 2005 . Antioxidant Capacity of Fruit Extracts of Blackberry (Rubus sp.) Produced in Different Climatic Regions . J. Food Sci. , 70 ( 7 ) : S497 – S503 .
- Aaby , K. , Skrede , G. and Wrolstad , R.E. 2005 . Phenolic Composition and Antioxidant Activities in Flesh and Achenes of Strawberries (Fragaria ananassa) . J Agric Food Chem , 53 ( 10 ) : 4032 – 4040 .
- Wong , S.P. , Leong , L.P. and Koh , J.H.W. 2006 . Antioxidant Activities of Aqueous Extracts of Selected Plants . Food Chem , 99 ( 4 ) : 775 – 783 .