Abstract
Five commonly consumed pulses, Mah (Vigna mungo), Green mung (Vigna radiata), Arhar (Cajanas cajan), Masur (Lens esculantus), and Moth (Vigna aconitifolia), were studied for their total phenolic content and antioxidant activity after germination (12 and 24 h) and pressure cooking. Arhar had the highest total phenolic content (6.71 mg ferulic acid/g flour) whereas Moth had the least (1.54 mg/g). All pulses, except Moth, showed a significant decrease in total phenolic content after germination. The antioxidant activity of the pulses varied from 10.61 to 36.38% (DPPH radical scavenging activity), which significantly decreased with germination in all pulses except Moth. The total phenolic content highly correlated with the antioxidant activity in the pulses. Cooking lowered the total phenolic content by 10–45% and antioxidant activity by 27–68% in the control and germinated pulses.
INTRODUCTION
Pulses belong to the family of leguminous plants that are grown for their dry fruit. They are an important source of macronutrients (such as proteins, carbohydrates, dietary fiber), micronutrients, vitamins, carotenoids, and phenolic compounds.Citation[1] India is the largest consumer and importer of pulses in the world and pulses are consumed regularly in every household at least with one meal. Pulses are also popular because animal proteins are scarce and expensive or are not consumed especially in India due to religious or cultural reasons. Cereal grains (which are themselves deficient in lysine) are commonly consumed along with pulses (relatively poor in the essential amino acid methionine) to form a complete protein diet leading to protein supplementation and complementation.
Germination is one of the most common and effective processes for improving the nutritional quality of pulses, not only by the reduction of anti-nutritive compounds, but by augmenting the levels of free amino acids, available carbohydrates, dietary fiber, and other components[Citation2–5] and increasing the functionality of the seeds due to the subsequent increase in the bio-active compounds.Citation[6] Phenolic compounds are a group of phytochemicals that exhibit a wide range of physiological properties, such as anti-allergenic, anti-artherogenic, anti-inflammatory, anti-microbial, antioxidant, cardio protective, and vasidilatory effects.[Citation7–9] They could be a major determinant of antioxidant potential of foodsCitation[10] and could, therefore, be a natural source of antioxidants. Antioxidants act as protective factors against oxidative damage in the human bodyCitation[11,Citation12] and prevent the development of chronic diseases, such as cancer, heart disease, stroke, and cataracts.Citation[13]
In India, pulses are the main source of protein and Mah, Green mung, Arhar, Masur, and Moth are commonly consumed pulses. The pulse along with water, salt, and some spice is cooked in a pot or pressure cooker till the grain bursts and a soup like consistency is formed. Pulses are carriers of phenolic compounds and have significant antioxidant potential. The changes occurring in the phenolic content and antioxidant activity upon germination and especially after pressure cooking have not been reported. Given the important role that pulses play in nutrition, the changes occurring in the total phenolic content and antioxidant activity as a result of germination and pressure cooking needed to be investigated.
MATERIALS AND METHODS
Germination
The whole seeds of Mah (Vigna mungo), Green mung (Vigna radiata), Arhar (Cajanas cajan), Masur (Lens esculantus), and Moth (Vigna aconitifolia) were procured from the local market. The pluses were cleaned and steeped for 24 h at 25°C and care was taken that water was changed at 2-h intervals. After soaking, pulses were allowed to germinate in an incubator at 25°C and 100% RH for 12 and 24 h. The germinated pulses were dried in a dryer at 40°C and were ground to pass through a 60 mesh sieve and packed in air tight bags for further analysis.
Cooking
Whole pulses 50 g (control or germinated) were taken in a 3 L capacity Hawkins pressure cooker (Hawkins Cookers Limited, Bombay, India) with four-fold of water and cooking done at a pressure of 3.3 N/m2 for optimum time. Preliminary trials were carried out to determine the optimum cooking time. The time at which the pulses split and showed no internal white core when pressed between two glass slides was taken as the cooking time. The cooking time was Mah (15 min), Arhar (14 min), Green mung (12 min), and Masur and Moth (11 min). The cooked pulses were freeze dried in a freeze dryer (Sim International, Newark, USA). The freeze-dried pulses were ground with a hand grinder and passed through a 60 mesh sieve and packed in air tight bags to prevent any moisture gain till further analysis.
Total Phenolic Content (TPC)
The TPC was determined according to the Folin-Ciocalteu spectrophotometric method as described by Singleton and Rossi.Citation[14] The ground sample (200 mg, dry weight basis) taken in centrifugal tubes was extracted with 4 ml of acidified methanol for 2 h. It was centrifuged for 10 min and 1.5 ml of ten-fold diluted Folin and Ciocalten phenol reagent (Sigma, St. Louis, MO, USA) was added to the supernatant followed by addition of ten-fold diluted 6% sodium carbonate solution (Qualikems Ltd., Vadodra, India). The samples were incubated at room temperature for 90 min and absorbance was read at 725 nm. The total phenolic content was calculated using a standard plot of ferulic acid and expressed as mg ferulic acid/g flour (dry weight basis).
Antioxidant Activity
The antioxidant activity was measured by the method of Brand-Williams et al.Citation[15] Methanol (1 ml) was added to ground sample (100 mg, dry weight basis) taken in a centrifuge tube and extracted for 2 h. The tube were centrifuged for 10 min and the supernatant was reacted with 3.9 ml of 6 × 10−5 mol/L 2,2-diphenyl-1-picrylhydrazyl (DPPH; Sigma, St. Louis, MO, USA) and incubated at room temperature for 30 min. Methanol was used as blank. Absorbance of the control sample at ‘0’ min was taken at 515 nm. Reduction in absorbance of samples was observed after 30 min. The AOA was expressed in percentage:
where t 0 and t 30 are absorbance at zero time and at 30 min.
Statistical Analysis
All tests were performed in triplicates on dry weight basis. Analysis of variance was carried out and Fishers least significant difference test was used to describe means with 95% confidence. The Pearson correlation coefficients were calculated by SPSS software (SPSS Inc., Chicago, IL, USA) at a probability level of (p ≤ 0.05).
RESULTS AND DISCUSSION
The ash content of the pulses was insignificantly (p ≤ 0.05) affected by germination (). Arhar, Moth, and Masur showed no significant change after 24 h of germination. Mah showed some decrease, whereas Green mung showed an insignificant increase in the ash content. Akpapunam and AchinewhuCitation[16] observed similar results for ash content in different germinated pulses and legumes. The protein content in Mah and Arhar increased significantly (p ≤ 0.05) up to 24 h germination (). The increase in protein content may be attributed to the synthesis of cell constituents and enzymes, which lead to degradation of other constituents.Citation[17] However, in Masur and Moth, the protein content significantly (p ≤ 0.05) decreased up to 12 h germination and then further increased upon 24 h germination. The protein content in Green mung significantly (p ≤ 0.05) increased up to 12 h germination and then significantly (p ≤ 0.05) decreased up to 24 h germination.
Table 1 Effect of germination duration on proximate composition of different pulses
The fat content increased significantly (p ≤ 0.05) in Mah, Green mung, Masur, Moth, and Arhar up to 24 h germination (). All germinated samples contained more ether extractable lipids than the raw, which may be attributed to dissociation of lipid complexes.Citation[16] Lee et al.Citation[18] reported that the crude fat and protein content increased significantly after germination in brown rice. Increase in these constituents has also been reported by Kim et al.Citation[19] for soybeans, Park et al.Citation[20] for mung beans, and by Jung et al.Citation[21] for germinated brown rice. The crude fiber content significantly (p ≤ 0.05) increased in Mah, Green mung, Masur, Moth, and Arhar up to 24 h germination (). An increase in the dietary fiber after germination has been reported by Lee et al.Citation[18] for brown rice, Lee et al.Citation[22] for buckwheat, and Kim et al.Citation[19] for soybeans.
Effect of Germination on TPC
The TPC varied from 1.54–6.71 mg/g in the different pulses with Arhar showing the highest content and Moth showing the least (). The TPC in Mah decreased by 35.79% up to 12 h germination and then showed an increase of 12.84% upon further 12 h germination. In Green mung, the TPC significantly (p ≤ 0.05) decreased by 16.72% upon 12 h germination and further decreased by 41.73% upon 24 h germination. Randhir et al.Citation[23] reported that germination causes a decrease of total phenolic content in Green mung. Barroga et al.Citation[24] reported similar total phenolic content values for raw and 24 h germinated Mung bean.
Table 2 Effect of germination on total phenolic content and antioxidant activity of pulses
When germinated for 12 and 24 h, the TPC in Arhar decreased by 23.39 and 13.61%, respectively. In Masur, a significant decrease of 48.7% was observed in the first 12 h of germination; however, further decrease of 4.4% after germination up to 24 h was not significant. Lopez-Amoros et al.Citation[25] reported decrease in trans ferulic acid and trans p-coumaric acid as germination time increased in legumes. The reduction in phenolic compounds could be due to physical leaching off the phenolic compounds into the soaking water.Citation[26] The decrease in TPC has also been attributed to enzymatic activity during germination.[Citation27–30
In Moth, the TPC significantly (p ≤ 0.05) increased by 20.12% in the first 12 h of germination and showed a significant increase of 48.10% upon germination for 24 h. Tian et al.Citation[31] reported that during germination, the bound phenolic compounds become free and lead to an increase in the total phenolic content. Fernandez-Orozco et al.Citation[32] reported that the total phenolic content significantly decreased after 2 days of germination but then increased as germination time increased to 4 days. During germination, the endogenous enzymes of the legumes are activated and the most important enzymes are the hydrolases and polyphenoloxydases, whose activity increases during germination depending on the type of legume. Khattak et al.Citation[33] reported that germination time up to 48 h significantly reduced the phytic acid content in chickpea.
Effect of Germination on Antioxidant Activity
The antioxidant activity in control samples ranged from 10.61–36.38% with the highest activity exhibited by Masur and the lowest exhibited by Moth (). Antioxidant activity is expressed as percent DPPH radical scavenging activity with higher values indicating greater antioxidant activity. During germination of Mah, antioxidant activity significantly decreased (p ≤ 0.05) by 56.43% at 12 h germination and as germination increased from 12 to 24 h, it significantly increased by 23.55%. The total phenolic content and antioxidant activity for Mah showed a positive correlation coefficient of 0.99.
In Green mung, the antioxidant activity insignificantly (p ≤ 0.05) decreased by 5.33% after 12 h germination and after 24 h germination, further significantly decreased by 13.68%. The total phenolic content and antioxidant activity showed a positive correlation coefficient of 0.99. Fernandez-Orozco et al.Citation[32] reported that as compared to raw seeds, sprouts of mung bean and soybean had more total phenolic compounds, and germination is a good process for obtaining functional flours with greater antioxidant capacity and more antioxidant compounds than the raw legumes.
The antioxidant activity in Arhar significantly (p ≤ 0.05) decreased by 48.94% after 12 h germination; after germination for 24 h, it showed a insignificant decrease of 3.53%. Arhar showed a positive correlation coefficient of 0.962 between the total phenolic content and antioxidant activity. ObohCitation[34] also studied the antioxidant activity of legumes and found a positive correlation between phenolic compounds and antioxidant activity. Upon germination for 12 and 24 h the antioxidant activity in Masur significantly (p ≤ 0.05) decreased by 42.85 and 15.15%, respectively. The total phenolic content and antioxidant activity showed a positive correlation coefficient of 0.99 in Masur. Lopez-Amoros et al.Citation[25] reported a decrease in antioxidant activity in Masur as germination time increased.
The antioxidant activity in Moth significantly (p ≤ 0.05) increased by 18.75% after germination for 12 h and further increased by 32.14% as germination time increased to 24 h. The total phenolic content and antioxidant activity showed a positive correlation coefficient of 0.99 in Moth. Correlation between the total phenolic content and antioxidant activity of some plant foods has been reported by Sun et al.Citation[35], Chu et al.Citation[13], and Yang et al.Citation[36]
Effect of Cooking on TPC
The total phenolic content after cooking () significantly (p ≤ 0.05) decreased by 26.31 and 43.44% in the control and 12-h germinated Mah and further decreased by 5.08% after 24 h germination but this decrease was insignificant. Rocha-Guzman et al.Citation[37] studied three common bean cultivars for phenolic content and free radical scavenging activity before and after autoclaving and reported that phenolic content in common beans after pressure cooking was reduced by 90%. Barroga et al.Citation[24] found that boiling and cooking reduced the amount of phenols in legumes by 73%.
Figure 1 Effect of cooking on total phenolic content (a) and antioxidant activity (b) in control, 12 h and 24 h germinated pulses, a and b superscripts are significantly (p ≤ 0.05) different within a treatment.
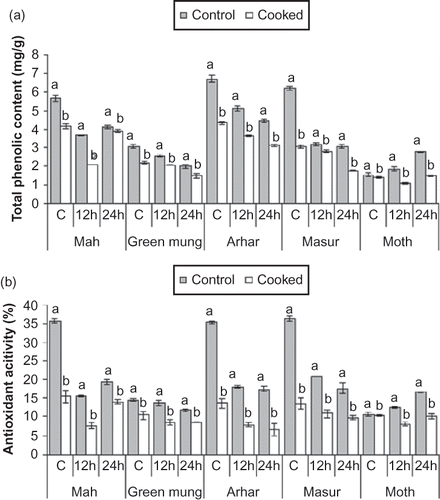
In Green mung, the total phenolic content in control significantly (p ≤ 0.05) decreased by 28.52% after cooking, whereas in 12- and 24-h germinated samples it significantly decreased by 19.68 and 25.25%, respectively (). After cooking the control, 12- and 24-h germinated Arhar in the pressure cooker, the TPC significantly decreased by 35.17, 28.98, and 29.95%, respectively (). In Masur, the total phenolic content after cooking significantly (p ≤ 0.05) decreased by 50.97, 12.58, and 42.11% in the control, 12- and 24-h germinated samples (). Vidal-Valverde et al.Citation[30] and Rocha-Guzman et al.Citation[37] reported similar results for cooked beans and observed a significant decrease in total polyphenols for soaked and germinated Masur.
Upon cooking, the total phenolic content decreased by 10.39, 40.54, and 45.99% in the control, 12- and 24-h germinated Moth (). Rocha-Guzman et al.Citation[37] also reported that cooking under pressure (autoclaving temperature 121°C and pressure 103.421 KPa) significantly decreased the level of total phenolic content in common beans.
Effect of Cooking on Antioxidant Activity
After cooking, the antioxidant activity significantly (p ≤ 0.05) decreased () by 56.56, 51.29, and 27.03% in the control, 12- and 24-h germinated samples. Mah showed a positive correlation coefficient of 0.99 between the total phenolic content and antioxidant activity. In Green mung, cooking significantly lowered the antioxidant activity (p ≤ 0.05) by 27.65, 37.7, and 27.48% in the control, 12- and 24-h germinated samples (). The total phenolic content and antioxidant activity showed a correlation coefficient of only 0.640 in Green mung.
In Arhar, the antioxidant activity significantly (p ≤ 0.05) decreased after cooking by 61.53, 56.64, and 61.47% in the control, 12- and 24-h germinated samples (). Arhar showed a positive correlation coefficient of 0.95 for the total phenolic content and antioxidant activity. Cooking lowered the antioxidant activity significantly (p ≤ 0.05) by 62.84, 47.33, and 44.5% in the control, 12- and 24-h germinated Masur samples (). Masur showed a positive correlation coefficient of 0.85 for the total phenolic content and antioxidant activity. In cooked ungerminated Moth, the decrease of 2.54% in the antioxidant activity was not significant, however, the decrease of 35.63 and 38.92% in the 12- and 24-h germinated samples was significant (). The total phenolic content and antioxidant activity showed a positive correlation coefficient of 0.947 in cooked Moth. Xu and ChangCitation[38] reported loss of antioxidant activity to leaching of soluble antioxidants in water and heat effect during cooking.
CONCLUSIONS
It was concluded that germination lowered the phenolic content and antioxidant activity in all pulses except Moth in which the TPC and antioxidant activity increased with germination. Cooking significantly lowered the total phenolic content and antioxidant activity in the pulses. There was a significant correlation between the total phenolic content and antioxidant activity both in germinated and cooked pulses.
REFERENCES
- Adsule , R.N. , Kadam , S.S. and Kadam , S.S. 1989 . “ World food legumes ” . In Legumes Nutritional Chemistry, Processing Technology and Utilization , Edited by: Salunkhe , D.K. Vol. II , 75 – 97 . Boca Raton, FL : CRC Press .
- Rubio , L.A. , Muzquiz , M. , Burbano , C. , Cuadrado , C. and Pedrosa , M.M. 2002 . High apparent digestibility of amino acids in raw and germinated faba bean (Vicia faba) and chick pea (Cicer arietinum) based diets for rats . Journal of the Science of Food and Agriculture , 82 : 1710 – 1717 .
- Vidal-Valverde , C. , Frias , J. , Sierra , I. , Blazquez , I. , Lambien , F. and Kuo , Y.H. 2002 . New functional legume food by germination: effect on the nutritive value of beans, lentils and peas . European Food Research and Technology , 215 : 472 – 476 .
- Vidal-Valverde , C. , Frias , J. , Hernandez , A. , Martin-Alvarez , P.J. , Sierra , I. , Rodriguez , C. , Blazquez , I. and Vicente , G. 2003 . Assessment of nutritive compounds and antinutritive factors in pea (Pisum sativum) seeds . Journal of the Science of Food and Agriculture , 83 : 1 – 4 .
- Zanabaria , E.R. , Katarzyna , N. , De Jong , L.E.Q. , Birgit , H.B.E. and Robert , M.J.N. 2006 . Effect of food processing on pearl millet (Pennisetum glaucum) IKMP-5 on the levels of phenolics, phytate, iron and zinc . Journal of the Science of Food and Agriculture , 86 : 1391 – 1398 .
- Frias , J. , Fernandez-Orozco , R. , Zielinski , H. , Piskula , M. , Kozlowska , H. and Vidal-Valverde , C. 2002 . Effect of germination on the content of vitamin C and E of lentils . Polish Journal of Food and Nutrition Science , 52 : 76 – 82 .
- Middleton , E. , Kandaswami , C. and Theoharides , T.C. 2000 . The effects of plant flavonoids on mammalian cells: Implications for inflammation, heart disease and cancer . Pharmacological Reviews , 52 : 673 – 751 .
- Mandic , A.I. , Dilas , M.S. , Letkovic , G.S. , Canadanovic-Brunet , M. and Tumbas , V.T. 2008 . Polyphenolic composition and antioxidant activities of grape seed extract . International Journal of Food Properties , 11 : 713 – 726 .
- Herken , E.N. , Erel , O. , Guzal , S. , Celik , H. and Ibanoglu , S. 2009 . Total antioxidant, phenolic compounds and total oxidant status of certified and uncertified Turkey's honey . International Journal of Food Properties , 12 : 461 – 468 .
- Parr , A.J. and Bolwell , G.P. 2000 . Phenols in plant and in man: The potential for possible nutritional enhancement of the diet by modifying the phenols content or profile . Journal of the Science of Food and Agriculture , 80 : 985 – 1012 .
- Cano , A. and Arnao , M.B. 2005 . Hydrophilic and lipophilic antioxidant activity in different leaves of three lettuce varieties . International Journal of Food Properties , 8 : 521 – 528 .
- Kikuzaki , H. , Hisamoto , M. , Hirose , K. , Akiyama , K. and Taniguchi , H. 2002 . Antioxidant properties of ferulic acid and its related compounds . Journal of Agricultural Food Chemistry , 50 : 2161 – 2168 .
- Chu , Y. , Sun , J. , Wu , X. and Liu , R.H. 2002 . Antioxidant and antiproliferative activities of common vegetables . Journal of Agricultural and Food Chemistry , 50 : 6910 – 6916 .
- Singleton , V.A. and Rossi , J.A. 1965 . Colorimetry of total phenolics with phosphomolybedic-phosphotungestic acid reagents . American Journal Enol Viticulture , 16 : 144 – 158 .
- Brand-Williams , W. , Cuvelier , M.E. and Berset , C. 1995 . Use of a free radical method to evaluate antioxidant activity . Lebensmittel-Wissenschaft and Technology , 28 : 25 – 30 .
- Akpapunam , M.A. and Achinewhu , S.C. 1985 . Effect of cooking, germination and fermentation on the chemical composition of Nigerian cow pea (Vigna unguiculata) . Plant Foods for Human Nutrition , 35 : 353 – 358 .
- Lee , C.K. and Karunanithy , R. 1990 . Effects of germination in the chemical composition of glycine and phaseolus beans . Journal of Science of Food and Agriculture , 51 : 437 – 445 .
- Lee , Y.R. , Kim , J.Y. , Woo , K.S. , Hwang , I.G. , Kim , K.H. , Kim , K.J. , Kim , J.H. and Jeong , H.S. 2007 . Changes in chemical and functional components of Korean rough rice before and after germination . Food Science and Biotechnology , 16 : 1006 – 1010 .
- Kim , S.D. , Kim , S.H. and Hong , E.H. 1993 . Composition of soybean sprout and its nutritional value . Korean Soybean Digest , 10 : 1 – 9 .
- Park , D.Y. , Cho , S.J. and Shin , Y.C. 1986 . Change of protein pattern of mung bean seeds (Phaseolus aureus) during germination . Korean Journal of Food Science and Technology , 18 : 162 – 168 .
- Jung , G.H. , Park , N.Y. , Jang , S.M. , Lee , J.B. and Jeong , Y.J. 2005 . Effect of germination in brown rice by addition of chitisan/glutamic acid . Korean Journal of Food Preservation , 4 : 538 – 543 .
- Lee , M.H. , Woo , S.J. , Oh , S.K. and Kwon , T.B. 1994 . Changes in content and composition of dietary fiber during buckwheat germination . Korean Journal of Food Nutrition , 7 : 274 – 283 .
- Randhir , R. , Lin , Y. and Shetty , K. 2004 . Stimulation of phenolics, antioxidant and antimicrobial activities in dark germinated mung bean sprouts in response to peptide and phytochemical elicitors . Process Biochemistry , 39 : 637 – 646 .
- Barroga , C.F. , Laurena , A.C. and Mendoza , E.M.T. 1985 . Polyphenols in mung bean (Vigna radiata L.): Determination and removal . Journal of Agricultural and Food Chemistry , 33 : 1006 – 1009 .
- Lopez-Amoros , M.L. , Hernandez , T. and Estrella , I. 2006 . Effect of germination on legume phenolic compounds and their antioxidant activity . Journal of Food Composition and Analysis , 19 : 277 – 283 .
- Dicko , M.H. , Gruppen , H. , Traore , A.S. , Van-Berkel , W.J.H. and Voragen , A.G.J. 2005 . Evaluation of the effect of germination on phenolic compounds and antioxidant activity in sorghum varieties . Journal of Agricultural and Food Chemistry , 53 : 2581 – 2588 .
- Ayet , G. , Burbano , C. , Cuadrado , C. , Pedrosa , M.M. , Robredo , L.M. , Muzquiz , M. , Cuadra , C. , Castano , A. and Osagis , A. 1985 . Effect of germination, under different environmental conditions, on saponins, phytic acid and tannins in lentils (Lens culinaris) . Journal of the Science of Food and Agriculture , 74 : 273 – 278 .
- Danisova , C. , Holotnakova , E. , Hozova , B. and Buchtova , V. 1995 . Effect of germination on a range of nutrients of selected grains and legumes . Acta Alimentaria , 23 : 287 – 298 .
- Urbano , G. , Lopez-Jurado , M. , Hernandez , J. , Fernandez , M. , Moren , M.C. , Frias , J. , Diaz-Pollan , C. , Prodanov , M. and Vidal-Valverde , C. 1995 . Nutritional assessment of raw, heated and germination in lentils . Journal of Agricultural and Food Chemistry , 43 : 1871 – 1877 .
- Vidal-Valverde , C. , Frias , J. , Estrella , I. , Gorospe , M.J. , Ruiz , R. and Bacon , J. 1994 . Effect of processing on some antinutritional factors of lentils . Journal of Agriculture and Food Chemistry , 42 : 2291 – 2295 .
- Tian , S. , Nakamura , K. and Kayahara , H. 2004 . Analysis of phenolic compounds in white rice, brown rice, and germinated brown rice . Journal of Agricultural and Food Chemistry , 52 : 4808 – 4813 .
- Fernandez-Orozco , R. , Frias , J. , Zielinski , H. , Piskula , M.K. , Kozlowska , H. and Vidal-Valverde , C. 2008 . Kinetic study of the antioxidant compounds and antioxidant capacity during germination of Vigna radiata cv. emerald, Glycine max cv. jutro and Glycine max cv. merit . Food Chemistry , 111 : 622 – 630 .
- Khattak , A.B. , Zeb , A. , Bibi , N. , Khalil , S.A. and Khattak , M.S. 2007 . Influence of germination techniques on phytic acid and polyphenols content of chick pea (Cicer arietinum L.) sprouts . Food Chemistry , 104 : 1074 – 1079 .
- Oboh , G. 2006 . Antioxidant properties of some commonly consumed and underutilized tropical legumes . European Food Research and Technology , 224 : 61 – 65 .
- Sun , J. , Chu , Y. , Wu , X. and Liu , R. 2002 . Antioxidant and antiproliferative activity of common fruits . Journal of Agricultural and Food Chemistry , 50 : 7449 – 7454 .
- Yang , J. , Lin , H. and Mau , J. 2002 . Antioxidant activity of several commercial mashrooms . Food Chemistry , 77 : 229 – 235 .
- Rocha-Guzman , N.E. , Gonzalez-Laredo , R.F. , Ibarra-Perez , F.J. , Nava-Berumen , C.A. and Gallegos-Infante , J.A. 2007 . Effect of pressure cooking on the antioxidant activity of extracts from three common bean (Phaseolus vulgaris L.) cultivars . Food Chemistry , 100 : 31 – 35 .
- Xu , B.J. and Chang , S.K.C. 2007 . A comparative study on phenolic profiles and antioxidant activities of legumes as affected by extraction solvents . Journal of Food Science , 72 : 159 – 166 .