Abstract
Termitomyces heimii is highly priced due to its unique taste unlike many other cultivated mushrooms. Termitomyces heimii, an edible mushroom, has high nutritive value. It is a fungus that usually lives in termite hills. There are limited reports on the chemical investigations of Termitomyces heimii. This may be due to difficulty in obtaining wild fruit bodies, as these are seasonal mushrooms. Twenty-eight compounds were identified from the extract of ethyl acetate fraction of the fruiting bodies of Termitomyces heimii using spectrometric and spectroscopic techniques. The components in the sub-fractions from the ethyl acetate extract were identifed by gas chromatography-mass spectrometry and NMR analysis. The compounds identified included fatty acids and the methyl and ethyl esters and sterols. To our knowledge, this is the first report on the isolation and identification of components from Termitomyces heimii.
INTRODUCTION
Mushrooms have long been valued as delicious and nutritious foods in many countries. Currently, mushrooms are appreciated, not only for texture and flavour but also for their chemical and nutritional characteristics.[Citation1,Citation2] Mushrooms are now becoming attractive as functional foods, and as a source of physiologically beneficial nutraceuticals. Recently, three species, including Grifola frondosa (maitake), Morchella esculenta (morel), and Termitomyces spp. (termite mushrooms also known as ‘cendawan busut’) have become highly valued, partially due to their rareness and difficulty in cultivation.[Citation3] Termitomyces spp remain elusive and even laboratory trials to culture and cultivate them are far from success stories. They are called termite mushrooms as they are only able to survive and continue their progeny while in symbiotic growth together with termites in their hills. These highly palatable fungi with high umami have a close association that has reached a high degree of specialization with termites. These termites construct ‘fungus-gardens’ within their nests and cultivate a basidiomycete fungus belonging to the genus Termitomyces. The fungus gardens produce numerous pearly white nodules known as primordial.[Citation4] Wild mushrooms of the genus Termitomyces, such as Termitomyces eurhizus (Berk) Heim, T. clypeatus Heim, T. striatus (Beeli) Heim, T. robustus (Beeli) Heim, T. microcapus (Berk and Broome) Heim, and T. heimii Natarajan have been identified as edible mushrooms with high nutritive values.[Citation5] Termitomyces robustus and T. striatus are two examples often used as pot–herbs.[Citation6]
Edible mushrooms have their characteristic smell and taste because of their chemical components. Termitomyces spp. fetches a high price due to the taste that is unlike many cultivated mushrooms. There are also reports on their medicinal properties including antioxidant potentials.[Citation7] However, reports on chemical constituents of Termitomyces spp. are limited.[Citation8] This could be due to difficulty in obtaining wild fruiting bodies, as these are seasonal mushrooms. In Malaysia, Termitomyces heimii is one of the Termitomyces spp. that is commonly encountered in the wild and is considered as a delicacy.[Citation9] It would be interesting to study the chemical compounds present in the fresh fruiting bodies of Termitomyces heimii.
MATERIALS AND METHODS
Mushroom Samples
The fresh fruiting bodies of Termitomyces heiimi were collected from Kuala Selangor (location: 3°21′19.20″ N; 101°14′36.35″ E), Selangor Darul Ehsan, Malaysia in March 2007 and was processed immediately.
Preparation of Extracts
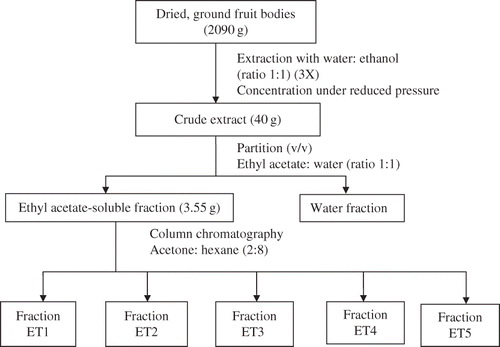
The fruiting bodies (5000 g) were washed, freeze-dried, and ground to a fine powder using a Waring blender (Waring Commercials, USA). The dried, ground sample (524 g) was then soaked in ethanol and water (8:2 ratio) (1.5 L) for 3 days at 27 + 2°C (room temperature). The solvent-containing extract was then decanted and filtered through cotton and filter paper. The extraction of the ground sample was further repeated (3×) each with 1.5 L of ethanol and water (8:2 ratio). The filtrate from each extraction was combined and the excess solvent was evaporated under reduced pressure using a rotary evaporator to give a yellowish brown thick extract (60 g). The crude extract was further extracted with a mixture of ethyl acetate and water (1:1) to give an ethyl acetate-soluble fraction (7.55 g).
Isolation of Secondary Metabolites
The ethyl acetate extract (5 g) was mixed with silica gel (0.063–0.200 nm; mesh: 70–230) (Merck, Germany). The extract mixture was further dried to a powder in an oven. The powdered mixture of ethyl acetate extract and silica was subjected to column chromatography initially eluting with 100% hexane followed by hexane enriched with increasing percentages of acetone. Fractions of 25 ml were collected in numbered vials. The eluted compounds were monitored using thin layer chromatography (TLC). TLC was carried out using pre-coated TLC plates 60 F254 (thickness of 20.25 mm) purchased from Merck and spots were visualized in UV light (254 and/or 365 nm) and/or iodine vapour. The fractions were pooled according to the spots on thin layer chromatography. The excess solvent in the pooled fractions was evaporated under reduced pressure using a rotary evaporator. Components in the isolated fractions were identified using gas chromatography-mass spectrometry (GC-MS) and NMR.
Instrument Used
The GC-MS analysis was performed using a 6890 N gas chromatograph (Agilent Technologies) equipped with a 5979 mass selective detector (70 eV direct inlet); a HP-5ms (5% phenylmethylpolysiloxane; Agilent Technologies, Germany) capillary column (30.0 m × 250 μm ID × 0.25 μm film thickness) initially set at 150°C, then programmed to 280°C at 5°C per min using helium as the carrier gas at a flow rate of 1 ml/min, was used. The total ion chromatogram obtained was auto-integrated by Chemstation and the constituents were identified by comparison with an accompanying mass spectral data base (NIST Library, 2005). NMR spectra were recorded on a Bruker Avance DPX-500 spectrometer (Bruker Bio Spin, USA) for 1H, 13C, COSY, DEPT, HMBC, and HMQC NMR. The internal standard used in 1H NMR spectra was TMS (δ: 0.00) for CDCl3; in 13C NMR it was CDCl3 (δ: 77.0).
RESULTS AND DISCUSSION
Column chromatography of the ethyl acetate extract yielded five fractions that were labeled as ET1, ET2, ET3, ET4, and ET5, respectively (). All components present in the fractions were identified by GC-MS analysis. Fraction ET1 (0.47 g) was light-yellow viscous oil containing tetracosane (8.29 g/100 g extract), methyl palmitate (0.19 g/100 g extract), ethyl palmitate (24.68 g/100 g extract), methyl linoleate (2.85 g/100 g extract), ethyl linoleate (47.66 g/100 g extract), ethyl oleate (8.51 g/100 g extract), ethyl eicosanoate (0.23 g/100 g extract), and ethyl tetracosanoate (31.28 g/100 g extract).
Fraction ET2 (0.43 g) was white vicious oil containing ebericol (23.26 g/100 g extract.), lanosterol (26.51 g/100 g extract), palmitic acid (25.58 g/100 g extract), oleic acid (10.93 g/100 g of extract), and stearic acid (32.09 g/100 g extract). Palmitic acid exhibited a parent ion at m/z 256 in the EI-MS spectrum that is consistent with the molecular formula C16H32O2. The base peak at m/z 60 is a characteristic peak resulting from the McLafferty rearrangement. Besides the McLafferty rearrangement peak, the spectrum also showed loss of clusters of 14 (CH2) mass units typical of long-chain carboxylic acid.
Fraction ET3 (0.54 g) consisted of colorless needle-like crystals containing ergosterol (25.51%) as the major constituent and neoergosterol (1.67 g/100 g extract), ergosta-5,8-dien-3-ol (5.14 g/100 g extract), ergosta-5,8(14)-dien-3-ol (0.74 g/100 g extract), 7-ergostenol (1.85 g/100 g extract), brassicasterol (0.92 g/100 g extract), and γ-egrostenol (1.85 g/100 g extract) as minor components. Ergosterol was isolated as colourless needles and identified using GC-MS analysis and NMR (1H, 13C, DEPT, COSY, HMBC, and HMQC NMR) spectral data. The proton NMR is consistent with the structure of ergosterol. Two singlets at δ0.61 and 0.92 were assigned to the 18- and 19-methyl protons, respectively, whilst doublets at δ0.78 (1 H, d, J = 2.7 Hz) and δ0.82 (1 H, d, J = 2.7 Hz) were consistent with the 26- and 27-methyl protons. The 21- and 28-methyl protons were expected to resonate at lower fields (δ0.90, 1.02) as they were in close proximity to the double bond at C-22. Both methyl protons appeared as doublet with a coupling constant of 8.1 Hz each. The two olefinic protons at δ5.56 (1 H, dd, J = 2.7, 5.4 Hz) and δ5.36 (1 H, m) were assigned to H-6 and H-7, respectively, whilst superimposed dd at δ5.15–5.32 were assigned to the olefinic protons H-22 and H-23. Multiplets centred at δ3.62 were consistent with H-3. The C-13 spectrum showed the presence of 28 carbons consistent with 10 methine carbons, 7 methylene and 6 methyl, 1 olefinic methine, 2 quatenary carbons, and 2 quatenary olefinic carbons.
Even though mushrooms are deficient in vitamin D2, earlier researchers have found them to be a rich source of ergosterol. Mushrooms are considered a delicacy, highly accepted by vegetarians and non vegetarians.[Citation10] Ergosterol was the major component in Termitomyces heimii and may act as a biological precursor to vitamin D2. It can form viosterol by ultraviolet light, irradiation temperature, and moisture,[Citation11] which is then converted to ergocalciferol, a form of vitamin D2 used for pharmaceutical applications and food supplements. Ergosterol, which is common in all mushrooms, has been shown to inhibit phorbol-12-myristate 13 acetate (TPA)-induced inflammatory conditions in mice,[Citation12] and vitamin D2 has been shown to offer protection against colon and prostate cancer.[Citation13] Ergosterol peroxide of selected mushrooms has been reported to supress inflammatory responses of macrophages and growth of colon adenocarcinoma cells[Citation14] and TPA-induced inflammation.[Citation12] However, despite being common in many mushrooms, ergosterol peroxide was not detected in all extracts analysed in this study.
Fraction ET4 (0.78 g) was a yellow viscous oil containing linoleic acid (88.47 g/100 g extract) as the major component. Linoleic acid is a polyunsatutared fatty acid and is a member of the group of essential fatty acids called omega-6 fatty acids because they are an essential dietary requirement for all mammals. Essential fatty acids (EFAs) are fatty acids that cannot be constructed within an organism from other components by any known chemical pathways; therefore, they must be obtained from the diet. Linoleic acid is the raw material for a number of compounds vital for health (e.g., arachidonic acid, which is involved in inflammation response). Linoleic acid is important for the proper growth and development of infants. It was once known as vitamin F but is no longer regarded as a vitamin.[Citation15] Linoleic acid produces compounds called prostaglandins, and prostaglandins are substances that are found in every cell for the body's health maintenance. In the body, EFAs are primarily used to produce hormone-like substances that regulate a wide range of functions, including blood pressure, blood clotting, blood lipid levels, the immune response, and the inflammation response to injury infection. In addition, linoleic acid also been proven to reduce tumor size through its effect on a gene that controls the apoptosis rate; in another study, multiple sclerosis patients supplemented with linoleic acid show a smaller increase in disability and reduced severity and duration of attacks than those without linoleic acid supplements.[Citation16] It has also been reported that unsaturated fatty acids are dominant fatty acids in apples and pears, thus also making T. heimii a good source of edible oils.[Citation17]
Fraction ET5 (0.68 g) was a yellow viscous oil containing myristic acid (1.32 g/100 g extract), linoleic acid (49.41 g/100 g extract), benzaldehyde, 4-hydroxy (0.40 g/100 g extract), benzeneacetamide (14.85 g/100 g extract), cinnamic acid (2.02 g/100 g extract), and nicotinamide (26.47 g/100 g extract). Nicotinamide, also known as niacinamide and nicotinic acid amide, is the amide of nicotinic acid (vitamin B3/niacin). Nicotinamide has demonstrated anti-inflammatory and anti-anxiety properties.[Citation18,Citation19] It rarely causes side effects, and is considered generally safe as a food additive, and as a component in cosmetics and medication. Nicotinamide also prevents immunosuppression caused by UVA and UVB radiation, and can, therefore, be added to sunscreen for added protection.[Citation20]
CONCLUSION
Termitomyces heimii is a rare and highly sought after edible fungus that grows seasonally, and in symbiosis with termites. It is difficult to cultivate. Twenty-eight compounds, mainly fatty acids esters of fatty acids and ergosterol were present in Termitomyces heimii extract. Ergosterol was found to be the major component followed by linoleic acid; thus, making Termitomyces heimii a good source of these two components. Ergosterol is the precursor of ergocalciferol, which is extensively used as a dietary calcium supplement in people suffering from hypocalcinaemia and osteoporosis. Linoleic acid is important for bodily growth; maintainance of health through the production of prostaglandins; regulation of blood pressure, blood lipid, immune response, inflammation, and apoptosis. Thus, the findings support the therapeutic claims of this mushroom. Further, the consumption of T. heimii would be beneficial for health purposes and may have chemopreventive properties of selected diseases of humankind. However, studies to elucidate the mechanisms of possible biological activity are needed to validate the claims.
ACKNOWLEDGMENT
The authors wish to acknowledge the University of Malaya for financial assistance received through OS2–285 and 66–02–03–0074 (Mushroom research).
REFERENCES
- Manzi , P. , Gambelli , L. , Marconi , S. , Vivanti , V. and Pizzoferrato , L. 1999 . Nutrients in edible mushrooms: an interspecies comparative study . Food Chemistry , 65 ( 4 ) : 477 – 482 .
- Guedes , P.D. , Ribeiro , B. , Goncalves , R.F. , Baptista , P. , Valentao , P. , Seabre , R.M. and Andrade , P.B. 2008 . Correlation between the pattern volatiles and the overall aroma of wild edible mushrooms . Journal of Agricultural and Food Chemistry , 56 : 1704 – 1712 .
- Tsai , S.H. , Wu , T.P. , Huang , S.J. and Mau , J.L. 2006 . Nonvolatile taste components of Agaricus bisporus harvested at different stages of maturity . Food Chemistry , 103 : 1457 – 1464 .
- Fauzi , D. and Yow , Y.Y. 1990 . A nutritional study of Malaysian termite mushroom . Sains Malaysiana , 19 ( 3 ) : 107 – 114 .
- Chakraborthy , I. , Mondal , S. , Rout , D. and Syed , S. 2006 . A water insoluable (1–3)-β-glucan from alkaline extract of an edible mushroom Termitomyces eurhizus . Carbohydrate Research , 341 : 2990 – 2993 .
- Adewusi , S.R.A. , Alofe , F.V. , Odeyemi , O. , Afolabi , O.A. and Oke , O.L. 1991 . Studies on some edible wild mushrooms from Nigeria: Nutritional, teratogenic and toxic considerations . Plants Food for Human Nutrition , 43 : 115 – 121 .
- Mau , J.L. , Chang , C.N. , Huang , S.J. and Chen , C.C. 2004 . Antioxidant properties of methanolic extracts from Grifola frondosa, Morchella esculenta and Termitomyces albuminosus mycelia . Food Chemistry , 87 : 111 – 118 .
- Tsai , S.Y. , Weng , C.C. , Huang , S.H. , Chen , C.C. and Mau , J.L. 2005 . Nonvolatile taste components of Grifola frondosa, Morchella esculenta and Termitomyces albuminosus mycelia . LWT-Science and Technology , 39 : 1066 – 1071 .
- Vikineswary , S. , Noorlidah , A. , Normah , I. , Tan , Y.H. , Fauzi , D. and Jones , E.B.G. 2007 . “ Edible and medicinal mushrooms ” . In Malaysian Fungal Diversity , Edited by: Vikineswary , S. , Jones , E.B.G. and Hyde , K.D. 287 – 305 . Malaysia : Mushroom Research Centre and Ministry of Natural Resources and Environment .
- Jasinghe , V.J. and Perera , C.O. 2005 . Distribution of ergosterol in different tissues of mushrooms and its effect on the conversion of ergosterol to vitamin D2 by UV radiation . Food Chemistry , 92 : 541 – 546 .
- Jasinghe , V.J. , Perera , C.O. and Sablani , S.S. 2007 . Kinetics of the conversion of ergosterol in edible mushrooms . Journal of Food Engineering , 79 : 864 – 869 .
- Yasukawa , K. , Aoki , T. , Takido , M. , Ikekawa , T. , Saito , H. and Matsuzawa , T. 1994 . Inhibitory effects of ergosterol isolated from the edible mushroom Hypsizigus marmoreus on TPA-induced inflammatory ear oedema and tumour promotion in mice . Phytotherapia Research , 8 : 10 – 13 .
- Guyton , K.Z. , Kensler , T.W. and Posner , G.H. 2003 . Vitamin D and vitamin D analogs as cancer chemopreventive agents . Nutrition Review , 61 : 227 – 238 .
- Kobari , M. , Yoshida , M. , Ohnishi-Kameyama , M. and Shinmoto , H. 2007 . Ergosterol peroxide from edible mushroom suppress inflammatory responses in RAW264.7 macrophages and growth of HT29 colon adenocarcinoma cells . British Journal of Pharmacology , 150 : 209 – 219 .
- Egmond , V. , Andreas , W.A. , Michael , R. , Kosorok , R.K. , Anita , L. and Philip , M.F. 1996 . Effect of linoleic acid intake on growth of infants with cystic fibrosis . American Journal of Clinical Nutrition , 63 : 746 – 752 .
- Dox , I.G. , Melloni , B.J. and Eisner , G.M. 1993 . The Harper Collins Illustrated Medical Dictionary , New York : Harper Perennial .
- Yukui , R. , Wenya , W. , Rashid , F. and Qing , L. 2009 . Fatty acids composition of apple and pear seed oil . International Journal of Food Properties , 12 ( 4 ) : 774 – 779 .
- Jaconello , P. and Niacin versus niacinamide . 1992 . Canadian Medical Association Journal , 147 ( 7 ) : 990
- Niren , N.M. 2006 . Pharmacologic doses of nicotinamide in the treatment of inflammatory skin conditions: A review . Cutis , 77 : 11 – 16 .
- Damian , D.L. , Patterson , C.R. , Stapelberg , M. , Park , J. , Barnetson , R.S. and Halliday , G.M. 2008 . UV radiation-induced immunosuppression is greater in men and prevented by topical nicotinamide . Journal of Investigation Dermatology , 128 ( 2 ) : 447 – 454 .