Abstract
The beneficial health effects of extra virgin olive oil are due to both its high content of monounsaturated fatty acids and its high content of phenolic compounds, which have recently attracted research interest. In this context, the aim of this work was to examine the in vitro antioxidant and antiproliferative activities of the phenolic extract obtained from extra virgin olive oil from O. europea cultivar ‘Frantoio’ (samples 1–4), one of the main varieties cultivated in Italy. The total phenolic content was determined by Folin–Ciocalteu method and instead the phenolic profile was obtained by HPLC coupled to a diode array detector and mass spectrometry. Extra virgin olive oil extracts exhibited high antioxidant activity through different mechanisms of action and these activities are related to the phenolic content. Sample 3 demonstrated the strongest free radical scavenging activity with IC50 value of 56.5 μg/mL. The reducing ability measured with FRAP assay revealed that samples ranged from 91.3 to 156 μM Fe(II)/g. The same interesting trend was observed with Trolox equivalent antioxidant capacity value. Moreover, the virgin olive oils showed a good oxidative stability ranging between 19 to 32 h. Antiproliferative activity evaluated by SRB assay revealed that phenolic extracts from the cultivar ‘Frantoio’ showed a strong antiproliferative activity against CORL-23 cell line with an IC50 value of 14.5 and 55.9 μg/mL for samples 3 and 1, respectively, and these results are comparable to the positive control vinblastine. Overall, these results showed that extra virgin olive oils from the cultivar ‘Frantoio’, may represent an interesting source of phenolic compounds as functional components that could be consumed in diets and/or used for the elaboration of functional food and pharmaceutical industries.
INTRODUCTION
It is reported that in 2005, 7.6 million people died of cancer out of 58 million deaths world-wide. Based on projections, cancer deaths will continue to rise with an estimated 9 million people dying from cancer in 2015, and 11.4 million dying in 2030. However, the incidence of cancer in Mediterranean countries is lower than in the rest of the European countries and the United States.Citation[1] This is mostly described by the lower rate of the large bowel, breast, endometrial, and prostate cancer by a number of epidemiological studies, and the major reason for this, apart from possible genetic factors, is attributed to the dietary practices. The traditional Mediterranean diet is characterized by high consumption of foods of plant origin, relatively low consumption of red meat, and high consumption of olive oil, particularly extra virgin olive oil (EVOO). There are a number of studies on health beneficial effects of olive oil. Several studies have been reported that olive oil is more favorable against cancer than other forms of added lipids due to its high content of monounsaturated fatty acids.Citation[2,Citation3]
Free radicals and oxidative stress are recognized as important factors in the etiology of many chronic diseases including cancer. Dietary components with antioxidant activity, therefore, have received particular attention because of their potential to modulate oxidative stress associated with chronic disease. In January 2009, the Mediterranean diet was declared as an UNESCO “Intangible Cultural Heritage,” for the benefits it brings to health and to quality of life. The major fat component of the so-called “Mediterranean diet” is olive oil and its nutritional properties are the main reason for the increment of its consumption all over the world in recent years.Citation[4]
EVOO is obtained exclusively by physical extraction methods from the fruit of Olea europea L. Its chemical composition is affected by environmental, genetic, agronomical, and technological factors.Citation4–9 In the European Union, olive oil production was 2,148,500 tons in the 2009/2010 campaign (data International Oil Council [COI]). The Italian production of virgin olive oil represents 26% in the world.Citation[7] In Italy, there are 395 cultivars of olive fruits, and ‘Frantoio’ is one of the main cultivars in the national olive oil production because it shows a high and constant productivity and adaptation capacity to different agronomical conditions although it is cold-sensitive.Citation[10] Its organoleptic characteristics and oxidative stability are much appreciated.Citation[11] Phenolic compounds in virgin olive oil, although considered among its several “minor” constituents, may play a major role in preventing chronic disease. Specific health-promoting effects of olive oil phenolics include inhibition of the oxidation of low density lipoproteins (LDLs) thought to be involved in the onset of atherosclerosis,Citation[12,Citation13] scavenging of free radicals,Citation[14] reducing inflammation through inhibition of lipoxygenase activity, sparing prostaglandin generation, lessening of the generation of reactive oxygen species by leukocytes, and enhancement of the macrophage-mediated response.Citation[15,Citation16]
The pharmaceutical interest in olive oil phenolic compounds due to their bioactivity on different cancer cells is also well known.Citation17–19 In this study, the in vitro antioxidant and antiproliferative activity by different analytical tests of phenolic olive oil extract from cultivar ‘Frantoio’ have been investigated. Moreover, the oxidative stability of virgin olive oil, the total phenolic content, and the characterization of the main phenolic classes have been analyzed.
MATERIALS AND METHODS
Chemicals
Ethanol, HCl, and CH3COOH were obtained from VWR International s.r.l. (Milan, Italy). DMSO, FeCl3, Na2CO3, FeSO4, CH3COONa, ascorbic acid, 2,2-diphenyl-1-picrylhydrazyl (DPPH), Folin–Ciocalteu reagent, tripyridyltriazine (TPTZ), ABTS solution, Trolox, sulphorodamine B (SRB), RPMI, DMEM, trichloroacetic acid (TCA), tris[hydroxymethyl]aminomethane, and vinblastine sulfate salt were purchased from Sigma–Aldrich S.p.a. (Milan, Italy).
Extra Virgin Olive Oils (EVOO)
The olive fruits of cultivar ‘Frantoio’ were harvested in the Campania region (Italy) in four different farms in the area of Salerno during the 2007/2008 season and, thus, four different EVOO batches were obtained using stone or continuous mills. All four oil samples accomplished the UNI10939, 2001 certification. EVOOs were stored at 10°C in darkness using green glass bottles without headspace up to analysis.
Extraction of the Phenolic Fraction
The phenolic fraction was extracted following the procedure of Loizzo et al.Citation[20] For spectrophotometric analysis, the dry extracts were re-suspended in 1 mL of methanol.
Determination of the Total Phenolic Content
The total phenolic content was evaluated colorimetrically using the Folin–Ciocalteu reagent and a spectrophotometer UV-Vis-Nir Cary 5000 (Varian, Leinì, Italy). Briefly, the phenolic extract was mixed with the Folin–Ciocalteau reagent and aqueous Na2CO3. The solution was kept in the dark at room temperature for 2 h and the total phenols were determined spectrophotometrically at λ = 765 nm.Citation[21] The results were expressed as gallic acid equivalents (mg/Kg oil), based on a calibration curve (r 2 = 0.996) obtained in a range from 2.5 to 500 mg/kg of gallic acid.
High Performance Liquid Chromatography of Phenols
The qualitative and quantitative characterization of the phenolic compounds was performed using high performance liquid chromatography (HPLC) with a diode array detector (DAD). For HPLC analysis, the methanol solutions of the phenolic extracts were filtered through 0.2 μm regenerated cellulose filters (Schleicher & Schuell, Dassel, Germany) and the sample was injected in a 20 μL loop. A Phenomenex (Torrance, CA, USA) 25 cm × 4.6 mm i.d. column packed with a Gemini C18 (5 μm particle size) and a ternary pump delivery system Varian 240 (Walnut Creek, CA, USA) were used. The mobile phase flow rate was 0.7 mL/min and the gradient elution was carried out using water/acetic acid (98:2 v/v) as the mobile phase A and methanol as the mobile phase B of the solvent system.Citation[19] The detector was a Varian Prostar PDA 330 and the data were acquired using the Varian Star 6.3 software. For structural elucidation, the HPLC system was coupled online to an LCQ ion-trap mass spectrometer (Thermoquest, San José, CA, USA) equipped with an electrospray ionization source suitable for tandem mass spectrometry (MS/MS). All phenolic compounds were quantified using a calibration curve obtained with 3,4-dihydroxyphenylacetic acid (r 2 = 0.998), whereas flavones were quantified as quercetin equivalents (r 2 = 0.997).
Determination of the Oxidative Stability
The oxidative stability of the oils was determined with a Rancimat apparatus (Metrohm model 679, Herisau, Switzerland), by measuring the induction period of a 5 g sample heated at 110°C under an air flow of 20 L h−1. The induction period was determined by drawing the two tangents of the time-conductivity curve and projecting the intersection onto the time-axis. The induction period was expressed in hours (h).
DPPH (2,2-Diphenyl-1-Picrylhydrazyl) Assay
Radical scavenging capacity was determined according to the technique reported by Servili et al.Citation[23] with some modifications. An aliquot of 1.5 mL of 0.25 mM DPPH solution in ethanol and 12 μL of phenolic extract at concentrations ranging from 25 to 150 μg/mL were mixed. The mixture was shaken vigorously and allowed to reach a steady state at room temperature for 30 min. Decolorization of DPPH was determined by measuring the absorbance at λ = 517 nm with a spectrophotometer. The DPPH radicals scavenging activity was calculated according to the following equation:
where A 0 is the absorbance of the control (blank, without extract) and A 1 is the absorbance in the presence of the extract.
FRAP Assay
The FRAP method measures the absorption change that appears when the TPTZ (2,4,6-tripyridyl-s-triazine)-Fe3+ complex is reduced to the TPTZ-Fe2+ form in the presence of antioxidant compounds.Citation[24] Briefly, the FRAP reagent contained 2.5 mL of 10 mM tripyridyltriazine (TPTZ) solution in 40 mM HCl plus 2.5 mL of 20 mM FeCl3 and 25 ml of 0.3 M acetate buffer (pH 3.6) was freshly prepared. Samples were dissolved in ethanol at a concentration of 1 μg/mL. An aliquot of 0.2 mL of solution was mixed with 1.8 mL of FRAP reagent and the absorption of the reaction mixture was measured at λ = 595 nm. Ethanolic solutions of known Fe (II) concentration, in the range of 50–500 μM (FeSO4), were used for obtaining the calibration curve. The FRAP value represents the ratio between the slope of the linear plot for reducing Fe3+–TPTZ reagent by extract compared to the slope of the plot for FeSO4.
ABTS Assay
ABTS assay was based on the method of Re et al.Citation[22] with slight modifications. ABTS radical cation (ABTS+) was produced by the reaction of a 7 mM ABTS solution with 2.45 mM potassium persulphate. The mixture was stored in the dark at room temperature for 12 h before use. The ABTS+ solution was diluted with ethanol to an absorbance of 0.70 ± 0.05 at 734 nm. After addition of 25 μL of sample or Trolox standard to 2 mL of diluted ABTS+ solution, absorbance at λ = 734 nm was measured at exactly 6 min. The decrease in absorption was used for calculating Trolox equivalent antioxidant capacity (TEAC) values. A standard curve was prepared by measuring the reduction in absorbance of ABTS•+ solution at different concentrations of Trolox. Appropriate blank measurements were carried out and the values recorded. Results were expressed as Trolox equivalent antioxidant capacity (TEAC).
Cell and Cell Culture
Five human cancer cell lines, large cell carcinoma COR-L23 (ECACC No. 92031919), colorectal adenocarcinoma Caco-2 (ATCC No. HTB-37), lung carcinoma A549 (ECACC No. 86012804), hepatocellular carcinoma Huh-7D12 (ECACC No. 01042712), renal cell adenocarcinoma ACHN (ATCC No. CRL-1611), and one normal cell line, such as skin fibroblasts 142BR (ECACC No. 90011806), were used in our experiments. The COR-L23 cells were cultured in RPMI 1640 medium, while 142BR, Caco-2, A549, and Huh-7D12 cells were cultured in DMEM. Both media were supplemented with 10% fetal bovine serum, 1% L-glutamine, and 1% penicillin/streptomycin. The cell lines were maintained at 37°C in a 5% CO2 atmosphere with 95% humidity.
Antiproliferative Bioassay
The antiproliferative assay was performed as previously described using the protein-staining sulphorodamine B (SRB) cell proliferation assay.Citation[23] Briefly, cells were plated over a range from 5 × 104 to 15 × 104 cells and incubated to allow for cell attachment. After 24 h the cells were treated with serial dilutions of the samples. One hundred μL/well of each sample dilution was added to the plates in six replicates to obtain the final concentrations ranging from 5 to 200 μg/mL. The final mixture used for treating the cells contained not more than 0.5% of the solvent (DMSO), the same as in the solvent-control wells. After 48 h of exposure, cells were fixed with trichloroacetic acid (TCA) and then treated with SRB in 1% acetic acid. For reading plate, the bound dye was solubilized with tris[hydroxymethyl]aminomethane. The absorbance of each well was read on a Molecular Devices SpectraMax Plus Plate Reader (Molecular Devices, CELBIO, Milan, Italy) at λ = 490 nm. Cell survival was measured as the percentage absorbance compared to the untreated control. Vinblastine sulfate salt was used as positive control. Antiproliferative activity of the sample was expressed in terms of its IC50 value calculated by nonlinear regression curve with the use of Prism GraphPad Prism version 4.0 for Windows, GraphPad Software (San Diego, CA, USA). The dose-response curve was obtained by plotting the percentage of inhibition versus the concentration.
Statistics
All experiments were carried out in triplicate. Data were expressed as mean ± standard deviation. Differences were evaluated by one-way analysis of variance (ANOVA) test completed by a Dunnett's test. Differences were considered significant at **p < 0.01. The inhibitory concentration 50% (IC50) was calculated by using Prism Graphpad version 4.0 for Windows (GraphPad Software, San Diego, CA, USA). The dose-response curve was obtained by plotting the percentage of inhibition versus the concentrations.
RESULTS AND DISCUSSION
Total Phenolic Content and HPLC-DAD-MS2 Profile
EVOO contains different classes of phenolic compounds. Phenolic acids, represented by caffeic, vanillic, syringic, p-coumaric, o-coumaric, protocatechuic, sinapic, p-hydroxybenzoic, and gallic acid, were the first group of phenols discovered in olive oil. Phenolic alcohols include (3,4-dihydroxyphenyl)ethanol 3,4-DHPEA) and (p-hydroxyphenyl)ethanol (p-HPEA) and are the most abundant simple phenols.Citation[25]Secoiridoids, aglycon derivatives of oleuropein and ligstroside, are peculiar to virgin olive oil. Flavonoids like luteolin and apigenin were also reported as phenolic components of EVOO by Tripoli et al.Citation[26]
The phenolic compounds are very important to preserve the quality of the EVOO and to discriminate these products. The performed extraction method for the phenolic fraction was carried out to obtain a total recovery of the minor polar compounds. The determination of the total phenolic compounds was carried out using the Folin-Ciocalteu method, which was integrated with the HPLC analysis. In , the total amount of the phenolic compounds with values ranging from 109 to 250 mg/L of gallic acid are shown.
Table 1 Oxidative stability, total phenolic content, and phenolic compound classes of EVOO from cultivar ‘Frantoio’ harvested in Campania (Italy)
The HPLC analysis of the classes of phenolic compounds of the four EVOO is reported in . The HPLC profile was recorded at a wavelength of 280 nm for simple phenols and secoiridoids, whereas flavones were detected at a wavelength of 350 nm. HPLC–DAD–MS/MS was used for quantification and to confirm the identification of the phenolic acids, phenolic alcohols, secoiridoids, and flavones. The content of the three classes (simple phenols, secoiridoids, and flavones) was determined as the sum of similar phenols species. As shown in , the content of simple phenols (3,4-DHPEA, p-HPEA, vanillic acid, vanillin, 3,4-DHPEA-Ac and p-coumaric acid) was different and ranged from 7.54 to 18.6 mg/kg (sample 4 and 1, respectively). In accordance with other authors, secoiridoids are the main phenolic compounds present in EVOO.Citation[9] Also, in this study, secoiridoids were very abundant if compared with the two other classes of phenols and their content ranged from 73.6 to 252 mg/Kg. The highest content of secoiridoids was found in sample 3 such as determined by Folin-Ciocalteau method. Finally, the two flavones detected in the samples were ascribed to luteolin and apigenin. The flavones were similar in the oil samples and their content reached 4.55 mg/Kg.
Determination of the Oxidative Stability
The oxidative stability of the oils was measured as the induction time in response to forced oxidation. This determination only gives a total estimation on findings of the antioxidant potential of the oil, without information on the possible contribution of single compounds. The oxidative stability was determined by Rancimat apparatus (Metrohm, Switzerland), ranging from 19 to 32 h (). The sample 3 showed a good and highest oxidative stability due to major content of total phenols; on the other hand, the sample 4 showed the lowest values.
These data showed a good positive correlation when they were correlated with total phenolic content quantified by Folin–Ciocalteu method (r 2 = 0.87) and secoiridoids compounds (r 2 = 0.99); a negative correlation was found between oxidative stability and flavones (r 2 = 0.77).
Antioxidant Activity
Several methods were recently developed for measuring the antioxidant capacity of food and beverages. These assays differ in the generation of different radicals and/or target molecules, and in the way end points are measured. Considering that different antioxidant compounds may act in vivo through different mechanisms, no single method can fully evaluate the antioxidant capacity of food since levels of single antioxidant in food do not necessarily reflect their antioxidant activity.Citation[27] Therefore, to investigate the antioxidant activity of chemicals choosing an adequate assay based on chemicals of interest is critical.Citation[28] The great interest in EVOO phenols can be attributed to the association of such substances with several biological activities including antioxidant activity.Citation[29]
The EVOO extracts were tested for their antioxidant activities employing various established in vitro systems. A rapid, simple, and inexpensive method to measure antioxidant capacity involves the use of the free radical 2,2-diphenyl-1-picrylhydrazyl (DPPH). DPPH is widely used to test the ability of compounds to act as free radical scavengers or hydrogen donors, and to evaluate antioxidant activity of food.Citation[30] It has also been used to quantify antioxidants in complex biological systems in recent years. A dose-response relationship was observed for all tested oils (). The IC50 value for DPPH scavenging by EVOO phenolic extracts are reported in . Sample 3 demonstrated the strongest radical scavenging activity with an IC50 value of 56.5 μg/mL. As previously described, sample 3 was characterized by high levels of secoiridoids derivatives that are strictly connected with the ability of these compounds to act as antioxidant.Citation[31]
Figure 1 Radical scavenging activity by DPPH assay of phenolic extract from extra virgin olive oils from cultivar ‘Frantoio’. Each data represents the mean ±SD (n = 3).
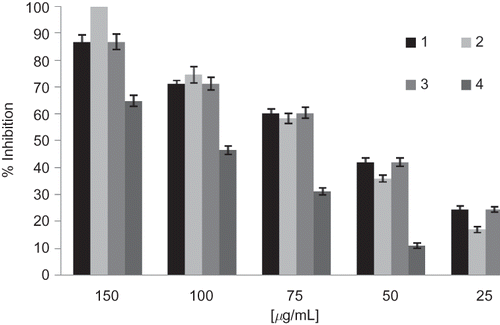
Table 2 Antioxidant activity of EVOO phenolic extracts from cultivar ‘Frantoio’
FRAP assay measures the reducing ability of antioxidant that react with ferric tripyridyltriazine (Fe3+–TPTZ) complex and produce a colored ferrous tripyridyltriazine (Fe2+–TPTZ).Citation[24] Using this assay, the FRAP value of samples ranged from 91.3 to 156 μM Fe(II)/g (). The reducing ability of the sample was strongly correlated with the phenolic levels. These results were in agreement with Song and Barlow,Citation[32] who found a strong correlation between phenolic content and FRAP assay. Some researchers reported that phenolic compounds exhibited redox properties (i.e., act as reducing agents, hydrogen donators, and singlet oxygen quenchers). The redox potential of phenolic phytochemicals plays a crucial role in determining the antioxidant properties.Citation[33] In addition, the antioxidant ability of EVOO to scavenge the blue-green colored ABTS+ radical cation was measured relative to the radical scavenging ability of Trolox. As shown in , the result clearly indicates that all the tested samples have an interesting reducing power with TEAC value ranging from 36.4 to 17.9.
Our samples obtained from cultivar ‘Frantoio’ exhibited interesting antioxidant and chelating properties and these activities are related to the phenolic content according to Bendini et al.Citation[34]
In a recent study, Carrasco-Pancorbo et al.Citation[35] demonstrated the antioxidant activity of different single phenols isolated from EVOO by several methods, including DPPH, accelerated oxidation in a lipid model system, and by an electrochemical method. Again, the ability to act as a hydrogen donor and the inhibition of oxidation enhanced by increasing the number of hydroxyl group in the phenols, were demonstrated. In particular, compounds with o-dihydroxyl functionalities have shown high antioxidant activity given the formation of intramolecular hydrogen bonds during the reaction with free radicals; also, electron-donating substituents in “ortho” position tend to weaken the O–H bond of phenol and furnish more stability to the phenoxyl radical. The results obtained for the three tests showed that 3,4-DHPEA, 3,4-DHPEA-EDA, and 3,4-DHPEA-EA are the strongest antioxidants confirming that the presence of a single hydroxyl group confers a limited amount of antioxidant activity; also the presence of the carboxymethyl group (COOCH3) as in 3,4-DHPEA-EA, seems to cause a decrease in the antioxidant power since it is not an electron donor group.Citation[35]
Antiproliferative Effects on Human Cancer Cells
The SRB assay was used for the screening of antiproliferative effect of phenolic extract from olive oil. Although there are a number of problems associated with the use of cell lines for testing antiproliferative effects, it does provide a viable option to the simplicity of in vitro model systems. In the present study large cell carcinoma (COR-L23), colorectal adenocarcinoma (Caco-2) lung carcinoma (A549), hepatocellular carcinoma (Huh-7D12), and renal cell adenocarcinoma ACHN were used as model experiments. Results have shown that after 48 h treatment with the phenolic extracts obtained from EVOO cultivar ‘Frantoio’, growth of human cancer cell lines are inhibited in a concentration-dependent manner and IC50 values were calculated and reported in . Interestingly, sample 2 demonstrated the strongest inhibitory activity on large cell carcinoma COR-L23, with IC50 of 14.5 μg/mL, and this value is 3.1-time lower that the IC50 of positive control vinblastine (45.5 μg/mL). A comparable activity on COR-L23 was observed with samples 1 and 3, with IC50 values of 55.9 and 65.1 μg/mL, respectively. Sample 3 exhibited an interesting activity also against Caco-2 cell with IC50 value of 65.5 μg/mL that was comparable with positive control.
Table 3 Antiproliferative activity (IC50 μg/ml) of phenolic extract from EVOO from cultivar ‘Frantoio’
This phenolic extract exhibited an IC50 value of 57.3 μg/mL against renal cell adenocarcinoma ACHN, which is about 2 times lower than the antiproliferative activity of samples 1 and 4 against the same cell line. Higher values of IC50 were found for A549 cells (IC50 values ranging from 62.6 to 119 μg/mL). Huh-7D12 cell cultures were not sensible to the antiproliferative activity of phenolic extract obtained from EVOO. Neither, samples analyzed in this study were able to exert antiproliferative activity against human skin fibroblast 142BR. This result suggests a specific mechanism of action interfering with abnormal proliferation. The therapeutic virtue of EVOO are attributed to their biophenolic contents.Citation[36] It is believed that the mechanism of action of these constituents in relation to “prevention” of several diseases including cancer involves an antioxidant component either directly or indirectly.Citation[37] The major phenolic compounds of olive oil, oleuropein and hydroxytyrosol, decreased cell viability, inhibited cell proliferation, and induced cell apoptosis in MCF-7 cells. The result of the MTT assay showed that 200 μg/mL of oleuropein or 50 μg/mL of hydroxytyrosol remarkably reduced cell viability of MCF-7 cells. Oleuropein or hydroxytyrosol decreased the number of MCF-7 cells by inhibiting the rate of cell proliferation and inducing cell apoptosis. Hydroxytyrosol and oleuropein also exhibited a statistically significant block of G1 to S-phase transition manifested by the increase of cell number in G0/G1 phase.Citation[38] Recently, Lozano-Sánchez et al. reported that crude EVOO phenolic extract rich in secoiridoids exhibited a remarkable cytotoxic activity against SKBR3 human breast cancer cells.Citation[39]
Previously, Babich and VisioliCitation[40] reported the cytotoxicity of oleuropein aglycone, oleuropein, caffeic acid, o-coumaric acid, cinnamic acid, tyrosol, syringic acid, protocatechuic acid, and vanillic acid against human cells isolated from tissues of the oral cavity. In support of the hypothesis that constituents of EVOO may contribute to the cancer protective activity of the Mediterranean diet, Juan et al.Citation[41] reported the antitumor activity of an olive fruits extract composed of maslinic acid and oleanolic acid in HT-29 human colon cancer cells. Obied et al.Citation[42] reported the effect of olive oil biophenols (caffeic acid, hydroxytyrosol, oleuropein, verbascoside, hydroxytyrosol acetate, 3,4-DHPEA-EDA, and comselogoside), and crude and ethyl acetate extracts from cultivar ‘Correggiola’ on digestive system tumor cell lines where these phytochemicals are expected to come in direct contact with the tumor cells at higher concentrations and where the effect of metabolism is minimal. None of the tested biophenols significantly affected the growth of colorectal adenocarcinoma cell (HT-29). Moreover, caffeic acid significantly promoted the growth of gastric adenocarcinoma (AGS) cells at all applied concentrations. Hydroxytyrosol, oleuropein, and verbascoside at 10 μM significantly reduced the proliferation of AGS cells by 27, 16, and 19%, respectively. On the contrary, extracts effectively inhibited the growth of both cell lines in a concentration-dependent manner (0.4–1.2 mg/mL). The observed low activity of individual biophenols can be due to a real resistance of the cells to their mechanism of action, lack of antiproliferative activity of these biophenols, or the degradation of the biophenols in the assay media. On the contrary, both crude extract and ethyl acetate extract effectively suppressed the growth of human cancer cells.
CONCLUSION
The antioxidant, metal chelating, and antiproliferative properties of phenol extracts at different concentration of EVOO cultivar ‘Frantoio’, one of main variety cultivated in Italy, have been investigated. The EVOOs presented good concentration of total phenolic content, particularly in secoridoids compounds. The phenol extracts have antioxidant properties, including free radical scavenging activity and reducing power and these activities are related to the phenolic content. Recently, phenolic compounds found in food and beverage, have begun to receive much attention among researchers as a new natural antioxidant. Moreover, treatment with EVOO extracts showed interesting antiproliferative activity against human colon cancer cell line and this activity is comparable to commercial drug vinblastine. These results shown that EVOOs from cultivar ‘Frantoio’, may represent an interesting source of phenolic compounds as functional components that could be consumed in diets and/or used for the elaboration of functional food and pharmaceutical industries. This work support the health benefits derived from another consumed product of Mediterranean diet: olive oil.
ACKNOWLEDGMENTS
The authors wish to thank Dr. Andrea Giomo, Università Politecnica delle Marche, for providing the olive oil samples and Dr. Annamaria Caufin, Facoltà di Farmacia, Scienze della Nutrizione e della Salute, Università della Calabria, for revision of the English version of the manuscript.
REFERENCES
- Keys , A. , Aravanis , C. and Van Buchem , F.S.P. 1981 . The diet and all-causes death rate in the seven countries study . Lancet , 8237 : 58 – 61 .
- Owen , R.W. , Mier , W. , Giacosa , A. , Hull , W.E. , Haubner , R. , Würtele , G. , Spiegelhalder , B. and Bartsch , H. 2000 . Olive-oil consumption and health: The possible role of antioxidants . Food and Chemical Toxicology , 38 : 647 – 659 .
- Owen , R.W. , Giacosa , A. , Hull , W.E. , Haubner , R. , Spiegelhalder , B. and Bartsch , H. 2000 . The antioxidant anticancer potential of phenolic compounds isolated from olive oil . European Journal of Cancer , 36 : 1235 – 1247 .
- Commission Regulation (EC) No 1989/2003 of 6 November 2003 amending Regulation (EEC) No 2568/91 on the characteristics of olive oil and olive-pomace oil and on the relevant methods of analysis . Official Journal of the European Union L295/57 of 13.11.2003 ,
- Nergiz , C. and Engez , Y. 2000 . Compositional variation of olive fruit during ripening . Food Chemistry , 69 : 55 – 59 .
- Morellò , J.R. , Romero , M.P. and Motilva , M.J. 2006 . Influence of seasonal conditions on the composition and quality parameters of monovarietal virgin olive oils . Journal American Oil Chemical Society , 83 : 683 – 688 .
- Romero , M.P. , Tovar , M.J. , Girona , J. and Motilva , M.J. 2002 . Changes in the HPLC phenolic profile of virgin olive oil from young trees (Olea europaea L. Cv. Arbequina) grown under different deficit irrigation strategies . Journal of Agricultural and Food Chemistry , 50 : 5349 – 5354 .
- Servili , M. , Esposito , S. , Lodolini , E. , Selvaggini , R. , Taticchi , A. and Urbani , S. 2007 . Irrigation effects on quality, phenolic composition, and selected volatiles of virgin olive oils cv. Leccino . Journal of Agricultural and Food Chemistry , 55 : 6609 – 6618 .
- Boselli , E. , Di Lecce , G. , Strabbioli , R. , Pieralisi , G. and Frega , N.G. 2009 . Are virgin olive oils obtained below 27°C better than those produced at higher temperatures? . LWT–Food Science and Technology , 42 : 748 – 757 .
- Fiorino , P. , Lombardo , N. and Marone , E. 2005 . Germplasm in olivicolture: New employment of “old” cultivars . Italus Hortus , 12 : 7 – 18 .
- Piscolla , G. and Zoppi , L. 2001 . The Olive Tree in Tuscany , Florence : Agenzia di Promozione Economica della Toscana .
- Fito , M. , Covas , M.I. , Lamuela-Raventos , R.M. , Vila , J. , Torrents , J. , de la Torre , C. and Marrugat , J. 2000 . Protective effect of olive oil and its phenolic compounds against low-density lipoprotein oxidation . Lipids , 35 : 633 – 638 .
- Visioli , F. and Galli , C. 2002 . Biological properties of olive oil phytochemicals . Critical Reviews in Food Science and Nutrition , 42 : 209 – 221 .
- Visioli , F. , Bellomo , G. and Galli , C. 1998 . Free radical-scavenging properties of olive oil polyphenols . Biochemical and Biophysical Research Communications , 247 : 60 – 64 .
- de la Puerta , R. , Ruiz Gutierrez , V. and Hoult , J.R. 1999 . Inhibition of leukocyte 5-lipooxygenase by phenolics from virgin olive oil . Biochemical Pharmacology , 57 : 445 – 449 .
- Visioli , F. , Bellosta , S. and Galli , C. 1998 . Oleuropein, the bitter principle of olives, enhances nitric oxide production by mouse macrophages . Life Science , 62 : 541 – 546 .
- Montedoro , G.F. , Servili , M. , Baldioli , M. and Miniati , E. 1992 . Simple and hydrolyzable phenolic compounds in virgin olive oil. Their extraction, separation and quantitative compounds and semiquantitative evaluation by HPLC . Journal of Agricultural and Food Chemistry , 40 : 1571 – 1576 .
- Singleton , V.L. and Rossi , J.A. 1965 . Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents . American Journal of Enology and Viticulture , 16 : 144 – 158 .
- Boselli , E. , Di Lecce , G. , Minardi , M. , Pacetti , D. and Frega , N.G. 2007 . La spettrometria di massa nell'analisi di componenti minori polari dell'olio vergine di oliva . Rivista Italiana delle Sostanze Grasse , 84 : 3 – 14 .
- Loizzo , M.R. , Menichini , F. , Tundis , R. , Bonesi , M. , Nadjafi , F. , Saab , A.M. , Frega , N.G. and Menichini , F. 2010 . Comparative chemical composition and antiproliferative activity of aerial parts of Salvia leriifolia Benth. and S. acetabulosa L. essential oils against human tumor cells in vitro models . Journal of Medicinal Food , 13 : 62 – 69 .
- Benzie , I.F. and Strains , J.J. 1996 . The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay . Analytical Biochemistry , 239 : 70 – 76 .
- Re , R. , Pellegrini , N. , Proteggente , A. , Pannala , A. , Yang , M. and Rice-Evans , C. 1996 . Antioxidant activity applying an improved ABTS radical cation decolorization assay . Free Radical Biology & Medicine , 26 : 1231 – 1237 .
- Servili , M. , Esposto , S. , Fabiani , R. , Urbani , S. , Taticchi , A. , Mariucci , F. , Selvaggini , R. and Montedoro , G.F. 2009 . Phenolic compounds in olive oil: Antioxidant, health and organoleptic activities according to their chemical structure . Inflammopharmacology , 17 : 76 – 84 .
- Rovellini , P. , Cortesi , N. and Fedeli , E. 1997 . Analysis of flavonoids from Olea europaea by HPLC–UV and HPLC–Electrospray–MS . Rivista Italiana delle Sostanze Grasse , 74 : 273 – 279 .
- Moon , J.K. and Shibamoto , T. 2009 . Antioxidant assays for plant and food components . Journal of Agricultural and Food Chemistry , 57 : 1655 – 1666 .
- Tripoli , E. , Giammanco , M. , Tabacchi , G. , DiMajo , D. , Giammanco , S. and La Guardia , M. 2005 . The phenolic compounds of olive oil: structure, biological activity and beneficial effects on human health . Nutrition Research Review , 18 : 98 – 112 .
- Hashim , Y. , Rowland , I.R. , McGlynn , H. , Servili , M. , Selvaggini , R. , Taticchi , A. , Esposto , S. , Montedoro , G. , Kaisalo , L. , Wahala , K. and Gill , C.I.R. 2008 . Inhibitory effects of olive oil phenolics on invasion in human colon adenocarcinoma cells in vitro . International Journal of Cancer , 122 : 495 – 500 .
- Bendini , A. , Cerretani , L. , Carrasco-Pancorbo , A. , Gomez-Caravaca , A.M. , Segura-Carretero , A. , Fernandez-Gutierrez , A. and Lercker , G. 2007 . Phenolic molecules in virgin olive oils: A survey of their sensory properties, health effects, antioxidant activity and analytical methods. An overview of the last decade . Molecules , 12 : 1679 – 1719 .
- Servili , M. , Selvaggini , R. , Esposto , S. , Taticchi , A. , Montedoro , G. and Morozzi , G. 2004 . Health and sensory properties of virgin olive oil hydrophilic phenols: Agronomic and technological aspect of production that affect their occurrence in the oil . Journal of Chromatgraphy A , 1054 : 113 – 127 .
- Ding , H. , Gao , Y. , Lei , H. , Luo , L. , Chao , H. and Ruan , R. 2010 . In vitro antioxidant effects of flavonoids of sweet potato vines . International Journal of Food Properties , 13 : 360 – 368 .
- Baldioli , M. , Servili , M. , Perretti , G. and Montedoro , G.F. 1996 . Antioxidant activity of tocopherols and phenolic compounds of virgin olive oil . Journal American Oil Chemical Society , 73 : 1589 – 1593 .
- Song , Y.Y. and Barlow , P.J. 2004 . Antioxidant capacity and phenolic content of selected fruit seeds . Food Chemistry , 88 : 411 – 417 .
- Rice Evans , C.A. , Miller , N.J. and Paganga , G. 1997 . Antioxidant properties of phenolic compounds . Trends Plants Science , 4 : 152 – 159 .
- Bendini , A. , Cerretani , L. , Salvador , M.D. , Fregapane , G. and Lercker , G. 2009 . Stability of the sensory quality of virgin olive oil during storage: An overview . Italian Journal Food Science , 21 : 389 – 406 .
- Carrasco-Pancorbo , A. , Cerretani , L. and Bendini , A. 2005 . Evaluation of the antioxidant capacity of individual phenolic compounds in virgin olive oil . Journal of Agricultural and Food Chemistry , 53 : 8918 – 8925 .
- Lopez , S. , Pacheco , Y.M. , Bermudez , B. , Abia , R. and Muriana , J.G. 2004 . Olive oil and cancer . Grasas Aceites , 55 : 33 – 41 .
- Owen , R.W. , Haubner , R. , Wurtele , G. , Hull , W.E. , Spiegelhalder , B. and Bartsch , H. 2004 . Olives and olive oil in cancer prevention . European Journal Cancer Prevention , 13 : 319 – 326 .
- Han , J. , Talorete , T.P. , Yamada , P. and Isoda , H. 2009 . Anti-proliferative and apoptotic effects of oleuropein and hydroxytyrosol on human breast cancer MCF-7 cells . Cytotechnology , 59 : 45 – 53 .
- Lozano-Sánchez , J. , Segura-Carretero , A. , Menendez , J.A. , Oliveras-Ferraros , C. , Cerretani , L. and Fernández-Gutiérrez , A. 2010 . Prediction of extra virgin olive oil varieties through their phenolic profile. Potential cytotoxic activity against human breast cancer cells . Journal of Agricultural and Food Chemistry , 58 : 9942 – 9955 .
- Babich , H. and Visioli , F. 2003 . In vitro cytotoxicity to human cells in culture of some phenolics from olive oil . Il Farmaco , 58 : 403 – 407 .
- Juan , M.E. , Wenzel , U. , Ruiz-Gutierrez , V. , Daniel , H. and Planas , J.M. 2006 . Olive fruit extracts inhibit proliferation and induce apoptosis in HT-29 human colon cancer cells . The Journal of Nutrition , 136 : 2553 – 2557 .
- Obied , H.K. , Prenzler , P.D. , Konczak , I. , Rahman , A. and Robards , K. 2009 . Chemistry and bioactivity of olive biophenols in some antioxidant and antiproliferative in vitro bioassays . Chemical Research in Toxicology , 22 : 227 – 234 .