Abstract
Potato chips fried in mid-oleic sunflower oil were stored in closed containers at ambient temperature (∼22°C) either in dark or under fluorescent light for 6 weeks. Lightness and yellowness of chips decreased with storage time. Peroxide values were influenced by storage conditions and time. Sixteen flavor volatiles were positively identified and concentrations of hexanal and 2-furaldehyde increased with storage time. However, t,t,-2,4-decadienal was not affected by storage time. The results indicated that potato chips fried in mid-oleic sunflower oil might retain the desirable fried flavor during 6 weeks of storage in a sealed container in the dark.
Introduction
Potato chips are one of the most popular snacks in the Unites States.Citation[1] The chips are commonly fried in cottonseed oil for desirable fried food flavor in the southern U.S.Citation[2, Citation3] However, cottonseed oil contains a higher amount of plamitic acid (C16:0), which is hypercholesteremic and associated with the increased incidence of coronary heart disease.Citation[4, Citation5] This factor has led to use of less saturated and hydrogenated oil for deep-fat frying. Furthermore, reliable scientific evidences support that increased levels of unsaturated fatty acid, such as linoleic (C18:2n6) and oleic (C18:1n9) acid, have health benefits to humans because they lower the levels of low-density lipoprotein (LDL)-cholesterol.Citation[4, Citation5] In response to health fat consumption patterns, researchers have sought to replace saturated and trans fats with unsaturated fats in potato chips.Citation[6, Citation7]
Frying oil containing a high amount of polyunsaturated fatty acids forms a higher ratio of oxidized products in deep-fat frying compared with the oil containing high monounsaturated or saturated fatty acids.Citation[3, Citation8, Citation9] The refined, bleached, and deodorized (RBD) high linoleic sunflower oil contains about 70% C18:2n6, whereas cottonseed oil used to fry potato chips contains 33 to 59% C18:2n6.Citation[7] The oxidation of C18:2n6 during frying produces t,t-2,4-decadienal that might impart desirable flavor to fried foods, but the higher level of C18:2n6 might provide less stable flavor of the sunflower oil-fried chips.Citation[6, Citation10] Research has been focused on increasing the oxidative stability of oil from oilseeds by plant breeding and transgenic applications.Citation[11] The resulting oilseeds increase oxidative stability of oils with increasing the content of C18:1n9 and decreasing C18:2n6 in the oilseeds. Accordingly, the C18:1n9 levels increased up to 90% to increase the stability of oil.Citation[12] However, attempts to improve the oxidation stability of the fried chips with using a higher C18:1n9 (∼78%) frying oils had very limited success because the desired flavor intensity of fried potato is determined by the C18:2n6 content in deep-fat frying oil.Citation[3, Citation11] However, the ideal proportion of C18:1n9 and C18:2n6 to produce the most desirable flavor in potato chips has not been suggested. Mid-oleic sunflower oil (65% C18:1n9 and 26% C18:2n6) has been developed to use for frying that has high stability without hydrogenation.Citation[13] Yet, it is not determined that potato chips fried in mid-oleic sunflower oil provide desirable fried flavor with good flavor stability. The purpose of this study was to evaluate the quality and flavor volatiles of fresh and stored potato chips fried in mid-oleic sunflower oil.
Materials and methods
Potato Chip Production and Storage
Sliced potatoes, 1.52 mm thick, were obtained from a local food processor and kept in sealed bags full of water until frying. Prior to being used for frying, mid-oleic sunflower oil from a commercial processor was break-in heated in a Dual Zone Deep Fryer (Model D14527DZ; DeLonghi America Inc., Saddle Brook, NJ, USA) at 163°C for 4 h. The temperature was raised to 194°C prior to frying potato chips. The sliced potatoes were removed from the bags and air-dried for 10 min under forced airflow at ambient temperature (∼22°C). A batch of 250-g air-dried potato slices was placed in a frying basket and fried for 4 min. All subsequent batches of potato slices in each day followed the same frying temperatures and times. The fried chips were placed on paper towels for 2 min, collected in a plastic container, and immediately added 1% (w/w) NaCl uniformly. Eight batches of fried chips were produced every day for 3 consecutive days. There were three replications in this experiment. A single replication consisted of potato chips fried in one day and storage fried chips for 0, 3, and 6 wk under fluorescent light or the dark at ambient temperature. Prior to packaging, all potato chips obtained from 8 batches in each day were mixed thoroughly. The chips were sealed in a 0.95-L glass jar (60 g chips/jar). For 0-wk storage, four jars of chips were flushed with nitrogen, sealed, and stored in the dark at −18°C until analyzed. The jars for 2-, 4-, or 6-wk storage (8 jars/storage) were stored either in the dark or under fluorescent light (16.1–18.3 lux) at ambient temperature. At the end of each storage time, the jars were flushed with nitrogen, sealed, and stored in the dark at −18°C until analyzed.
Physical and Chemical Analysis
At the end of each storage time, the color of potato chips was measured directly from the surface of chips using a HunterLab Color instrument with illuminant D65 as the light source (Hunter Associates Laboratory, Reston, VA, USA). The CIE L* a* b* color space notation was used to represent the color coordinate values of potato chips. Five measurements were taken from each sample as described by Gokem and SenyuvaCitation[14] and the average of five measurements was recorded as color coordinate values of each sample.
The moisture content of fresh potato chips (0-wk storage) was determined by the vacuum oven-drying AOACCitation[15] method. Total lipids were extracted from each sample by means of the chloroform/methanol (2:1 v/v) method of Melton et al.Citation[16] and measured gravimetrically. The extracted lipid was transferred to a screw-cap brown bottle, flushed with nitrogen gas, and stored at −18°C until further analyzed. The peroxide values (PV) of the lipid extracted from the potato chips were determined by the method of Mehlenbacher.Citation[17] Fatty acid composition of total lipids extracted from fresh potato chips (0-wk storage) was analyzed by preparing fatty acid methyl esters (FAME) from 0.1 g of the extracted total lipid.Citation[18] The FAME were analyzed with a TRACE GC Ultra (Thermo Electron Corp., Austin, TX, USA) coupled with an autosampler AS3000 (Thermo Electron Corp.). A 30 m × 0.25 mm i.d., df 0.2 μm fused silica capillary column (SP-2330; Supelco Inc., Bellefonte, PA, USA) was used to separate the methyl esters, which were detected with a flame ionized detector (FID). The injection temperature was 220°C, and the column temperature was programmed from 130 to 220°C at 2°C/min. Helium was the carrier gas with a flow rate at 1.5 mL/min and a split ratio of 30:1. The FAME were identified by matching their retention times with those of known standards (Sigma Chemical Co., St. Louis, MO, USA) and the relative weight percents of individual FAME (C10:0–C22:0) in each sample were calculated using corrected areas.Citation[18]
The flavor volatiles of chips were extracted by a solid phase microextraction (SPME) methodCitation[19] and identified using a gas chromatography and mass spectrometry (GC-MS). The chips were homogenized with a mortar and pestle. A portion (4.0 g) of the homogenized samples was transferred into a 15-mL vial and then sealed with a PTFE silicon septum (Supelco Inc.). The vial was heated at 60°C on a SPME sampling stand using a heat/stir plate (Model PC-400; Corning Inc., Corning, NY, USA). Subsequently, the volatiles in the headspace were collected for 15 min on a 50/30-μm divinylbenzene/carboxen/polydimetylsiloxane fiber (Supelco Inc.) inserted through the silicon septum. The volatiles were desorbed for 5 min by inserting the SPME needle and exposing the fiber directly into the injection port (230°C) of a gas chromatography (TRACE GC Ultra; Thermo Electron Corp., Austin, TX, USA), separated on a Supelcowax column (60 m × 0.25 mm i.d., df 0.2 μm; Supelco Inc.), and detected with a mass selective detector (Finnigan TRACE DSQ MS; Thermo Electron Corp.). Helium was used as a carrier gas with a flow rate of 1.6 mL/min. The injection port was in the splitless mode and the column temperature was programmed from 50 to 230°C (3°C/min) and holding at 230°C for 15 min. The mass spectrometer operated in the electron impact mode with an electron energy of 70 eV, a multiplier voltage of 1100 V, and data collection rate of 1.5 scan/s over a range of m/z 40–450. Volatile compounds were positively identified by comparing their mass spectra and retention times with those from standard compounds (Sigma-Aldrich Corp., St. Louis, MO, USA). Flavor volatiles extracted from the homogenized chips by the SPME method were also analyzed with a Thermo Electron gas chromatography (TRACE GC Ultra; Thermo Electron Corp.) equipped with a flame ionization detector (FID) using the same column and conditions described in the GC-MS. Volatiles were extracted with the same SPME and conditions described in the SPME method. The concentration of individual flavor volatiles in each sample was determined as percentage of total area of the peaks in the GC chromatogram.
Statistical Analysis
All data were analyzed as a randomized block design (RBD), block on day, with factorial treatment arrangement using the MIXED procedure of SAS (SAS Institute Inc., Cary, NC, USA), with an individual container containing chips as an experimental unit. The effects of storage conditions (dark and light) and storage times (0, 2, 4, 6 wk) as well as their two-way interaction were considered to be fixed. Significant differences among means were determined by the Least-Squares Means generated and separated using the PDIFF (p-values for difference) option of SAS for main or interaction effects. Significance was determined at P < 0.05, but differences of 0.05 ≤ P < 0.1 were considered as trends.
Results and discussion
Physicochemical Properties of Potato Chips
Moisture and lipid contents are important quality parameters of potato chips. Moisture and lipid contents of fresh potato chips in the present study were in the acceptable ranges (). The moisture content of potato chips depends on the thickness of potato slices. According to Gamble and Rice,Citation[20] thin slices resulted in shorter distances for moisture to diffuse to the surface during frying that led to rapid moisture loss. Lipid contents of potato chips were affected by not only the thickness of slices but also the specific gravity of raw potatoes. Oil absorption of the potato chips generally decreases with increasing specific gravity and slice thickness of the potatoes.Citation[20, Citation21] Increasing frying time and oil temperature also caused a slight increase in the final oil content of the chips.Citation[8] Potato chips generally contain 35.4 to 44.5% of lipid to provide a unique flavor and texture; moreover, crispness of potato chips is lost if the moisture content of chips is higher than 3.5%.Citation[22]
Table 1 Chemical composition of fresh potato chips fried in mid-oleic sunflower oil.
Cottonseed oil is the most frequently used oil for frying potato chips in the southern U.S. It contains about 25% palmitic (C16:0), 18% oleic (18:1n9), and 57% linoleic (C18:2n6) acids. Compared with cottonseed oil, the total lipids from fresh chips fried in mid-oleic sunflower oil contained a higher percentage of C18:1n9 and a lower percentage of C18:2n6 and C16:0 (); therefore, the chips fried in mid-oleic sunflower oil may have a healthier fatty acid profile than the chips fried in cottonseed oil. Dietary saturated fatty acids, such as lauric (C12:0), myristic (C14:0), and C16:0 acids, have plasma LDL-cholesterol raising characteristics, whereas unsaturated fatty acids, including C18:1n9 and C18:2n6, decrease LDL-cholesterol.Citation[23]
Color measurements (CIE L* a* b*) of potato chips fried in mid-oleic sunflower oil, stored in the dark or under fluorescent light for 0 (fresh), 2, 4, and 6 wk are presented in Color is an important quality factor of potato chips. It is mainly influenced by the chemical composition of potatoes and processing factors, such as slice thickness as well as frying temperature and time. The following three chemical reactions have been suggested to develop the color of potato chips during frying, such as caramelization, a reaction of malonaldehyde, and Maillard reaction.Citation[24] In caramelization, sugars dehydrate and undergo a series of reactions to form caramel, dark-colored pigments. A high temperature (above 160°C) during frying may accelerate caramelization. Subsequently, malonaldehyde formed during frying may react with amino acids to form brown-colored complexes. Reducing sugars reacts with amino acids and protein to form dark brown colored substances in the Maillard reaction. The color of chips is also changed due to the oxidation of chips during storage that depends on the storage time and condition.
Figure 1 Changes of CIE L*, a*, and b* values (n = 12) in potato chips fried in mid-oleic sunflower oil and stored in the dark or under fluorescent light (16.1–18.3 lux) for 6 wk; points with no common letters are different (P < 0.05). (Color figure available online.)
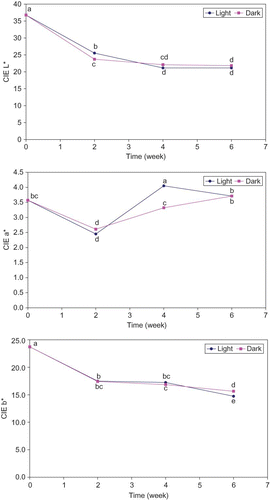
Without consideration of the storage time, no significant differences were found in the CIE L* a* b* values of the chips stored in the dark or under fluorescent light (). However, the CIE L* a* b* values of the chips were influenced (P < 0.05) by the storage time (0-, 2-, 4-, and 6-wk) across storage conditions (dark and light). The CIE L* (lightness) and b* (yellowness) values of the chips were decreased (P < 0.05) as the storage time progressed. The CIE L* and b* values of the chips stored either in the dark or under the light decreased sharply over the first 2 weeks (). The chips stored under the light further decreased (P < 0.05) the CIE L* values until week 4, while the CIE L* values of chips stored in the dark remained the same. Then there was no further decrease of the CIE L* and b* values of chips stored either in the dark or under the light until week 6. The CIE a* (redness) value of the chips were increased (P < 0.05) with some variation during the 6-wk storage time (). The CIE a* values of the chips stored either in the dark or under the light decreased (P < 0.05) sharply during the first 2 weeks, then increased (P < 0.05) slightly to stabilize at week 6. However, the CIE a* values of the chips stored under the light continued to increase (P < 0.05) until week 4, then decreased slightly until week 6. Changes in color values (CIE L* a* b*) of the chips in this study might be due to the lipid oxidation because lipid oxidation generally occurred in the fried potato chips during storage. In the presence of basic groups in proteins, aldol condensation of carbonyls produced from lipid oxidation may catalyze and result in formation of brown pigments. The change in color of potato chips fried in mid-oleic sunflower oil with storage time might be due to the interaction of the amino group with reducing sugar, which is a non-oxidative browning also known as Millard reaction. In the present study, potato chips fried in mid-oleic sunflower oil were darker (lower L*) and less yellowish (lower b*) as storage time processed, while redness (higher a*) of chips was increased with some variation.
Storage conditions and times as well as their interactions significantly affected the peroxide values (PV) of lipids extracted from the chips (). Fried potato chips are generally oxidized during storage that is dependent upon the storage time and condition. The PV of lipid extracted from the chips fried in mid-oleic sunflower oil were progressively increased (P < 0.05) during storage, which is indicative of occurrence of oxidation of the chips (). The PV of chips stored under the light were higher (P < 0.05) than those stored in the dark; moreover, the chips stored in the dark were oxidized at a much slower rate than those stored under fluorescent light. The greater increase in PV indicates that the potato chips stored under light experienced photo- and auto-oxidation. After 2-, 4-, and 6-wk storages in the dark, the PV of chips increased (P < 0.05) from 0.26 to 1.14, 1.96, and 3.18 meq peroxide/kg oil, respectively, while those of chips stored under the light for 2-, 4-, and 6-wk increased (P < 0.05) to 2.13, 3.6, and 4.96 meq peroxide/kg oil, respectively. The findings in the present study agreed with those reported by LinCitation[25] and PangloliCitation[7] who found similar trends in chips fried in high linoleic sunflower or cottonseed oil and then stored in either dark or light.
Table 2 Flavor volatiles (n = 12) isolated from potato chips fried in mid-oleic sunflower oil and stored for 0 (fresh), 2, 4, or 6 wk either in the dark or under fluorescent light (16.1–18.3 lux).
Flavor Volatiles in Potato Chips
Twenty-one flavor volatiles were isolated from potato chips fried in mid-oleic sunflower oil, but only 16 compounds were positively identified (). Pentanal, hexanal, 2-hexanal, 2-heptenal, 2-octenal, 2-nonenal, and 2,4-decadienal are formed from the oxidation of C18:2n6.Citation[24, Citation26] In the present study, these volatiles in potato chips were expected because of the presence of C18:2n6 in the mid-oleic sunflower oil (). However, pentanal was not detected from the chips in the current study. A previous study also showed that pentanal was not detected from the chips fried in either high linoleic sunflower or cottonseed oil.Citation[7] Noticeable amounts of hexannal, octanal, nonanal, 2-furaldehyde, trans-2-decenal, tertradecanal, and hexadecanal were detected from the chips fried in mid-oleic sunflower oil (). Octanal, nonanal, and trans-2-decenal are derived from the oxidation of C18:1n9,Citation[24, Citation26] whereas tertradecanal and hexadecanal are formed via thermal oxidation of the saturated fatty acids.Citation[10] The 2-furaldehyde is derived from the dehydration of pentose or Maillard reaction that occurred in the potato chips.Citation[27] Relatively small amounts of trans-2-pentenal, heptanal, decanal, and benzaldehyde were found in the chips fried in mid-oleic sunflower oil (). According to Belitz,Citation[26] heptanal and decanal are derived from the oxidation of C18:1n9. However, trans-2-pentenal and benzaldehyde are formed by the oxidation of C18:3n3.Citation[10, Citation24] The volatiles isolated in fresh chips (0-wk) either already existed in the frying oils due to thermal oxidation and then absorbed by potato chips or were formed from reactions in the chips during frying. With exception of 2-furaldehyde, the most abundant identified volatiles in the fresh chips were oxidation products of the C18:1n9 and C18:2n6, which were the most abundant fatty acids presented in the frying oil.
Concentrations of volatiles were generally affected by storage conditions. Pangloli et al.Citation[7] reported that chips stored in the dark had lower (P < 0.05) concentrations of volatiles than those stored under the light because of the low rate of lipid oxidation in the chips stored in the dark. However, this trend was not clearly observed in the current study, except hexanal and 2-furaldehyde volatiles (). Significant differences were found in the contents of hexanal, heptanal, nonanal, and 2-furaldehyde among chips stored either in the dark or under the light during 6 wk. The content of hexanal progressively increased (P < 0.05) with storage times when the chips were stored under the light (), but the mean concentrations of hexanal in the chips stored for 4- and 6-wk were similar. The concentration of hexanal in the chips was not changed until the first 2 weeks of storage in the dark, but increased after the 4 wk of storage. After 4- and 6-wk storage time in the dark, the mean concentration of hexanal in the chips did not change (P > 0.05). The concentration of heptanal tended to decrease (P < 0.1) at 2-wk storage and then to increase (P < 0.1) by 4-wk storage in the dark. The concentration of nonanal in chips was significantly lower at 4-wk storage under the light than in fresh chips (0-wk storage). The flavor volatile 2-furaldehyde in the chips increased steadily during the storage time. Furthermore, it increased faster in the chips stored under the light, especially from 4- to 6-wk storage. LinCitation[25] suggested that malonaldehyde (lipid oxidation product) reacts with amino acids in potato chips to form 2-furaldehyde. Accordingly, concentration of malonaldehyde may increase in potato chips as storage time progressed. The volatile t,t,-2,4-decadienal that imparts desirable flavor to fresh potato chipsCitation[10] did not significantly change during 6-wk storage either in the dark or under the light. The results of the present study agreed with those reported by Pangloli.Citation[7] However, a previous study showed that the concentration of t,t,-2,4-decadienal significantly increased after 4-wk storage of potato chips under the light.Citation[25] In addition, this volatile compound significantly decreased after 4-wk storage of potato chips in the dark.Citation[28] These contradicting results indicated that volatile compounds might be constantly formed and degraded as well as reacting with other compounds during storage of the chips. These ongoing chemical reactions of volatiles from the lipid oxidation might explain the different pattern of volatile compounds among potato chips storage studies.
Conclusion
Mid-oleic sunflower oil could produce potato chips with a healthier fatty acid profile than other chips produced with partially hydrogenated canola and soybean oils, commonly used to prepare potato chips in the U.S. This indicates that mid-oleic sunflower oil can be used in a new way to provide healthier fried snack foods for humans. According to the peroxide and chromatic values, chips fried in mid-oleic sunflower oil were degraded progressively during storage. However, the chips might retain the desirable fried flavor (t,t,-2,4-decadienal) during 6 wk storage in a sealed container. Additional research is needed to investigate ways to retard the lipid oxidation of chips fried in mid-oleic sunflower oil during storage.
References
- Clark , J.P. 2003 . Happy birthday, potato chip! and other snack developments . Food Technology , 57 : 89 – 92 .
- Weiss , T.J. 1983 . Food Oils and Their Uses , 2nd , 310 AVI Publishing: Westport, Connecticut .
- Warner , K. , Orr , P. , Parrott , L. and Glynn , M. 1994 . Effect of frying oil composition on potato chip stability . Journal of the American Oil Chemists’ Society , 71 : 1117 – 1121 .
- Vanderveen , J.E. 1996 . “ Dietary recommendation for lipids and measure designed to facilitate implementation ” . In Food Lipids and Health , Edited by: McDonald , R.E. and Min , D.B. 1 – 18 . New York : Marcel Dekker .
- Wolfarm , G. 2003 . Dietary fatty acids and coronary heart disease . European Journal of Medical Research , 8 : 321 – 324 .
- Melton , S.L. , Trigiano , M.K. , Penfield , M.P. and Yang , R. 1993 . Potato chips fried in canola and/or cottonseed oil maintain high quality . Journal of Food Science , 58 : 1079 – 1083 .
- Pangloli , P , Melton , S.L. , Collins , J.L. , Penfield , M.P. and Saxton , A.M. 2002 . Flavor and storage stability of potato chips fried in cottonseed and sunflower oils and palm olein/sunflower oil blends . Journal of Food Science , 67 : 97 – 103 .
- Saguy , I.S. and Pinthus , E.J. 1995 . Oil uptake during deep-fat frying: Factors and mechanism . Food Technology , 49 : 142 – 145 .
- Singh , R.P. 1995 . Heat and mass transfer in foods during deep-fat frying . Food Technology , 49 : 134 – 137 .
- Pokorny , J. and Smouse , T.H. 1989 . “ Flavor chemistry of deep fat frying oil ” . In Flavor Chemistry of Lipid Food , Edited by: Min , D.B. 113 – 155 . Champaign, Illinois : AOCS Press .
- Warner , K. and Gupta , M. 2005 . Potato chip quality and frying oil stability of high oleic acid soybean oil . Journal of Food Science , 70 : S395 – S400 .
- Warner , K. and Knowlton , S. 1997 . Frying quality and oxidative stability of high-oleic corn oils . Journal of the American Oil Chemists’ Society , 74 : 1317 – 1321 .
- Kiatsrichart , S. , Brewer , M. , Cadwallader , K. and Artz , W. 2006 . Pan-frying stability of NuSun oil, a mid-oleic sunflower oil . Journal of the American Oil Chemists’ Society , 80 : 479 – 483 .
- Gokem , V. and Senyuva , H.Z. 2006 . Study of color and acrylamide formation in coffee, wheat flour and potato chips during heating . Journal of Food Chemistry , 99 : 238 – 243 .
- AOAC . 1995 . Official Methods of Analysis of the AOAC International , 16th , Arlington, Virginia : AOAC International .
- Melton , S.L. , Moyers , R.E. and Playford , C.G. 1979 . Lipid extracted from soy products by different procedures . Journal of the American Oil Chemists’ Society , 56 : 489 – 497 .
- Mehlenbacher , V.C. 1966 . Analysis of Fats and Oils , 616 Champaign, Illinois : The Garrand Press .
- AOCS . 1993 . Official Methods and Recommended Practices of American Oil Chemists’ Society , 4th , Champaign, Illinois : AOCS Press .
- Harmon , A.D. 1997 . “ Solid-phase microextraction for the analysis of flavor ” . In Techniques for Analyzing Food Aroma , Edited by: Marsili , R. 81 – 112 . New York : Marcel Dekker .
- Gamble , M.H. and Rice , P. 1988 . The effect of slice thickness on potato crisp yield and composition . Journal of Food Engineering , 8 : 31 – 46 .
- Smith , O. 1987 . “ Potato chips ” . In Potato Processing , 4th , Edited by: Tarbut , W.F. and Smith , O. 371 – 490 . Van Nostrand Reinhold, Co.: New York .
- Kubiak , C.L. , Austin , J.A. and Lindsay , R.C. 1982 . Influence of packaging construction on stability of potato chips exposed to fluorescent lighting . Journal of Food Protection , 45 : 801 – 805 .
- Noakes , M.N. , Nestle , P.J. and Clifton , T.M. 1996 . Modifying the fatty acids profile of dairy products through feedlot technology lowers plasma cholesterol of humans consuming the products . American Journal of Clinical Nutrition , 63 : 42 – 46 .
- deMan , J.M. 1999 . Principles of Food Chemistry , 3rd , 342 Gaithersburg, Maryland : Aspen Publishing .
- Lin , H-W. 1993 . Flavor and stability of potato chips fried in canola, high oleic acid sunflower, sunflower, and cottonseed oils , Knoxville , TN : Master Thesis, The University of Tennessee .
- Belitz , H.D. and Lipids . 1987 . Food Chemistry , Edited by: Belitz , H.D. and Grosch , W. 158 – 247 . New York : Springer-Verlag .
- Heath , H.B. and Reineccius , G. 1986 . Flavor Chemistry and Technology , 463 AVI Publishing: Westport, Connecticut .
- Han , M.J. 1989 . Flavor and stability of potato chips fried in soybean, cottonseed, and palm olein oils , Knoxville , TN : Doctoral Dissertation, The University of Tennessee .