Abstract
Acid and pepsin-soluble collagen was isolated from the skin of horse mackerel (Magalaspis cordyla) and croaker (Otolithes ruber) using 0.5 M acetic acid followed by precipitation with 0.9 M NaCl. The yields of acid soluble collagen were 17.3 ± 0.4 and 21.9 ± 0.6% and pepsin soluble collagen (22.5 ± 0.8 and 25.7 ± 0.3%) as per wet weight basis, respectively. The extracted acid soluble collagen and pepsin soluble collagen were characterized by SDS-PAGE, amino acid composition and FTIR spectroscopy. SDS-PAGE showed that all the extracted collagen contained two alpha components (α 1 and α 2) and one beta component (β); all the collagens were typical type I and maintained their triple helical structures well with slight molecular structure differences. The amino acid profiles of these collagens were similar with a low imino acid content. Therefore, there is a possibility to use horse mackerel and croaker skin collagen as an alternative source for commercial collagen and may find applications in the food, cosmetic, biomedical, and pharmaceutical industries.
INTRODUCTION
India is one of the largest marine fish producers in the world. The yield of marine capture production in 2005 was 84.2 million tons, accounting 59.5% of global production. Commonly available marine fishes in India include ribbon fish, croaker fish, eel fish, horse mackerel, katti fish, leather jacket, and mahi mahi. With the rapid development of fishery, various processing companies were established in and around the coastal areas. Moreover, during harvest season, many fishes are discarded, due to not being processed on time. Hence, comprehensive utilization of marine fish, especially the production of value-added products, has both environmental and economical importance.
Collagen is one of the predominant proteins in the living body and comprises about 30% of total animal protein. It is chiefly present in animal skin, bone, cartilage, tendon, and blood vessels and plays a major role in extracellular matrix (ECM). Due to its triple-helical nature it forms fibril and network-like structures and supports the ECM. Currently, 27 variants of collagen, named type I–XXVII have been reported.[Citation1] However, type I collagen is the most frequent fiber forming one in the tissues.[Citation2]
Collagen has a broad range of applications in the fields of food, pharmaceutical, cosmetic, biomedical materials, photographic film, and leather industries. Furthermore, it serves in tissue engineering due to its excellent biocompatibility and biodegradability.[Citation3, Citation4] The main sources of industrial collagen are those from skin and bones of bovine or porcine origin. However, the outbreaks of bovine spongiform encephalopathy (BSE) and transmissible spongiform encephalopathy (TSE) have resulted in anxieties among the users of cattle collagen-derived products. Besides, the collagen from pig's skin and bone is not allowed to be used in some religious groups. Fish waste, such as bones and scales, as well as skins, is very rich in collagen.[Citation5] Although the physical and chemical properties of fish collagen are different from those of mammalian collagen, it is unlikely to be related to BSE and TSE and will not be forbidden for religious reasons.[Citation6]
Thus, fish processing wastes may be alternative collagen sources and this has attracted the attention of the scientific community all over the world.[Citation7–11 Citation Citation Citation Citation11 Therefore, the objective of this study was to extract and characterize the acid and pepsin soluble collagen from the skin of horse mackerel and croaker for making more effective use of available resources.
MATERIALS AND METHODS
Sample Collection
Marine fishes, horse mackerel (Magalaspis cordyla) and croaker (Otolithes ruber), were collected from the Royapuram sea coast (13° 6′ 26″ N, 80° 17′ 43″ E), Tamilnadu, India in March 2009. Three fishes (average body weight of 450 g) of horse mackerel and croaker were washed thoroughly with running water and packed in polythene bags and brought to the laboratory. The collected samples were dissected and skin was cut into small pieces and stored at −20°C until use.
Extraction of Collagen
The extraction was carried out as per the protocol of Zhang et al.[Citation12] All procedures were carried out at 4°C with gentle stirring. Skins were soaked in 0.1 M NaOH with a sample/solution ratio of 1:20 (w/v), containing 0.5% non-ionic detergent for 24 h. The solution was changed once to get rid of non-collagenous proteins, partial pigments, and fat. The samples were then washed with distilled water until the pH of the water became neutral. Residual fat was removed in 15% (v/v) butyl alcohol with a sample/solution ratio of 1:20 for 24 h with a change of solution after12 h. Defatted skins were thoroughly washed with distilled water. To remove pigments more effectively, defatted skins were bleached with 3% H2O2 solution for 24 h and the solution was changed once. The bleached skin was thoroughly washed with distilled water and then stirred in 15 volumes (w/v) of 0.5 M acetic acid for 24 h. The suspensions were centrifuged at 9000× g for 15 min at 4°C. The supernatants obtained were salted-out by the addition of NaCl to a final concentration of 0.7 M. The resultant precipitate was collected by centrifugation at 9000× g for 15 min at 4°C and then dissolved in 0.5 M acetic acid. The solution obtained was dialyzed against 0.1 M acetic acid for 2 days with a change of solution after every 6 h and one day with double distilled water, followed by lyophilization in a freeze-dryer (Alpha 1–2 LD, SciQuip Ltd., Shropshire, UK) and finally acid-soluble collagen (ASC) was obtained. Undissolved residue, obtained after acid extraction, was suspended in 2 volumes (w/v) of 0.5 M acetic acid containing 1.5% (w/w) pepsin for 30 h at 4°C with continuous stirring. The pepsin-soluble collagen (PSC) was obtained by the same method as ASC. The extraction of collagens was performed three times and the yield value was the average of triplicate analysis.
UV-Vis Absorption Spectra
The UV-visual absorption spectra of collagens were recorded by an Ultraspec 2100 Pro (Amersham Biosciences, Piscataway, NJ, USA). One mg of the sample was dissolved in 2 ml of 0.5 M acetic acid and the collagen solutions were centrifuged at 5000× g for 10 min at 4°C. The clarified samples were determined for absorbance at different wavelengths (from 200 nm to 420 nm) using the mode wave scan to get UV-Vis spectra of each sample.[Citation13]
Sodium Dodecyl Sulphate Polyacrylamide Gel Electrophoresis (SDS-PAGE) Analysis
SDS-PAGE was performed according to the standard method of Laemmli[Citation14] with a slight modification, using 8% resolving gel and 5% stacking gel. The collagen samples were dissolved in a sample buffer, with 10% β-mercaptoethanol, to reach a final concentration of 1 mg/ml, and the mixed solution was boiled for 5 min. After electrophoresis (100 v), the gel was stained for 2 h using 0.25% Coomassie brilliant blue R250 solution and destained using 7.5% acetic acid and 5% methanol. Molecular weight markers (myosin [205 kDa], phosphorylase B [97 kDa], bovine serum albumin [66 kDa], ovalbumin [43 kDa], and carbonic anhydrase [29 kDa]) were purchased from GENEI (Bangalore, India) and were used as marker proteins, and standard type I collagen from calf skin (Sigma Chemical Co., St. Louis, MO, USA) was used as the reference.
Fourier Transform Infrared Spectroscopy (FTIR)
FTIR spectra were obtained from tablets containing 2 mg collagen samples in approximately 100 mg potassium bromide (KBr). All spectra were recorded using an FTIR spectrophotometer (Perkin Elmer, MA, USA) from 4000 to 500 cm−1 at a data acquisition rate of 2 cm−1 per point.[Citation10]
Amino Acid Composition
The amino acid composition was identified by using the methods of Yanhong et al.[Citation15] with small modifications. The lyophilized collagen was digested with HCl (6 N) at 110°C for 24 h and neutralized with NaOH and loaded onto high performance liquid chromatography (HPLC). HPLC analysis was carried out in an Agilent 1100 assembly system (Hewlett-Packardt Boeblingen, Germany) after precolumn derivatization with o-phthaldialdehyde (OPA). Each sample (1 μl) was injected on a Zorbax 80 A C18 column (Hewlett-Packard, Boeblingen, Germany) at 40°C with detection at 338 and 262 nm. Mobile phase A was 7.35 mmol/l sodium acetate/triethylamine (500:0.12, v/v), adjusted to pH 7.2 with acetic acid, while mobile phase B (pH 7.2) was 7.35 mmol/l sodium acetate/methanol/acetonitrile (1:2:2, v/v/v). The amino acid composition was expressed as percentage of protein.
Statistical Analyses
Statistical data for the properties of collagen from horse mackerel and croaker were conducted with three replicates. Data was expressed as mean ± standard deviation. The statistical analysis was performed using SPSS 10.0 software (SPSS Inc., Chicago, IL, USA).
RESULTS AND DISCUSSION
Isolation of ASC and PSC from Horse Mackerel and Croaker Skin
The skin of horse mackerel and croaker was hardly soluble in 0.5 M acetic acid and the yield of ASC was about 17.3 ± 0.4 and 21.9 ± 0.6% on a wet weight basis. On the contrary, pepsin soluble collagen was readily soluble from the residue of the acetic acid extraction and was then purified by differential salt precipitation and dialysis. The yield of PSC was comparatively high, about 22.5 ± 0.8 and 25.7 ± 0.3% on wet weight basis. But the percentages of collagen obtained from the fish skin viz., Japanese sea bass (51.4%), chub mackerel (49.8%), bullhead shark (50.1%);[Citation16] ocellate puffer fish, 44.7%,[Citation7] channel catfish acid-soluble (25.8%) and pepsin-soluble collagens (38.4%),[Citation17] deep-sea redfish acid-solubilized (47.5%), and pepsin-solubilized collagens (92.2%).[Citation10] In comparison with these reports, the yield of collagen from the present study was slightly low.
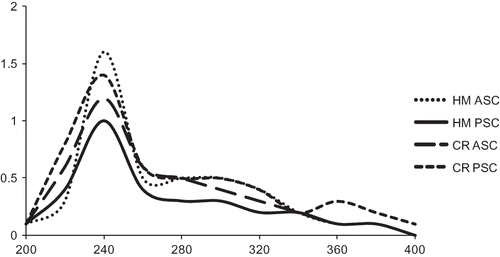
UV-Vis Spectra
The basic and simple way to characterize the collagen is to wave scan the sample from 200 to 400 nm, because collagen has maximum absorbance at 230 nm.[Citation18] Generally, the maximum absorption of protein is at 280 nm, since tryptophan did not exist in the collagen extracted from the skin of horse mackerel and croaker, also the contents of tyrosine was low; all the samples showed maximum absorbance in between 230–240 nm (Fig. 1). This was in agreement with those of collagen from the skins of largefin longbarbel catfish,[Citation12] walleye pollock,[Citation13] and channel catfish.[Citation17]
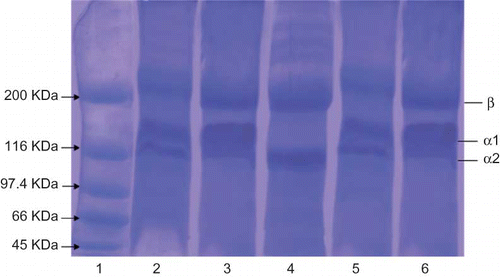
SDS-PAGE Pattern of Collagen
The acid and pepsin solubilized collagen of horse mackerel and croaker was characterized by SDS-PAGE using 8% resolving gel. Skin ASC and PSC had at least two different α-chains (α 1 and α 2) and their cross-linked chains (). In both species, the molecular mass of skin ASC and PSC subunit was about ∼135 kDa for α 1, ∼115 kDa for α 2, and the existence of α 3 chain was not identified clearly under the electrophoretic conditions. There were no significant differences in subunit molecular mass between the two fish's collagen and, moreover, a small amount of β-chain was obtained in these collagens. The existence of at least two different subunits shows that a major collagen from the fish skin was type I collagen. In electromobility, the positions of the chains of collagen from ASC and PSC of both the fishes were similar to those of standard type I collagen from calf skin. Hence, a major component of collagen extracted was type I collagen and, in addition, the electrophoretic patterns and migration of ASC and PSC were similar to the electrophoretic patterns of collagens isolated from the skin of other reported fish species.[Citation10, Citation11, Citation16, Citation17, Citation19]
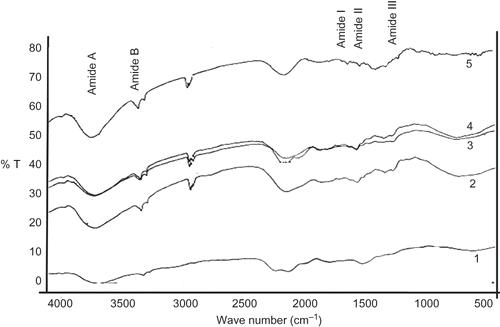
Fourier Transform Infrared Spectroscopy (FTIR)
FTIR spectra of calf skin collagen, ASC and PSC of horse mackerel and croaker are shown in . The regions of amides I, II, and III are known to be directly related with the shape of a polypeptide. Amide A band (3400–3440 cm−1) is related to N–H stretching vibrations. Amide I band (1600–1660 cm−1) is associated with stretching vibrations of carbonyl groups in peptides, being the most important factor in investigating the secondary structure of a protein. Amide II (∼1550 cm−1) is associated with NH bending and CN stretching. Amide III (1320–1220 cm−1) is related to CN stretching and NH, and is involved with the triple helical structure of collagen.[Citation20–22 Citation Citation22 In this study, regions of amides A, I, II, and III for calf skin, horse mackerel and croaker collagens were 3333 and 3427 cm−1 (amide A); 1660 and 1651 cm−1 (amide I); 1547 and 1544 cm−1 (amide II); and 1238 and 1240 cm−1 (amide III), respectively, exhibiting peaks in similar wave numbers. The amide I band, which is the absorption band of C=O stretching, is associated with the confirmation of the secondary structure of the protein. The absorption band of amide III is authentication for the existence of helical structure.[Citation17] Characterization by FTIR spectroscopy is reliable, rapid, nondestructive, and a precise method,[Citation23] and the current obtained FTIR spectrum was similar to that of collagen extracted from silver carp, Cyprinus carpio, large fin long barbell cat fish, minke whale, yellowfin tune, black drun, and sheepshead sea bream.[Citation6, Citation8, Citation11, Citation12, Citation24]
Amino Acid Composition
The amino acid composition of collagen from horse mackerel and croaker skin was expressed in percentage, as shown in . Glycine (29.6–31.7%) was the most abundant amino acid in ASC and PSC from horse mackerel and croaker skin. In general, glycine occurred as every third residue in collagen molecules, except for the first 14 amino acid residues from the N-terminus and the first 10 from the C-terminus.[Citation25] There were relatively high contents of proline, glutamic acid, serine, and hydroxyproline, while isoleucine, methionine, phenylalanine, arginine, and histidine were very low. Tryptophan and cysteine were not detected. It is well known that imino acids, such as proline and hydroxyproline, exist in collagen. The higher the imino acid content, the more stable are the helices.[Citation26] However, the degree of hydroxylation of proline residues will also influence the stability of the helix structure of collagen,[Citation27] and is also maintained partially by the hydrogen bonding ability of the hydroxy group of hydroxyproline. The total contents of imino acid were between 15.4–17.6%, and had minor variations to those in collagens from fish scale, such as sardine, red sea bream, and Japanese sea bass (19.3–19.7%), Japanese sea bass caudal fin (19.3%), and bigeye snapper skin (19.3%), but were significantly higher than those of collagens from edible jellyfish exumbrella (12.2%).[Citation28–31 Citation Citation Citation31
Table 1 Amino acid composition (%) of ASC and PSC from horse mackerel (HM) and croaker (CR) skin
CONCLUSIONS
This study investigated acid and pepsin soluble extraction conditions for production of horse mackerel and croaker skin collagen and characterized the chemical properties of the extracted collagen by amino acid analysis, SDS-PAGE, UV-Vis, and FTIR spectrophotometer. The collagen extracted from both the fishes was identified as type I collagen and is thought to be a viable substitute for bovine and porcine collagens and may further find applications in the functional food, cosmetic, biomedical, and pharmaceutical industries. However, further detailed in vivo studies are required to observe its complete efficiency regarding its application.
ACKNOWLEDGMENT
The authors gratefully acknowledge Dr. K. Ramasamy, Dean, School of Bioengineering, SRM University, for his constant support throughout the project. They also extend their gratitude to the Dean, School of Pharmacy, and Mr. K. Manikandan for availing the FTIR facility and the management, SRM University for providing the facilities.
REFERENCES
- Birk , D.E. and Bruckner , P. 2005 . Collagen suprastructures . Topics in Current Chemistry , 247 : 185 – 205 .
- Michelacci , Y.M. 2003 . Collagens and proteoglycans of the corneal extracellular matrix . Brazilian Journal of Medical and Biological Research , 36 : 1037 – 1046 .
- Hassan , F. and Sherief , P.M. 1994 . Role and application of fish collagen . Seafood Export Journal , 25 : 19 – 24 .
- Zhang , Z.K. , Li , G.Y. and Shi , B. 2006 . Physicochemical properties of collagen, gelatin and collagen hydrolysate derived from bovine limed split wastes . Journal of the Society of Leather Technologists and Chemists , 90 : 23 – 28 .
- Gomez-Guillen , M.C. , Turnay , J. , Fernandez-Diaz , M.D. , Ulmo , N. , Lizarbe , M.A. and Montero , P. 2002 . Structural and physical properties of gelatin extracted from different marine species: A comparative study . Food Hydrocolloids , 16 : 25 – 34 .
- Zhang , J. , Duan , R. , Tian , Y. and Konno , K. 2009 . Characterisation of acid-soluble collagen from skin of silver carp (Hypophthalmichthys molitrix) . Food Chemistry , 116 : 318 – 322 .
- Nagai , T. , Araki , Y. and Suzuki , N. 2002 . Collagen of the skin of ocellate puffer fish (Takifugu rubripes) . Food Chemistry , 78 : 173 – 177 .
- Ogawa , M. , Moody , M.W. , Portier , R.J. , Bell , J. , Schexnayder , M.A. and Losso , J.N. 2004 . Biochemical properties of bone and scale collagens isolated from the subtropical fish black drum (Pogonia cromis) and sheepshead seabream (Archosargus probatocephalus) . Food Chemistry , 88 : 495 – 501 .
- Jongjareonrak , A. , Benjakul , S. , Visessanguan , W. , Nagai , M. and Tanaka , M. 2005 . Isolation and characterisation of acid and pepsin-solubilised collagens from the skin of brownstripe red snapper (Lutjanus vitta) . Food Chemistry , 93 : 475 – 484 .
- Wang , L. , An , X. , Xin , Z. , Zhao , L. and Hu , Q. 2007 . Isolation and characterization of collagen from the skin of deep-sea redfish (Sebastes mentella) . Journal of Food Science , 72 : 450 – 455 .
- Nagai , T. , Suzuki , N. and Nagashima , T. 2008 . Collagen from common minke whale (Balaenoptera acutorostrata) unesu . Food Chemistry , 111 : 296 – 301 .
- Zhang , M. , Liu , W. and Li , G. 2009 . Isolation and characterisation of collagens from the skin of largefin longbarbel catfish (Mystus macropterus) . Food Chemistry , 115 ( 3 ) : 826 – 831 .
- Yan , M. , Li , B. , Zhao , X. , Ren , G. , Zhuang , Y. , Hou , H. , Zhang , X. , Chen , L. and Fan , Y. 2008 . Characterization of acid-soluble collagen from the skin of walleye pollock (Theragra chalcogramma) . Food Chemistry , 107 : 1581 – 1586 .
- Laemmli , U.K. 1970 . Cleavage of structural proteins during assembly of head of bacteriophage T4 . Nature , 277 : 680 – 685 .
- Li , Y. , Zhang , T. , Mu , W. and Liu , J. 2008 . Antioxidant and free radical-scavenging activities of chickpea protein hydrolysate(CPH) . Food Chemistry , 106 : 444 – 450 .
- Nagai , T. and Suzuki , N. 2000 . Isolation of collagen from fish waste material-skin, bone and fins . Food Chemistry , 68 : 277 – 281 .
- Liu , H.Y. , Li , D. and Guo , S.D. 2007 . Studies on collagen from the skin of channel catfish (Ictalurus punctaus) . Food Chemistry , 101 : 621 – 625 .
- Liu , Y.K. and Liu , D.C. 2006 . Comparison of physical-chemical properties of type I collagen from different species . Food Chemistry , 99 : 244 – 251 .
- Ogawa , M. , Moody , M.W. , Portier , R.J. , Bell , J. , Schexnayder , M.A. and Losso , J.N. 2003 . Biochemical properties of black drum and sheepshead seabream skin collagen . Agricultural and Food Chemistry , 51 : 8088 – 8092 .
- Jakobsen , R.L. , Brown , L.L. , Hutson , T.B. , Fink , D.J. and Veis , A. 1983 . Intermolecular interactions in collagen self-assembly as revealed by Fourier transform infrared spectroscopy . Science , 220 : 1288 – 1290 .
- Muyonga , J.H. , Cole , C.G.B. and Duodu , K.G. 2004 . Characterisation of acid soluble collagen from skins of young and adult Nile perch (Lates niloticus) . Food Chemistry , 85 : 81 – 89 .
- Li , H. , Liu , B.L. , Gao , L.Z. and Chen , H.L. 2004 . Studies on bullfrog skin collagen . Food Chemistry , 84 : 65 – 69 .
- Kulmyrzaev , A.A. , Karoui , R. , De Baerdemaeker , J. and Dufour , E. 2007 . Infrared and fluorescence spectroscopic techniques for the determination of nutritional constituents in foods . International Journal of Food Properties , 10 ( 2 ) : 299 – 320 .
- Duan , R. , Zhang , J. , Du , X. , Yao , X. and Konno , K. 2009 . Properties of collagen from skin, scale and bone of carp (Cyprinus carpio) . Food Chemistry , 112 : 702 – 706 .
- Foegeding , E.A. , Lanier , T.C. and Hultin , H.O. 1996 . “ Collagen ” . In Food Chemistry , 3rd , Edited by: Fennema , O.R. 902 – 906 . New York : Marcel Dekker .
- Ikoma , T. , Kobayashi , H. , Tanaka , J. , Walsh , D. and Mann , S. 2003 . Physical properties of type (I) collagen extracted from fish scales of Pagrus major and Oreochromis niloticas . International Journal of Biological Macromolecules , 32 : 199 – 204 .
- Ramachandran , G.N. 1988 . Stereochemistry of collagen . International Journal of Peptide and Protein Research , 31 : 1 – 16 .
- Nagai , T. , Izumi , M. and Ishii , M. 2004 . Fish scale collagen preparation and partial characterization . International Journal of Food Science and Technology , 39 : 239 – 244 .
- Kittiphattanabawon , P. , Benjakul , S. , Visessanguan , W. , Nagai , T. and Tanaka , M. 2005 . Characterisation of acid-soluble collagen from skin and bone of bigeye snapper (Priacanthus tayenus) . Food Chemistry , 89 : 363 – 372 .
- Nagai , T. 2004 . Characterization of collagen from Japanese sea bass caudal fin as waste material . European Food Research and Technology , 218 : 424 – 427 .
- Nagai , T. , Ogawa , T. , Nakamura , T. , Ito , T. , Nakagawa , H. , Fujiki , K. , Nakao , M. and Yano , T. 1999 . Collagen of edible jellyfish exumbrella . Journal of the Science of Food and Agriculture , 79 : 855 – 858 .