Abstract
The antioxidant properties of crude acetone extract and its fractions separated using n-hexane, chloroform, ethyl acetate, and butanol of Cynomorium songaricum Rupr. were examined by 2,2′-azino-bis (3-ethylbenzthiazoline-6-sulfonic acid) and 1,1-diphenyl-2-picrylhydrazyl radical scavenging activity assay. The activity of the crude acetone extract was significantly higher than that of vitamin E and lower than that of ascorbic acid. The ethyl acetate fraction had the highest activity among the four fractions. Catechins and proanthocyanidins were identified as the predominant components in ethyl acetate fraction by high performance liquid chromatography–mass spectrometry. Catechin was demonstrated as the major phenolic compound in ethyl acetate fraction of C. songaricum after high-speed counter-current chromatography isolation. In summary, C. songaricum exhibiting high antioxidant activity and abundant procyanidins might be considered as a potential source of antioxidants.
INTRODUCTION
Increasing evidence indicates that the imbalance between production and consumption of reactive oxygen species, leading to oxidative stress, can ultimately result in various diseases, including aging, cancer, neurological degeneration, and arthritis.[Citation1] Therefore, the potential of antioxidants to prevent oxidation in the body has attracted much attention,[Citation2] and dietary supplements containing antioxidants are available to protect the body from the attack of oxidative stress.[Citation3]
Cynomorium songaricum Rupr., also known as “Suoyang” in China, has been widely used in traditional Chinese medicine, which is an obligate root parasitic plant. It is mainly distributed in Northwestern China and Central Asia and is used as healthy foods and nutrients by local people.[Citation4] Nowadays, the stems of C. songaricum are widely used as functional food ingredients or supplements. For example, the wine containing extract of C. songaricum as an ingredient is popular in China. Pharmacological research had demonstrated that anti-oxidation is one of the main activities of C. songaricum. The ethyl acetate fraction of C. songaricum significantly attenuated staurosporine-induced cell death of SK-N-SH human neuroblastoma cells potentially through their radical scavenging activity.[Citation5] The methanol extract of C. songaricum possessed potent SOD-like activity and moderate α-glucosidase inhibitory activity.[Citation6] C. songaricum also have other activities, including anti-HIV,[Citation7] anti-aging,[Citation8] improving sexual function, and immunity.[Citation9] Among the chemical constituents of C. songaricum, steroids, triterpenes, flavanoids, condensed tannins, and polysaccharide have been reported previously.[Citation7, Citation10, Citation11] However, there is little evidence for the chemical constituent with responsibility for radical scavenging potential of C. songaricum.
In this article, we investigated phenolic compounds in crude acetone extract and fractions of C. songaricum and evaluated their antioxidant activity by using 2,2′-azino-bis (3-ethylbenzthiazoline-6-sulfonic acid) (ABTS) and 1,1-diphenyl-2-picrylhydrazyl (DPPH) radical-scavenging assays. On the other hand, we analyzed the major phenolic compounds in ethyl acetate fraction of C. songaricum by high performance liquid chromatography-mass spectrometry (HPLC–MS), and isolated and purified the predominant antioxidant compounds by high speed counter current chromatography (HSCCC) guided by in vitro activity assays.
MATERIALS AND METHODS
Plant Material
The stems of C. songaricum were collected in the marginal zone of Hobq Desert, Inner Mongolia, China, at 39.83 N, 108.7 E, on May 7, 2008, and were identified by Professor Gui-Lin Chen, College of Life Sciences, Inner Mongolia University, where a voucher specimen (No. 0807025) was deposited. Dried plant material was stored at ambient temperature until used.
Chemicals and Regents
All solvents used for preparation of samples and HSCCC separation were of analytical grade (Tianjin Chemical Factory, Tianjin, China). Methanol for HPLC was of HPLC grade (Tianjin Chemical Factory, Tianjin, China). Distilled water was prepared using MILLI-Q A10 water system (Millipore, Billerica, MA, USA). DPPH (1,1-diphenyl-2-picrylhydrazyl radical), ABTS (2,2′-azino-bis(3-ethylbenzthiazoline-6-sulfonic acid) diammonium salt), Trolox (6-hydroxy-2,5,7,8-tetramethychroman- 2-carboxylic acid), Folin–Ciocalteau phenol reagent, and vitamin E were from Sigma-Aldrich (St. Louis, MO, USA). Ascorbic acid was purchased from Tianjin Chemical Factory (Tianjin, China).
Extraction Procedure
The stems of C. songaricum (1 kg) were ground to powder (about 30 mesh) and extracted three times at room temperature with 3 l of 70% aqueous acetone (v/v) for 24 h per extraction. Extracts were filtered, combined, and concentrated at 40°C under reduced pressure. A representative sample of the crude acetone extract (CAE) was lyophilized to determine the extraction yield (w/w) and to measure the total phenol and the antioxidant capacity. The resulting aqueous extract was then partitioned with solvents of increasing polarity resulting in n-hexane fraction (HF), chloroform fraction (CF), ethyl acetate fraction (EAF), and butanol fraction (BF). The ethyl acetate extracts were combined and evaporated to dryness under reduced pressure at 40°C for further analysis and isolation.
Measurement of Total Phenolic Content
The content of total phenolic in the CAE and its fractions of C. songaricum were determined using the Folin-Ciocalteu colorimetric method.[Citation12] Briefly, 0.1 ml aliquots of fractions were diluted to 2 ml with distilled water. To this solution, 1 ml of Folin–Ciocalteu reagent and 1 ml of 10% sodium carbonate solution was added, and then the mixture was incubated for 2 h at 25°C. The absorbance of blue-colored mixture was measured at 765 nm by using UV-visible spectraphotometer of Shimadzu UV-2450 (Shimadzu Corporation, Kyoto, Japan). The amount of total phenol was calculated as mg of gallic acid equivalents per gram of dry weight of extract (mg GAE/g) from the calibration curve of gallic acid standard solution. For the gallic acid, the curve absorbance versus concentration is described by the equation y = 0.036x - 0.0006 (R 2 = 0.9997), where y = absorbance and x = concentration.
ABTS Radical-Scavenging Assay
The free radical-scavenging activity was determined by ABTS radical cation decolorization assay described by Re et al.[Citation13] Briefly, ABTS+ was generated by oxidation of ABTS with potassium persulphate. ABTS was dissolved in water to 7 mM, and potassium persulphate was added to a concentration of 2.45 mM. The reaction mixture was left to stay at room temperature overnight (12–16 h) in the dark before using it for the study of plant extracts. The ABTS+ solution was then diluted with ethanol to obtain an absorbance of 0.70 ± 0.02 at 734 nm. Next, 30 μl of Trolox standard or test samples was added to 2.97 ml of diluted ABTS+ solution, and the absorbance was measured at 6 min after mixing. Three different concentrations of each sample were chosen to test the ABTS+ scavenging activity. Antioxidant properties of samples solution were expressed as Trolox equivalent antioxidant capacity (TEAC mM). The results were compared with the standard curve for calculation of TEAC: y = 4.5534x + 0.8186 (R 2 = 0.9978), where y = inhibition and x = concentration.
DPPH Radical-Scavenging Assay
DPPH scavenging activity was measured by the modified spectrophotometric method of Lebeau et al.[Citation14] Solution of DPPH in methanol (1 × 10−6 M) was prepared freshly. An aliquot of 1 ml of this solution was mixed with 3 ml of the samples diluted with ethanol at three varying concentrations (50–200 μg/ml). The mixture in the test tubes was shaken for 30 min at room temperature, and then the decrease in absorbance was measured at 515 nm. Ascorbic acid and vitamin E were used as positive controls. The scavenging activities of the samples were measured as the decrease in absorbance of DPPH expressed as a percentage of the absorbance of a control DPPH solution without test samples. The percentage inhibition was calculated by the following equation: The percentage inhibition of the radicals due to the antioxidant property of the extracts was calculated using the formula:
where AB = absorbance of blank and AA = absorbance of test. IC50 value was determined to be the effective concentration at which DPPH radical was scavenged by 50%. The IC50 value was obtained by interpolation from linear regression analysis.
HPLC–DAD System for Analysis of EAF and Compounds
EAF was analyzed using a Shimadzu LC-20A RP-HPLC (Shimadzu Corporation, Kyoto, Japan) including a LC-20AT pump, a SIL-20A automated sample injector, a CTO-10AS temperature-controlled column compartment, a SPD-M20A detector, and Shimadzu LC solution software. Elution was performed on a Shim-pack VP-ODS, 250 mm × 4.6 mm and 5 μm column. The mobile phase was composed of solvent A (water with 0.1% acetic acid) and solvent B (methanol with 0.1% acetic acid). The solvent gradient was as follows: initially 2% B, to 10% B in 8 min, from 10 to 12% B in 12 min, from 12 to 20% B in 10 min and continuing at 20% B for a further 10 min, from 20 to 40% B in 20 min, continuing at 40% B for a further 10 min until completion of the run. A flow rate of 0.8 ml/min was used and 20 μl of sample were injected and detected at 254 nm.
HPLC-ESI-MS Analysis
HPLC-ESI-MS analysis of sample was carried out using a Surveyor Plus LC system and LCQ Fleet ion trap mass spectrometer (Finnigan, Thermo Electron Corporation, San Jose, CA, USA). HPLC separation was performed under the same conditions described above. A mass spectrometer was operated in positive electrospray ionization (ESI) mode with N2 as the nebulizing gas at 275 kPa; drying gas flow rate and temperature were 10 l/min and 340°C, respectively. The electrospray voltage was 5 kV. The capillary temperature was 200°C. Fragmentor voltage was 100 V. Mass spectra was recorded from m/z 100 to 2000 amu.
HSCCC Isolation
Purification of EAF was carried out with a TBE–300A preparative HSCCC (Tauto Biotechnique Company, Shanghai, China). The HSCCC system was equipped with a Model NS-1007 constant-flow pump (Beijing Institute of New Technology Application, Beijing, China), a model 8823B UV detector (Beijing Institute of New Technology Application) at 254 nm, and a model N2000 workstation (Zhejiang University, Hangzhou, China). An HX 1050 constant temperature circulating implement (Beijing Boyikang Lab Instrument Company, Beijing, China) was used to control the separation temperature.
The solvent system utilized in the first separation was prepared by mixing n-hexane, ethyl acetate, methanol, and water (1:3:1:3, v/v/v/v), and shaken vigorously in a separatory funnel. The upper phase was pumped into the coils until the columns were filled up. The upper phase serves as the stationary phase. The lower layer (mobile phase) was then pumped into the head end of the column at a flow-rate of 2 ml/min. The centrifuge was set at 850 rpm. A sample (500 mg), dissolved in 20 ml of the mixture of n-hexane–ethyl acetate–methanol–water (1:3:1:3, v/v/v/v), was injected after the system reached the hydrodynamic equilibrium. The effluent was continuously monitored by a UV detector at 254 nm, and the peak fractions were collected according to the elution profile. Each collected fraction was lyophilized and dissolved by methanol for subsequent purity analysis by HPLC. Those containing a mixture of compounds (fraction 1) were pooled and used for further isolation and separation with a reformulated n-hexane–ethyl acetate–methanol–water (1:25:1:25, v/v/v/v) system at 900 rpm followed by HPLC analysis to examine the purity of the fractions. Antioxidant activity assays with ABTS and DPPH were used to guide the isolation and purification procedures. The compound 2 was subjected to Sephadex-LH 20 for further purification. The purity of fractions in each characterized compound was estimated by an area normalization method from the integration of these chromatograms (% of total area). Identification of the target components was carried out by Bruker 400 NMR spectrometer with DMSO-d6 as solvent and tetramethylsilane (TMS) as the internal standard.
Statistical Analysis
All analyses were conducted in three repetitions and data reported as means ± SD. One way analysis of variance (ANOVA) was used to compare the means. Differences were considered to be significant at P < 0.05. Results were calculated by employing the statistical software (SPSS 8.0, SPSS Inc., Chicago, IL, USA).
RESULTS AND DISCUSSION
Total Phenol Content
Phenolic compounds are closely associated with antioxidant activity,[Citation15] and those compounds can be extracted effectively by aqueous acetone from a lot of plants.[Citation16] Hence, 70% aqueous acetone was chosen as the extraction solvent. As the initial step of bioassay-guided fractionation process, this extract was subjected to continuous solvent extractions to yield HF, CF, EAF, and BF as shown in , the amount of total extractable compounds increased with increasing polarity of the solvent. The content of the total phenolic compounds of CAE and its different fractions from C. songaricum determined using Folin–Ciocalteau method and expressed as mg of gallic acid equivalents per gram of extract (mg GAE/g) were shown in . The phenolic content of C. songaricum was 65.2 mg/g (dry weight of root), which was similar to black tea (from 35.6 to 92.4 mg/g) but higher than most of the fruit and vegetables, such as grape (from 1.2 to 13.8 mg/g), orange (14.6 mg/g), and tomato (14.9 mg/g).[Citation17, Citation18] The estimation of phenolic content among different fractions of C. songaricum revealed that EAF contained the highest amount of total phenolic compounds followed by BF, CF, and HF, respectively. It has been suggested that phenolic compounds are secondary metabolites, and in part, are produced as a result of the plant's interaction with the environment.[Citation19] C. songaricum is distributed in a desert marginal area, the drought and saline-alkaline habitat is a potential positive factor to the accumulation of phenolic compounds. Phenolic compounds in plants are usually found in conjugated forms through hydroxyl groups with sugar as glycosides.[Citation20] Therefore, there was also high phenolic content in BF.
Table 1 Extraction yield (g), total phenolic content (mg GAE/g dry weight of extract), TEAC (mM), DPPH IC50 (mg/ml) of different extract/fractions obtained from the dry powder of C. songaricum stems
HPLC-MS Analysis of EAF
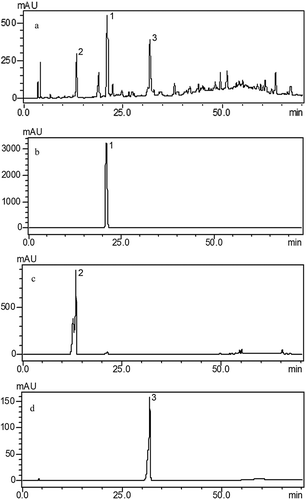
To understand the whole profile of EAF, different mobile phase conditions were examined including gradient elution mode. shows the HPLC profile of the ethyl acetate fraction of C. songaricum monitored at 254 nm. It shows that ethyl acetate fraction of C. songaricum contains several compounds, including gallic acid, protocatechuic acid, and catechin. HPLC-MS analysis was performed to collect structural information about the molecular weight and typical fragmentation of peaks eluted. Identified compounds in positive mode are shown in . Two isomeric forms of 1 (epi) gallocatechin (GC)/2 (epi) catechin (C) proanthocyanidin trimer (molecular weight, 882), two isomeric forms of procyanidin trimers (molecular weight, 866), two procyanidin dimers (molecular weight, 578), catechin (molecular weight, 290), two procyanidin dimer gallate isomers (molecular weight, 730), catechin-gallate (molecular weight, 442), and rutin (molecular weight, 610) fragmentation patterns in a positive mode were shown.
Table 2 Mass spectral characteristics of the main phytochemicals detected in ethyl acetate fraction of C. songaricum by HPLC-MS
Figure 1 HPLC profiles of the ethyl acetate fraction (EAF) from C. songaricum (a) and LC chromatogram of each compound from EAF (b–d). Peaks: 1 = protocatechuic acid; 2 = gallic acid; 3 = catechin.
Peak 1 and peak 6 have been identified as two procyanidin dimers (molecular weight, 578) by observing the molecular ion at m/z 579 ([M + H]+) in positive mode. Fragmentation of this anion yielded a monomer catechin (m/z 289) due to the cleavage of the inter-flavanoid C–C linkages. The fragment of m/z 427 resulted from a retro Diels-Alder (RDA) cleavage of the flavonoid nucleus. The fragment at m/z 409 corresponds to the loss of a water molecule. The fragmentation data obtained by positive electrospray ionization confirmed the data reported by de Pascual-Teresa et al.[Citation21] Peak 2 and peak 4 gave spectra showing [M + H]+ at m/z 867 and a fragment ion m/z 579 corresponding to [(M − C) + H]+. They were identified to be C–C–C procyanidin trimers in accordance with Gu et al.[Citation22] Peak 3 was identified as catechin (molecular weight, 290). ESI-MS analysis in the positive mode revealed a fragment ion at m/z 139 obtained from RDA reaction of catechin.
Peak 5 and peak 8 were identified as procyanidin dimer gallates, which presented [M + H]+ at m/z 731. The fragment at m/z 579 corresponded to the loss of a gallic acid moiety. In addition, the product resulting from the loss of a flavan-3-ol unit was also observed at m/z 443.[Citation23] Peak 7 and peak 10 gave spectra showing [M + H]+ at m/z 883, which were suggested to be digallate procyanidin dimer, or trimeric proanthocyanidin constituted by two (epi) catechin units and one epigallocatechin unit. The fragment ion signal at m/z 595 corresponded to the loss of an upper unit of (epi) catechin ([1 GC/1 C prodelphinidin dimer fragment + H]+). Those two compounds were tentatively identified as 1 GC/2 C proanthocyanidin trimers.[Citation24] Peak 9 had a positive molecular ion [M + H]+ at an m/z of 442 from an epi/catechin gallate and a fragment at an m/z of 289 from (epi) catechin. Peak 11 was identified as rutin (molecular weight, 610). The positive ion ESI spectrum showed an abundant [M + H]+ ion peak at m/z 611. The peak at m/z 303 [M – 308 + H]+ corresponding to quercetin [M + H]+ (the aglycone of rutin), the peak at m/z 465 resulted from loss of rhamnose from rutin.[Citation25]
HSCCC Isolation
Ethyl acetate extract was designed to be separated according to the partition coefficient (K) of the target component. In the first separation, three solvent systems composed of n-hexane, ethyl acetate, methanol, and water (1:2:1:2, 1:3:1:3, 1:4:1:4) was conducted and the partition coefficient was tested. Among the solvent systems, n-hexane–ethyl acetate–methanol–water (1:3:1:3) gave suitable partition coefficients for compound 1 (K = 1.06), Therefore, the volume radio of 1:3:1:3 was used for the first separation of ethyl acetate fraction. The preliminary separation of ethyl acetate fraction by HSCCC resulted in two fractions (). Fraction 2 mainly consisted of a single component with high purity of 97.2%, while fraction 1 was composed of several peaks after HPLC purity analysis. Fraction 2 was obtained as white needle crystals and identified by 1H and 13C NMR. Comparing the findings with the literature,[Citation26] the obtained compound was identified as protocatechuic acid.
Figure 2 HSCCC chromatogram showing the fractionation of the ethyl acetate fraction (EAF) from C. songaricum. (a) Fractionation of EAF. Two-phase solvent system: n-hexane–ethyl acetate–methanol–water (1:3:1:3, v/v/v/v). (b) Two-phase solvent system: n-hexane–ethyl acetate–methanol–water (1:25:1:25, v/v/v/v). Compound 1: protocatechuic acid; compound 2: gallic acid; compound 3: catechin.
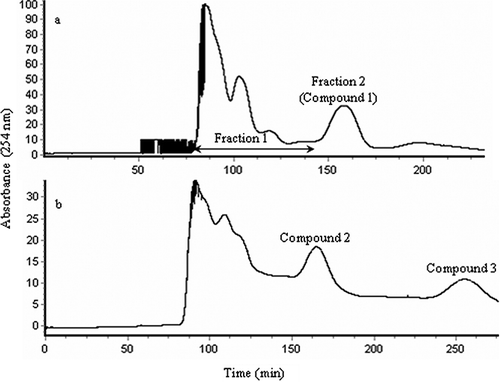
Radical scavenging activity assay showed that fraction 1 had strong activity against ABTS and DPPH radicals (). Fraction 1 was subsequently subjected to further separation by HSCCC with a reformulated n-hexane–ethyl acetate–methanol–water (1:25:1:25, v/v/v/v) system. Actually, two solvent systems at different volume ratios (n-hexane–ethyl acetate–methanol–water, 1:9:1:9 and 1:25:1:25 v/v/v/v) were designed. In the case of volume ratio of 1:25:1:25, the retention times of compounds 2 and 3 were more suitable than that of 1:9:1:9, whose retention time was too short. Therefore, the volume ratio of 1:25:1:25 was used for further experiments. High revolution speed (900 rpm) was used in order to get a higher retention percentage of the stationary phase. Two single peeks were obtained (); the purity of the first peek was not good enough to identify the structure, and the purity of the other one was high. Therefore, the first peak fraction was pooled and subjected to Sephadex-LH20 for further purification (purity of 92.3%). Compound 2 was identified by 1H and 13C NMR as gallic acid by comparison with a previous report.[Citation27] The structure of compound 3 (purity of 94.7%) was also identified by examining 1H and 13C NMR spectra. The results were similar with those in the literature.[Citation28] Thus, the structure of Compound 3 was positively identified as catechin. In this study, catechin was found as the predominant compound in ethyl acetate fraction of C. songaricum and the amount of catechin in ethyl acetate fraction was 69.85 mg/g. This result was in agreement with a previous publication, which also showed that catechin was the major phenolic compound in C. songaricum.[Citation7]
Table 3 Yield (mg), TEAC (mM), DPPH IC50 (μg/ml) of compounds isolated from C. songaricum stems by HSCCC
Antioxidant Activity
In order to obtain more reliable results, Fukumoto and Mazza suggested that antioxidant capacity should be measured by using more than one method.[Citation29] In this study, ABTS and DPPH radical scavenging assay were used to determine the free radical scavenging activities of samples. ABTS assay is based on the antioxidant ability to react with ABTS+ generated in the system. This method is widely used for evaluation of antioxidant activity in foods and biological systems, and a high value of TEAC indicated a high level of antioxidant activity.[Citation13] DPPH is a chromogen-radical-containing compound that can directly react with antioxidants. Stable radical DPPH has been widely used for the determination of primary antioxidant activity, which is the free radical scavenging activities of pure antioxidant compounds, plant and fruit extracts, and food materials.[Citation14] The ABTS radical scavenging activities of extracts and compounds were shown in and . The activity of the crude acetone extracts (CAE) was 1.5-fold higher than that of vitamin E, and lower than ascorbic acid. The EAF had the highest activity with 4.27 ± 0.42 mM among the four fractions. This is in accordance with Lu et al.,[Citation5] who observed that the ethyl acetate extract showed a good scavenging activity in a xanthine/xanthine oxidase system. The order in terms of the strength of radical-scavenging activity was as follows: catechin >gallic acid > protocatechuic acid > ascorbic acid > vitamin E. The three compounds isolated were significantly more active than controls, and catechin showed the highest activity. But there was no significant difference between catechin and gallic acid.
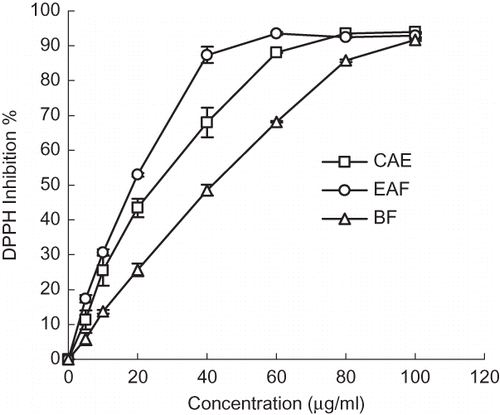
Similar results were obtained from the DPPH system as shown in and and . The crude extracts exhibited strong radical scavenging activity. The EAF showed higher activity than that of CAE, BF, CF, and HF. The highest scavenging activity of the EAF (93.5 ± 0.41%) was 60 μg/ml, while the CAE (93.4 ± 0.02%) and BF (91.7 ± 0.3%) were 80 and 100 μg/ml, respectively. After completing the reaction, the final solution was always yellowish and, therefore, its absorbance inhibition compared to the colorless methanol solution could not reach 100%.[Citation30] A linear increase with increasing of concentration (from 0 to 40 μg/ml) in free radical scavenging ability was observed (). A direct correlation of the total phenol content with the radical scavenging activity of the extracts and fractions from C. songaricum (R 2 = 0.983) was observed. This indicated that phenolic compounds were the major active components responsible for the antioxidant activity of C. songaricum. The effect of gallic acid was the strongest among the compounds isolated and controls. The decreasing order efficiencies in the DPPH system is as follows: gallic acid > catechin > ascorbic acid > protocatechuic acid > vitamin E. and the difference among gallic acid, catechin, and ascorbic acid was not significant (p > 0.05). The results of the antioxidant activity analysis () strongly support the idea that the scavenging activity can be attributed to their free hydroxyl groups, and the phenolic hydroxyl structural group in benzene ring contributes much to the free radical scavenging activity.[Citation31] Since catechin shows high radical scavenging activity and its yield is significantly higher than the other components, it is one of the major antioxidative compounds of C. songaricum.
CONCLUSION
Total phenolic content and antioxidant activity of crude extract and various fractions of C. songaricum were investigated in this article. The authors' results revealed that C. songaricum had abundant penolic components and exhibited high antioxidant activity. The total phenolic content of the partition fractions revealed that the EAF had the highest phenolic content. EAF and BF were more effective than ascorbic acid and vitamin E in scavenging radical. HPLC-MS analysis indicated that catechins and proanthocyanidins were the major phenolic compounds in EAF. Three phenolic compounds were isolated in EAF by HSCCC and identified as catechin, protocatechuic acid, and gallic acid. Catechin exhibiting high yield and activity is the predominant antioxidant compound in C. songaricum. More studies on isolation and quantification of individual phenolic compounds are needed to elucidate their antioxidant mechanisms and the existence of possible synergism, if any, among the compounds in C. songaricum, and effects of these compounds on the body for utilizations as a potential natural functional food remain to be studied.
ACKNOWLEDGMENTS
This research was supported by the National Key Technologies R & D Program of China (2011BAI07B07), the National Natural Science Foundation of China (30660015), the Key Technologies R & D Program of Inner Mongolia Autonomous Region of China, and the “211 Project” for Postgraduate Student Programme of Inner Mongolia University.
Present address: Ordos Center for Disease Control and Prevention, No.1 Ningxin Road, Kangbashi District, Ordos 017000, China.
REFERENCES
- Marx , J.L. 1987 . Oxygen free radicals linked to many diseases . Science , 235 ( 4788 ) : 529 – 531 .
- Siddhuraju , P. and Becker , K. 2007 . The antioxidant and free radical scavenging activities of processed cowpea (Vigna unguiculata (L.) Walp.) seed extracts . Food Chemistry , 101 ( 1 ) : 10 – 19 .
- Al-Duais , M. , Müller , L. , Böhm , V. and Jetschke , G. 2009 . Antioxidant capacity and total phenolics of Cyphostemma digitatum before and after processing: use of different assays . European Food Research Technology , 228 : 813 – 821 .
- Ma , L.J. , Chen , G.L. , Jin , S.W. and Wang , C.X. 2006 . The anti-aging effect and the chemical consttuents of Cynomorium songaricum Rupr . Acta Horticultural , 765 : 23 – 30 .
- Lu , Y. , Wang , Q.G. , Melzig , M.F. and Jenett-Siems , K. 2009 . Extracts of Cynomorium songaricum protect SK-N-SH human neuroblastoma cells against staurosporine-induced apoptosis potentially through their radical scavenging activity . Phytotherapy Research , 23 ( 2 ) : 257 – 261 .
- Ma , C.M. , Sato , N. , Li , X.Y. , Nakamura , N. and Hattori , M. 2010 . Flavan-3-ol contents, anti-oxidative and a-glucosidase inhibitory activities of Cynomorium songaricum . Food Chemistry , 118 ( 1 ) : 116 – 119 .
- Ma , C.M. , Nakamura , N. , Miyashiro , H. , Hattori , M. and Shimotohno , K. 1999 . Inhibitory effects of constituents from Cynomorium songaricum and related triterpene derivatives on HIV-1 protease . Chemical Pharmaceutical Bulletin , 47 ( 2 ) : 141 – 145 .
- Ma , L.J. , Chen , G.L. , Nie , L.S. and Ai , M. 2009 . Effect of Cynomorium songaricum polysaccharide on telomere length in blood and bra in of D-galactose-induced senescence mice . China Journal of Chinese Materia Medica , 34 ( 10 ) : 1257 – 1260 .
- Zhang , X.R. , Jia , Z.P. , Li , M.X. , Wang , J. , Yin , Q. , Luo , J.D. and Liu , H.Y. 2008 . Study on the effect of Part Ш from Cynomorium songaricum on immunosuppressive mice induced by cyclophosphamide . Journal of Chinese Medicinal Materials , 31 ( 3 ) : 407 – 409 .
- Ma , C.M. , Wei , Y. , Wang , Z.G. and Hattori , M. 2009 . Triterpenes from Cynomorium songaricium—Analysis of HCV protease inhibitory activity, quantification, and content change under the influence of heating . Journal of Natural Medicines , 63 ( 1 ) : 9 – 14 .
- Jiang , Z.H. , Tanaka , T. , Sakamoto , M. , Jiang , T. and Kouno , I. 2001 . Studies on a medicinal parasitic plant: Lignans from the stems of Cynomorium songaricum . Chemical Pharmaceutical Bulletin , 49 ( 8 ) : 1036 – 1038 .
- Kaur , R. , Arora , S. and Singh , B. 2008 . Antioxidant activity of the phenol rich fractions of leaves of Chukrasia tabularis A . Juss. Bioresource Technology , 99 ( 16 ) : 7692 – 7698 .
- Re , R. , Pellegrini , N. , Proteggente , A. , Panala , A. , Yang , M. and Rice-Evans , C. 1999 . Antioxidant activity applying an improved ABTS radical cation decolorization assay . Free Radical Biology and Medicine , 26 ( 9–10 ) : 1231 – 1237 .
- Lebeau , J. , Furman , C. , Bernier , J.L. , Duriez , P. , Teissier , E. and Cotelle , N. 2000 . Antioxidant properties of di-tert-butylhydroxylated flavonoids . Free Radical Biology and Medicine , 29 ( 9 ) : 900 – 912 .
- Okmen , B. , Sigva , H.O. , Mutlu , S. , Doganlar , S. , Yemenicioglu , A. and Frary , A. 2009 . Total antioxidant activity and total phenolic contents in different Turkish eggplant (Solanum melongena L.) cultivars . International Journal of Food Properties , 12 ( 3 ) : 616 – 624 .
- Hatano , T. , Tsugawa , M. , Kusuda , M. , Taniguchi , S. , Yoshida , T. , Shiota , S. and Tsuchiya , T. 2008 . Enhancement of antibacterial effects of epigallocatechin gallate, using ascorbic acid . Phytochemistry , 69 ( 18 ) : 3111 – 3116 .
- Turkmen , N. , Sari , F.Y. and Velioglu , S. 2006 . Effects of extraction solvents on concentration and antioxidant activity of black and black mate tea polyphenols determined by ferrous tartrate and Folin–Ciocalteu methods . Food Chemistry , 99 ( 4 ) : 835 – 841 .
- Cieślik , E. , Anna Gręda , A. and Adamus , W. 2006 . Contents of polyphenols in fruit and vegetables . Food Chemistry , 94 ( 1 ) : 135 – 142 .
- Snyder , B.A. and Nicholson , R.J. 1990 . Synthesis of phytoalexins in sorghum as a site-specific response to fungal ingress . Science , 248 ( 4963 ) : 1637 – 1639 .
- Rice-Evans , C.A. , Miller , N.J. and Paganga , G. 1996 . Structure-antioxidant activity relationships of flavonoids and phenolic acids . Free Radical Biology and Medicine , 20 ( 7 ) : 933 – 956 .
- de Pascual-Teresa , S. , Rivas-Gonzalo , J.C. and Santos-Buelga , C. 2000 . Prodelphinidins and related flavanols in wine . International Journal of Food Science and Technology , 35 : 33 – 40 .
- Gu , L. , Kelm , M.A. , Hammerstone , J.F. , Beecher , G. , Holden , J. , Haytowitz , D. and Prior , R.L. 2003 . Screening of foods containing proanthocyanidins and their structural characterization using LCMS/MS and thiolytic degradation . Journal of Agriculture Food Chemistry , 51 : 7513 – 7521 .
- Monagas , M. , Gómez-Cordovés , C. , Bartolomé , B. , Laureano , O. and Ricardo Da Silva , J.M. 2003 . Monomeric, oligomeric, and polymeric flavan-3-ol composition of wines and grapes from Vitis vinifera L. Cv. Graciano, Tempranillo, and Cabernet Sauvignon . Journal of Agriculture Food Chemistry , 51 : 6475 – 6481 .
- Sun , B.S. , Leandro , M.C. , de Freitas , V. and Spranger , M.I. 2006 . Fractionation of red wine polyphenols by solid-phase extraction and liquid chromatography . Journal of Chromatography A , 1128 : 27 – 38 .
- Stobiecki , M. , Malosse , C. , Kerhoas , L. , Wojlaszek , P. and Einhorn , J. 1999 . Detection of isoflavonoids and their glycosides by liquid chromatography/electrospray ionization mass spectrometry in root extracts of lupin (Lupinus albus) . Phytochemical Analysis , 10 : 198 – 207 .
- An , L.J. , Guan , S. , Shi , G.F. , Bao , Y.M. , Duan , Y.L. and Jiang , B. 2006 . Protocatechuic acid from Alpinia oxyphylla against MPP+-induced neurotoxicity in PC12 cells . Food and Chemical Toxicology , 44 ( 3 ) : 436 – 443 .
- Chen , Y. , Wang , M.F. , Rosen , R.T. and Ho , C.T. 1999 . 2, 2-Diphenyl-1-picrylhydrazyl radical-scavenging active components from Polygonum multiflorum Thunb . Journal of Agriculture Food Chemistry , 47 : 2226 – 2228 .
- Mendoza-Wilson , A.M. and Glossman-Mitnik , D. 2006 . Theoretical study of the molecular properties and chemical reactivity of (+)-catechin and (-)-epicatechin related to their antioxidant ability . Journal of Molecular Structure: THEOCHEM , 761 ( 1–3 ) : 97 – 106 .
- Fukumoto , L.R. and Mazza , G. 2000 . Assessing antioxidant and prooxidant activities of phenolic compounds . Journal of Agriculture Food Chemistry , 48 ( 8 ) : 3597 – 3604 .
- Miliauskas , G. , Venskutonis , P.R. and Van Beek , T.A. 2004 . Screening of radical scavenging activity of some medicinal and aromatic plant extracts . Food Chemistry , 85 ( 2 ) : 231 – 237 .
- Shahidi , F. , Janitha , P.K. and Wanasundara , P.D. 1992 . Free radical scavenging activity of phenolics are mainly due to the presence of free hydroxyl groups . Phenolic antioxidants. Critical Reviews in Food Science and Nutrition , 32 ( 1 ) : 67 – 103 .