Abstract
Sixty Brazilian honey samples were analysed for their total phenolic content with the Folin-Denis reagent, total flavonoid content by aluminium chloride method, and antioxidant activity by reaction with 2,2-diphenyl-1-picrylhydrazyl radical. Colour was also classified according to visual analysis and Pfund scale. Linear relationships were observed between colour and flavonoid content, total phenolics and antioxidant capacity, and total flavonoid and phenolic contents. The white-coloured Citrus honey showed the lowest antioxidant activity, while the light ambar Verbenaceae honey showed the highest total phenolics and antioxidant activity. Dark-coloured and polyfloral honeys, though less popularized among consumers, showed average to high antioxidant capacity.
INTRODUCTION
Many components of honey, such as water, carbohydrates, organic acids, and pigments, are derived from nectar, while many others are inserted by the bees or can result from structural modifications by the bee enzymes during nectar maturation. Honey is basically a supersaturated sugar solution of fructose and glucose (38 and 31 g/100 g, respectively). As these sugars are directly absorbed by the intestine and have similar percentages of intake, the high invert sugar concentration makes honey a potent energetic food. In addition to its high energy potential, honey can be used as a nutritive food. Although they are present in small quantities, honey has enzymes, amino acids, and phenolic compounds that make it much healthier than sucrose.
In Brazil, the most common bee is the Africanized bee, which is a hybrid between the African and European Apis mellifera honeybee species. Honey is an extremely variable food. Even if it is made from the same floral origin or the same bee species, honey can vary in texture, colour, and composition depending on the geographical origin, soil, weather conditions, and even the age of the bees, which greatly affects the enzymatic activity that produces the honey.[Citation1]
Small quantities of phenolic compounds are one of the most studied honey constituents because they have well-known biological activities and can potentially be used to identify the botanical origin of honeys. Most of the floral markers in honey are flavonoids or phenolic acids, which come from the nectar or pollen of specific plants. The identification of these compounds in honey can be an important tool for the recognition of the honey's floral type, especially because melissopalynology can sometimes be ambiguous due to the variations in pollen content of some vegetal species.[Citation2–4 Citation Citation4
Phenolic compounds can also greatly contribute to the colour and antioxidant activity of honey, especially when they are complexed with metals. Darker honeys usually have more phenolic compounds and minerals compared with light-coloured honeys.[Citation5] Although potassium is the major mineral in honey, iron contributes more to its colour and is found at higher concentrations in darker honeys. When associated with phenolic compounds, iron can darken honey even more because it forms its complexation with phenolic hydroxyls or carbonyls. Metals can also donate a free electron, which easily stabilises free radicals. Due to their complex formation with phenolic hydroxyls, these new radicals readily promote resonance of the aromatic structure of phenolic compounds, thus forming stable ortho- or para- quinones and enhancing the antioxidant capacity of honey.[Citation6,Citation7]
Antioxidant activity is very appealing for consumers, as it can help prevent many diseases, such as cancer and coronary and cardiovascular problems.[Citation8] Although the antioxidant capacity of honey is inferior to most fruits and vegetables, it can also be used as a healthy substitute for commercial sugar, which solely consists of sucrose. Honey is also less fattening because it is sweeter, so less is required for taste. Besides its use as a functional food, honey has been largely used in cosmetics for its antioxidant and moisturising capabilities. For these industrial purposes, dark heterofloral honeys are generally used; they are more common, easier to produce, and less expensive. Fortunately, dark honeys tend to be richer in phenolic constituents and possess higher antioxidant activity, which makes them a good choice for enriching these products.
Besides the floral and geographical origin, honey colour can also be affected by heat, time of storage, and antioxidant activity. Beehives generally reach temperatures between 32 and 40 °C, and temperatures within this range generally do not affect honey quality. However, if honey is warmed to greater than 40 °C, a series of undesirable reactions can occur, such as the degradation of volatile compounds, sugar caramelisation or dehydration and the production of polymeric dark pigments, which are known as melanoidins. These pigments are the condensation products of phenolic compounds or 5-hydroxymethylfurfural, a substance formed by the dehydration of sugars and some amino acids present in honey. The degradation of sugars, especially fructose, and the loss of many volatile compounds during heat treatment may explain the decreased sweetness and aroma of many darker honeys. There are many publications, however, on the antioxidant activity of these compounds, which, in addition to the action of metals, also contribute to the superior antioxidant activity of darker honeys, especially honeys that have been heat treated.[Citation9–11 Citation Citation11 The aim of this work was to study the antioxidant activity of 60 Brazilian Apis mellifera honeys from different floral origins and obtained from different parts of the state of Rio de Janeiro. The antioxidant activity was then correlated with the total flavonoid and phenolic contents, which have been shown to contribute to this property.
MATERIALS AND METHODS
Honey Samples and Reagents
All of the 60 honey samples and their melissopalynological information were provided by Laboratório Abelha-Natureza (UFRRJ). The samples were obtained from the local market or directly from beekeepers with 37 monofloral and 23 heterofloral honeys. The samples were acquired from 2009 to 2010 at different mesoregions of Rio de Janeiro and stored at 4 °C until analysis. The methanol used for the analyses was spectrophotometric grade (VETEC, Rio de Janeiro, Brazil), and the water had been previously passed through a Millipore filter (Millipore Direct-Q UV with pump, Direct-Q UV, Merck, Darmstadt, Germany). Analytical grade phosphomolybdic acid, anhydrous sodium carbonate, hexahydrated aluminum chloride, sodium tungstate, and phosphoric acid were purchased from VETEC. 2,2-diphenyl-1-picrylhydrazyl was purchased from Sigma-Aldrich Chemie (Munich, Germany), and D(+) maltose monohydrated was purchased from VETEC.
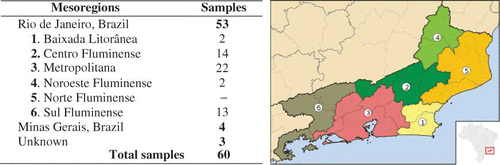
Artificial honey was prepared by dissolving 2.25 g of sucrose, 11.25 g of maltose, 60.75 g of fructose, and 50.25 g of glucose in 25.5 mL of Milli-Q water.[Citation5] This artificial honey was included in the analyses to evaluate the interference of an average sugar content of honey on the methods used. The results were also classified by the mesoregion where the samples were obtained. Mesoregions are subdivisions that are specific to Brazilian states. These divisions were created by the Brazilian Institute of Geography and Statistics (IBGE) by classifying the municipalities based on proximity and common characteristics and, therefore, facilitates statistical analysis. Rio de Janeiro has six mesoregions as shown in . The only region from which a honey sample was not collected was Norte Fluminense. The floral sources of the honey samples were determined by melissophalynology. The honey was considered as unifloral when more than 45% of pollen was collected from one plant species.[Citation12,Citation13]
Spectrophotometric Determination of Honey Colour
The Pfund scale was used to spectrophotometrically measure the colour of the honey samples, following the method described by Naab et al.[Citation14] The samples were diluted to a 50% (w/v) honey solution with the aid of ultrasound, and triplicate samples were evaluated on a UV-Vis NOVA 2000UV spectrophotometer (NI 2000UV, Novainstruments, São Paulo, Brazil) at 635 nm. The mean absorbance values were converted to the Pfund scale with the formula Pfund = −38.7 + 371.39 × Abs, and the results were classified as described in .
Table 1 Pearson correlation matrix for the colour, antioxidant activity, and total phenolic and flavonoid contents of honeys
Determination of Total Phenolic Compounds
The total phenolic compounds were determined by adapting the method described by Meda et al.[Citation15] First, 0.5 mL of each 100 mg/mL aqueous honey solution was reacted with 2.5 mL of the Folin-Denis reagent. After 5 min, 2.0 mL of a freshly prepared 14% aqueous sodium carbonate solution was added to the mixture. After 2 h, the absorbances of triplicate samples were measured at 760 nm using a water blank, and the total phenolic contents were calculated by comparison to a gallic acid calibration curve. The total phenolic content of artificial honey was also analysed in order to evaluate the interference of the sugars on the analysis. The results were expressed in mg of gallic acid equivalent per 100 g of honey (mg EGA/100 g).
Determination of Total Flavonoid Content
The total flavonoid content was also determined by adapting the method described by Meda et al.[Citation15] and Ahn et al.[Citation16] To 2.0 mL of each 500 mg/mL MeOH:H2O (1:1) honey solution, an equal volume of a freshly prepared 2% methanolic aluminium chloride solution was added. The absorbances of triplicate samples were measured at 415 nm using a methanol blank. The total phenolic content of artificial honey was also analysed in order to evaluate the interference of the sugars on the analysis. The flavonoid contents were calculated by comparison to a quercetin calibration curve, and the results were expressed in mg of quercetin equivalent per 100 g of honey (mg EQC/100 g).
Determination of the Antioxidant Activity
The antioxidant activity in terms of EC50 (effective concentration needed to neutralise 50% of the free radicals present) was determined in vitro following the method described by Pérez et al.[Citation17] MeOH:H2O (1:1) honey solutions at 5, 10, 20, 30, 40, and 50 mg/mL were prepared. For each concentration, an aliquot of 71 μL was reacted with 0.29 μL of a 0.3 mM 2,2-diphenyl-1-picrylhydrazyl (DPPH) methanol solution. The solutions were incubated in the dark for 30 min, and the absorbances were measured at 520 nm on an ELISA 680 Microplate Reader (Bio-Rad, CA, USA). The results were compared with the absorbance of the blank, which consisted of 71 μL of the honey solution and 29 μL of methanol, and with the control (29 μL of DPPH solution and 71 μL of methanol) using the formula %AA = 100 – [(Abssample – Absblank) × 100]/Abscontrol. The obtained %AA values were plotted on a curve and correlated with the corresponding final concentrations. The EC50 was calculated by substituting the Y value for fifty on the linear regression curve. An artificial honey solution was also analysed in the assay to evaluate the contribution of the predominant sugars to the antioxidant activities.
RESULTS AND DISCUSSION
Colour
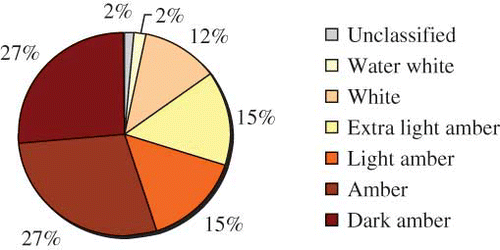
Most of the honeys were considered to be amber or dark amber by the Pfund classification (). Only one sample (corresponding to 2%) remained unclassified, as it had an absorbance value of less than 0.104 (Pfund values lower than zero). The results of the colour analysis performed here were different from the work described in Lacerda et al.,[Citation18] who analysed 24 honey samples obtained from southwest Bahia. In their work, although most of the samples were dark amber (29%), there was a greater range of colours on the Pfund scale. In this work, the percentage of honey samples decreased with the colour intensity, showing that honeys from Rio de Janeiro tend to be darker. Honey colour is usually correlated with its mineral content, especially that of iron. González-Miret et al.[Citation19] showed that light coloured honeys generally have less than 2.79 mg/kg of iron, while amber honeys have an average of 2.84 mg/kg and dark coloured honeys have more than 4.26 mg/kg. Colour can be also related to heat exposure or storage temperature, as an increase in temperature catalyses melanoidin formation and caramelisation reactions.
Total Phenolic, Flavonoid Contents, and Antioxidant Activity
The total phenolic compounds varied from 58.26 to 152.52 mg EGA/100 g of honey, with an average value of 102.83 mg EGA/100 g. The lowest total phenolic content corresponded to the visually light-coloured Eucalyptus honey from Banco de Minas–MG, which was the only sample that could not be classified by the Pfund scale because its absorbance value was too low (0.064). The highest value corresponded to a metropolitan heterofloral dark amber honey. The total flavonoid content varied from 1.99 to 11.86 mg EQC/100 g with an average value of 6.11 mg EQC/100 g. The lowest value corresponded to the only water-white Eucalyptus honey from Sul Fluminense, and the highest value corresponded to a dark amber Eucalyptus honey from Centro Fluminense.
As shown in , the total phenolic compounds were strongly negatively correlated with the EC50 (r = −0.8; ρ < 0.0001). This trend was expected because the amounts of phenolic acids are higher than flavonoids in Brazilian honeys. The negative correlation is due to the antioxidant activity values, which were expressed in terms of the concentration needed to stabilise 50% of the free radicals of DPPH. While the aluminium chloride method is specific for flavonoids, Folin-Denis quantifies all substances that can reduce molybdenum ions. Many of these compounds do not have chromophores, which explains the lower correlation between flavonoids and colour (r = 0.4; ρ < 0.0006) despite its high correlation with antioxidant capacity. Flavonoids, together with carotenoids, are responsible for the colour of flowers and are also present in low quantities in nectar and pollen. The strong positive relationship between flavonoid content and the colour of honey (r = 0.6; ρ < 0.0001) shows that they are also responsible for the colour of honey, possibly due to highly extended conjugated systems, especially when complexed with minerals. The moderate linear correlation between the total flavonoids and total phenolic compounds (r = 0.5; ρ = 0.0002) might be because flavonoids are one of the major phenolic compounds in honey.
Table 2 Average values for each mesoregion of the state of Rio de Janeiro, Brazil
The graphs in show the linear equations and the determination coefficients for the highest correlations obtained in (total phenolic content versus antioxidant activity and total flavonoid content versus colour). The bottom graphic (linear correlation between flavonoid content and colour) showed four honey samples that are very far from the trend line. One sample (1) corresponded to a metropolitan dark amber Montanoa honey with a relatively low flavonoid content (5.21 mg EQC/100 g) for its high Pfund value (225 mmPfund), the second (2) corresponded to a dark amber Ambrosia honey obtained from Sul Fluminense with a Pfund value of 235.3 mmPfund and flavonoid content of 7.63 mg EQC/100 g, and the third (3) corresponded to a dark amber Eucalyptus honey obtained from Centro Fluminense with a Pfund value of 266.3 mmPfund and a flavonoid content of 11.86 mg EQC/100 g, and the last one (4) was a heterofloral white honey with a relatively low Pfund value (27.6 mmPfund) for its high flavonoid content (11.59 mg EQC/100 g). In these cases, there might be another factor that increased or decreased the honey's colour, such as the mineral content, melanoidin or caramelisation products. A comparison of the average results for each mesoregion () showed that the metropolitan samples had the highest antioxidant activity and the highest total phenolic content, while the samples from Sul Fluminense had the highest phenolic content and the highest colour intensity. Significant variations were not observed for the other regions.
Figure 3 Correlation between total phenolic compounds and antioxidant capacity (top) and total flavonoid content and colour (bottom). (Colour figure available online.)
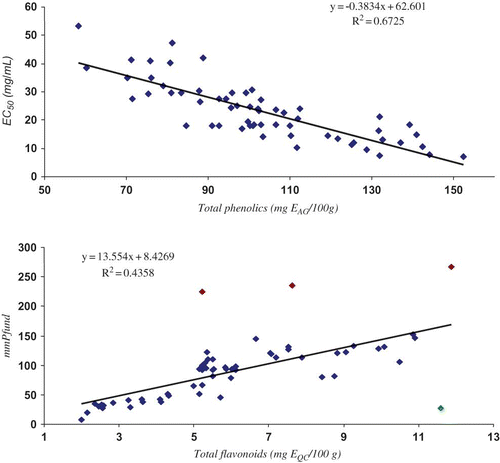
Table 3 Average values and standard deviation for each floral origin
These results for total phenolics and flavonoids in this work were compared with others from different countries,[Citation20–23 Citation Citation Citation23 and the Brazilian honeys studied in this work had a similar profile to those obtained from South Africa[Citation15] and Venezuela[Citation24] and a very different profile compared with the honeys obtained from Europe and Australia. These differences can be due to climate conditions, botanical differences, or even the type of bee. In these Southern countries, the Africanized bee is common, while other Apis mellifera species are more commonly found in the Northern Hemisphere. shows the average results for the total phenolic and flavonoid contents, antioxidant activity, and colour for each botanical origin and the number of samples analysed.
Table 4 Pearson correlation matrix for the colour, antioxidant activity, and total phenolic and flavonoid contents of honeys
Although it was white to translucent in colour, artificial honey could not be classified by the Pfund scale, as it had an absorbance of 0.047. This absorbance resulted in a Pfund value of −21.2 mmPfund, which was much less than the minimum value that is required to be classified as a water-white honey (the lowest honey colour classification). The total phenolic content also could not be quantified because the concentration measured was −2.32 mg EGA/100 g. In contrast to floral honeys, the artificial honey solution remained green after the addition of Folin-Denis reagent, which has a green colour. The apparent absence of blue colour formation and the spectrophotometric result indicated that the honey sugars, though mostly present as reducing sugars, were unable to reduce the molybdenum complexes present in the Folin-Denis reagent. The total flavonoid result was also very low compared to floral honeys (0.25 mg EQC/100 g), indicating that there was low interference of the sugar solution on the method. As expected, the antioxidant activity of the artificial honey, which had an EC50 of 404.64 mg/mL, was much lower than floral honeys. This value demonstrates that this sugar solution had a low capacity for free radical reduction and that the minor compounds in honey contribute more to the antioxidant activity.
Most of the honey samples collected were heterofloral (27 samples), followed by monofloral Eucalyptus (9 samples), Anadenanthera, and Myrcia (6 samples each), which are characteristic trees from the Atlantic Forest. As shown in , Ambrosia honey was the darkest, which was followed by Montanoa, Vernonia, Copaifera, and Eucalyptus. Citrus honey was the lightest coloured, the lowest in phenolic compounds, and one of the honeys with the lowest antioxidant activity (41.40 mg/mL), which is consistent with previously reported data.[Citation25–27 Citation Citation27 Despite its low phenolic content, Citrus honey has been shown to increase vitamin C absorption from the action of hesperidin, an aglycone that is usually present in this type of honey.[Citation28,Citation29] This property shows that, although antioxidant activity is an important and perhaps the best-known honey biological property, there are other compounds that enrich the quality of honey. These characteristics can also vary significantly with floral origin.
Anadenanthera honeys showed the highest total phenolic compounds (112.60 mg EAG/100 g of honey), followed by Eucalyptus (105.93 mg EAG/100 g of honey), Myrcia (103.05 mg EAG/100 g of honey), Montanoa (100.70 mg EAG/100 g of honey), heterofloral (98.80 mg EAG/100 g of honey), and Asteraceae honeys (97.11 mg EAG/100 g of honey). Overall, a comparison of the samples from each floral origin showed that the phenolic compounds increased with the colour intensity, which is consistent with other findings.[Citation23,Citation25,Citation30–32 Citation Citation32 In , an increase in the total phenolic compounds, total flavonoids, and antioxidant activity along with an increase in the Pfund grade could be observed.
Table 5 Number of visually light, medium, and dark samples by each Pfund classification
Brazilian honeys tend to be darker, which was expressed in the number of light- and dark-coloured samples that were obtained. Although darker honeys tend to be superior in antioxidant capacity, consumers usually reject them and tend to buy the most expensive light-coloured honey, especially unifloral honeys. There are still many misconceptions about honey quality in Brazil, and there must be further analysis and more publicity to popularise the use of honey as a functional food, especially dark-coloured honeys.
Honey Colour and Correlation with Antioxidant Capacity
Prior to the Pfund analysis, honey colour was classified by visual analysis as light-, medium-, or dark-coloured. shows the comparison between the visual and Pfund results, where the number of visually light, medium, or dark samples were compared to the values spectrophotometrically measured and classified by Pfund. The dark amber honeys were the most controversial because they were evenly split between medium and dark visual categories. This difference could be due to a slight visual transition, which indicates the limitations of visual perception, or even a limitation of the Pfund method, where the opacity of the samples could interfere with their absorbance. The presence of coloured substances that absorb wavelengths other than 635 nm, which is used for the Pfund analysis, could also have interfered with the results.
To determine which method correlated best with the other analysed parameters (flavonoid content, total phenolic content, and antioxidant activity), a principal component analysis (PCA) graph was constructed (), where shows the visual classification and shows the Pfund classification. For the latter, all possible combinations were converted to the Pfund scale based on a simpler classification (light-, medium-, and dark-coloured honeys), and the combination that best separated the samples based on the PCA analysis is shown in . Water-white and white honeys were classified as light-coloured honeys; extra light amber, light amber, and amber samples were classified as medium and dark amber honeys were classified as dark.
Figure 4 PCA graph for colour discrimination. (a) Visual classification and (b) Pfund classification of honey colour. (Colour figure available online.)
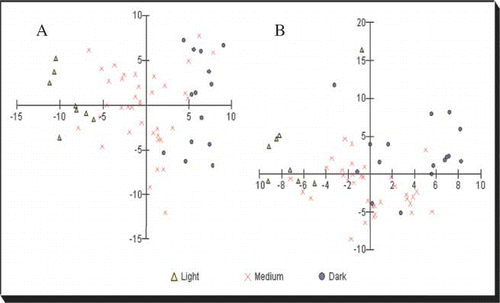
As seen in (visual analysis), a better colour discrimination was observed where there was a wider distance between the light- and dark-coloured honeys. Medium honeys are concentrated in the centre of the graph with some medium-coloured samples that tended to have a lighter colour and others with a darker colour. In , there is less definition among the colour classifications. Data in the literature have shown a linear relationship between phenolic compounds and honey colour with antioxidant activity.[Citation5,Citation23,Citation30–33 Citation Citation33 As shown in , the total flavonoid content had a strong positive correlation with colour (r = 0.6), and total phenolic had a strong negative correlation with EC50 (r = −0.8). These relationships show how these compounds can increase the colour and antioxidant activity. However, flavonoids generally absorb lower to 415 nm, which is significantly different than the wavelength used in the Pfund analysis (635 nm). The presence of coloured compounds that absorb at different wavelengths, such as flavonoids, can be masked by the Pfund analysis. This confounding element does not occur with visual analysis because the human eyes can detect light from about 400 to 700 nm. Although there are intrinsic problems with subjectivity with human perception, visual analysis is better associated with the flavonoid content, total phenolic compounds, and antioxidant activity of honey.
CONCLUSIONS
The phenolic and flavonoid profiles of the honeys studied in this work varied significantly with the floral type and the mesoregions from which these samples were obtained. Linear relationships between the flavonoid content and colour and the total phenolic content and antioxidant activity were observed. As honey is still consumed little in the country and most of the honey produced is exported, these properties should be publicised to help promote honey consumption and increase the popularity of local honeys among consumers, especially dark-coloured honeys, which are less appealing to Brazilian consumers.
ACKNOWLEDGMENTS
The authors are grateful to the CNPq, CAPES, and FAPERJ for financial support given to this work.
REFERENCES
- Ozcan , M. , Arslan , D. and Ceylan , D.A. 2006 . Effect of inverted saccharose on some properties of honey . Food Chemistry , 99 : 24 – 29 .
- Alvarez-Suarez , J.M. , Tulipani , S. , Romandini , S. , Bertoli , E. and Battino , M. 2010 . Contribution of honey in nutrition and human health: A review . Mediterranean Journal of Nutrition and Metabolism , 3 : 15 – 23 .
- Bogdanov , S. , Jurendic , T. and Sieber , R. 2008 . Honey for nutrition and health: A review . American Journal of the College of Nutrition , 27 : 677 – 689 .
- Polissiou , M. 2001 . Investigation of organic extractives from unifloral chestnut (Castanea sativa L.) and eucalyptus (Eucalyptus globulus Labill.) honeys and flowers to identification of botanical marker compounds . LWT–Food Science and Technology , 44 : 1042 – 1051 .
- Alvarez-Suarez , J. M. , Tulipani , S. , Diaz , D. , Estevez , Y. , Romandini , S. , Giampieri , F. , Damiani , E. , Astolf , P. , Bompadre , S. and Battino , M. 2010 . Antioxidant and antimicrobial capacity of several monofloral Cuban honeys and their correlation with color, polyphenol content and other chemical compounds . Food Chemical Toxicology , 48 : 2490 – 2499 .
- Kostyuk , V.A. , Potapovich , A.I. , Kostyuk , T.V. and Cherian , M.G. 2007 . Metal complexes of diatary flavonoids: Evaluation of radical scavenger properties and protective activity against oxidative stress in vivo . Cellular and Molecular Biology , 53 : 61 – 68 .
- Maselev , D. and Kuntic , V. 2007 . Investigation of metal-flavonoid chelates and the determination of flavonoids via metal-flavonoid complexing reactions . Journal of the Serbian Chemical Society , 72 : 921 – 939 .
- Herrera , E. , Jiménez , R. , Aruoma , O.I. , Hercberg , S. , Sánches-Garcia , I. and Fraga , C. 2009 . Aspects of antioxidant foods and supplements in health and disease . Nutrition Reviews , 67 ( Suppl. 1 ) : S140 – S144 .
- Maillard , M.N. , Billaud , C. , Chow , Y.N. , Ordonaud , C. and Nicolas , J. 2007 . Free radical scavenging, inhibition of polyphenoloxidase activity and copper chelating properties of model Maillard systems . Food Science and Technology , 40 : 1434 – 1444 .
- Rufián-Henares , J.A. and Morales , F.J. 2007 . Functional properties of melanoidins: In vitro antioxidant, antimicrobial and antihypertensive activities . Food Research International , 40 : 995 – 1002 .
- Turkmen , N. , Sari , F. , Poyrazoglu , E.S. and Velioglu , Y.S. 2006 . Effects of prolonged heating on antioxidant activity and colour of honey . Food Chemistry , 95 : 653 – 657 .
- Barth , O.M. 1989 . O Pólen no Mel Brasileiro , 1st , Gráfica Luxor : Rio de Janeiro .
- Louveaux , J. , Maurizzio , A. and Vorwohl , G. 1978 . Methods of Melissopalynology. Bee World , 59 : 139
- Naab , O.A. , Tamame , M.A. and Caccavari , M.A. 2008 . Palynological and physicochemical characteristics of three unifloral honey types from central Argentina. Spanish . Journal of Apiculture Research , 6 : 566 – 576 .
- Meda , A. , Lamien , C.E. , Romito , M. , Millogo , J. and Nacoulma , O.G. 2005 . Determination of the total phenolic, flavonoid and proline contents in Burkina Fasan honey, as well as their radical scavenging activity . Food Chemistry , 91 : 571 – 577 .
- Ahn , M.R. , Kumazawa , S. , Usui , Y. , Nakamura , J. , Matsuka , M. , Zhu , F. and Nakayama , T. 2007 . Antioxidant activity and constituents of propolis collected in various areas of China . Food Chemistry , 101 : 1383 – 1392 .
- Pérez , R.A. , Iglesias , M.T. , Pueyo , E. , González , M. and Lorenzo , C. 2007 . Amino acid composition and antioxidant capacity of Spanish honeys . Journal of Agricultural and Food Chemistry , 55 : 360 – 365 .
- Lacerda , J.J.J. , Santos , J.S.S. , Santos , S.A. , Rodrigues , G.B. and Santos , M.L.P. 2010 . Influência das características físico-químicas e composição elementar nas cores de méis produzidos por Apis mellifera no sudoeste da Bahia utilizando análise multivariada . Química Nova , 33 ( 5 ) : 1022 – 1026 .
- Gonzalez-Miret , M.L. , Terrab , A. , Hernanz , D. , Fernandez-Recamales , M.A. and Heredia , F.J. 2005 . Multivariate correlation between color and mineral composition of honeys and by their botanical origin . Journal of Agricultural and Food Chemistry , 53 : 2574 – 2580 .
- Al , M.L. , Daniel , D. , Moise , A. , Bobis , O. , Laslo , L. and Bogdanov , S. 2009 . Physico-chemical and bioactive properties of different floral origin honeys from Romania . Food Chemistry , 112 : 863 – 867 .
- Kaskoniene , V. , Maruska , A. and Kornysova , O. 2009 . Quantitative and qualitative determination of phenolic compounds in honey . Cheminé Technologija , 52 : 54 – 80 .
- Pichichero , E. , Canuti , L. and Canini , A. 2009 . Characterization of the phenolic and flavonoid fractions and antioxidant power of Italian honeys of different botanical origin . Journal of the Science of Food and Agriculture , 89 : 609 – 616 .
- Socha , R. , Juszczak , L. , Pietrzyk , S. and Fortuna , T. 2009 . Antioxidant activity and phenolic composition of herbhoneys . Food Chemistry , 113 : 568 – 574 .
- Vit , P. , Rodrígues-Malaver , A. , Roubik , D.W. , Moreno , E. , Souza , B.A. , Sanchos , M.T. , Fernández-Muiños , M. , Almeida-Anacleto , D. , Marchini , L.C. , Gil , F. , González , C. , Aguilera , G. and Nieves , B. 2009 . Expanded parameters to assess the quality of honey from Venezuelan bees (Apis mellifera) . Journal of ApiProduct and ApiMedical Science , 1 : 72 – 81 .
- Bertoncelj , J. , Dobersek , U. , Jamnik , M. and Golob , T. 2007 . Evaluation of the phenolic content, antioxidant activity and colour of Slovenian honey . Food Chemistry , 105 : 822 – 828 .
- Regina , L.P.L. , Luiza , D.S. , Aurea , E. and Rosane , N.C. 2012 . Antioxidant activity and phenolic composition of Brazilian honeys and their extracts . Journal of the Brazilian Chemical Society , 23 ( 4 ) : 618 – 627 .
- Ulusoy , E. , Kolayli , S. and Sarikaya , A.O. 2010 . Antioxidant and antimicrobial activity of different floral origin honeys from Turkiye . Journal of Food Biochemistry , 34 : 321 – 335 .
- Boines , G.J. and Horoschak , S. 1956 . Hesperidin and ascorbic acid in the prevention of upper respiratory infections . International Record of Medicine and General Practice Clinics , 169 : 73 – 76 .
- Tomás-Barberán , F.A. , Ferreres , F. , Garcia-Vigueira , C. and Hesperetin , Tomás-Lorente, F. 2006 . A marker of the floral origin of citrus honey . Journal of the Science of Food and Agriculture , 61 : 121 – 123 .
- Ferreira , I.C.F.R. , Aires , E. , Barreira , J.C.M. and Estevinho , L.M. 2009 . Antioxidant activity of Portuguese honey samples: Different contributions of the entire honey and phenolic extract . Food Chemistry , 114 : 1438 – 1443 .
- Frankel , S. , Robinson , G.E. and Berenbaum , M.R. 1998 . Antioxidant capacity and correlated characteristics of 14 unifloral honeys . Journal of Apicultural Research , 37 : 27 – 31 .
- Luiza , D.S. , Juliana , P.L.M.S. , Fernanda , B.S. , Maria Cristina , A.L. and Rosane , N.C. 2012 . Characterization of multifloral honeys with multivariate analysis of their chemical profile and antioxidant activity . Journal of Food Science , 77 ( 1 ) : C135 – C140 .
- Herken , E.N. , Erel , O. , Guzel , S. , Celik , H. and Ibanoglu , S. 2010 . Total antioxidant, phenolic compounds, and total oxidant status of certified and uncertified Turkey's honeys . International Journal of Food Properties , 13 ( 3 ) : 599 – 607 .