Abstract
Citrus fruit peels contain a large variety of bioactive components and are considered as potential sources of functional components. The purpose of this study was to evaluate the peroxynitrite-scavenging activities of different citrus fruit peels. The peroxynitrite-scavenging activities of methanolic extracts of nine dried citrus fruit peels were evaluated as determined by their ability to attenuate the peroxynitrite-mediated nitrotyrosine formation in albumin. The results showed that all of the tested citrus fruit peels had the ability to attenuate the formation of nitrotyrosine. The total phenolics contents highly correlated with the peroxynitrite-scavenging activities. The results also indicated that the peroxynitrite-scavenging activity of citrus fruit peels was mainly attributed to the total phenolics.
INTRODUCTION
In response to inflammation, ischemia injury, reperfusion injury, and a variety of environmental toxins, the human body will simultaneously produce superoxide anion (O2 −) and nitric oxide (NO). Superoxide anion and nitric oxide could further react with each other to form peroxynitrite. The cytotoxicity of peroxynitrite is more than its parent molecules. Peroxynitrite could damage a large variety of cellular components, including proteins, lipids, and DNA.Citation[1] Tyrosine residues of protein are especially susceptible to undergo peroxynitrite-mediated nitration and such nitration processes have been reported to contribute to the pathogenesis of several different diseases, including atherosclerosis, neuro-degeneration, and chronic respiratory diseases.Citation2–4 Citation Citation4] Due to lacking a specific enzyme to decompose peroxynitrite, the inhibition of sinister peroxynitrite activity in vivo relies solely upon the actions of certain nonenzymatic compounds.Citation[5] Therefore, development of a natural way to eliminate peroxynitrite would be a promising stratagem for therapeutic and healthy applications.
Citrus fruits are the largest produced fruit of the world and a large portion is consumed as juice. However, citrus fruits have a small edible portion and consequently, a considerable amount of by-products are formed every year from juice processing plant.Citation[6]The by-products, which comprise mainly citrus peels and exhibit potent antioxidant and anti-inflammatory activities, would be an exploitable natural resource of functional components.Citation7–9 Citation Citation9] In south China, dried citrus peels are usually infused by boiling water and consumed as a health-promoting beverage. Also, in traditional Chinese medicine, the dried citrus fruit peels, chen-pi, have been widely used as remedies to treat indigestion and reduce phlegm.Citation[10]
Three inhibitory effects on peroxynitrite in vivo are: (1) preventing the formation of peroxynitrite, (2) directly scavenging the presence of peroxynitrite, and (3) repairing any peroxynitrite-damaged biomolecules.Citation[11] According to our previous studies, certain citrus fruit peels exhibit potent activities to inhibit the formation of peroxynitrite including direct scavenging the NO radical and eliminating NO production by suppressing inducible nitric oxide synthase (iNOS) expression.Citation[12,Citation13] However, it seems that comprehensive and comparative information regarding the peroxynitrite-scavenging ability of various citrus fruit peels is still somewhat scant. In this study, the peroxynitrite-scavenging abilities of nine commonly consumed citrus fruits peels, including lemon (Citrus limon L. Bur), wendun (Citrus grandis Osbeck), peiyou (Citrus grandis Osbeck CV), grapefruit (Citrus paradis), liucheng (Citrus sinensis L. Osbeck), murcott (Citrus reticulata × C. sinensi), ponkan (Citrus reticulata Blanco), tonkan (Citrus tankan Hayata), and kumquat (Fortunella margarita) were investigated based on their inhibitory effects of the peroxynitrite-mediated nitrotyrosine formation.
MATERIALS AND METHODS
Chemicals Used for Investigation
Fluorescein, anti-3-nitrotyrosine antibodies, bovine serum albumin (BSA), dimethyl sulfoxide (DMSO), Folin-Ciocalteu reagent, and nitro blue tetrazolium chloride/5-bromo-4-chloro-3′-indolyphosphate p-toluidine salt (NBT/BCIP), sulphanilamide, naphthylethylenediamine, catechin, gallic acid, and the reference authentic standards (i.e., hesperidin, naringin, neohesperidin, nobiletin, and tangeretin) used in HPLC analyses, were purchased from Sigma Chemical Co. (St. Louis, MO, USA). The other authentic reference standards, narirutin and sinensetin, were purchased from ChromaDex Inc. (Irvine, CA, USA). All chemicals used in this study were of analytical grade.
Synthesis of Peroxynitrite
Peroxynitrite was synthesized as described follow. Briefly, 20 mL of acidified hydrogen peroxide (1 M H2O2 in 0.5 M HCl ) and 20 mL of sodium nitrite (200 mM) in two syringes were simultaneously injected, through a triangle stop-flow device, into a beaker, which contained 40 mL of well-stirred, and cooled (4°C) 1.5 M KOH solution. After that, manganese dioxide was added to decompose the excess hydrogen peroxide, and then be removed by centrifugation at 12,000×g, 4°C for 20 min. The supernatant was frozen at −20°C overnight and a yellow top layer was formed. The concentration of the synthesized peroxynitrite in the yellow top layer was determined by measuring the absorbance at 302 nm (e 302 nm = 1670 M−1 cm−1).
Preparation of Citrus Peel Extract
Nine citrus fruits, including lemon (Citrus limon (L.) Bur), wendun (Citrus grandis Osbeck), peiyou (Citrus grandis Osbeck CV), grapefruit (Citrus paradis), liucheng (Citrus sinensis (L.) Osbeck), murcott (Citrus reticulata × C. sinensi), ponkan (Citrus reticulata Blanco), tonkan (Citrus tankan Hayata), and kumquat (Fortunella margarita) were purchased from a local market (Hsinchu, Taiwan) in the winter of 2008 and hand-peeled immediately after purchase. After oven dried at 45°C for 48 h, the peels were then ground into powders. Aliquots (10 g) of the dried citrus peel powders were placed into glass flasks and extracted with 300 mL of methanol for 20 h, under vigorous shaking. Afterwards, the samples were filtrated through Whatman No. 1 filters, and the filtrates were then evaporated under a vacuum at <50°C using a rotary evaporator to obtain the solid extract. The solid extract was then dissolved in 10 mL of DMSO. Control experiments were carried out by adding an equivalent amount of DMSO without the peel extract.
Total Phenolic Compounds
Total phenolics content in the citrus fruit peels was determined by the Folin-Ciocalteu colorimetric method. Briefly, 0.1 mL of optimal diluted sample and 0.5 mL of Folin-Ciocalteu phenol reagent were reacted with 0.4 mL of 7.5% sodium carbonate for 1 h. The absorbance of the solution at a wavelength of 765 nm was measured using a spectrophotometer. Estimation of the phenolics content was carried out using gallic acid as a standard and the results were expressed in terms of milligrams of gallic acid equivalents (GAE) per 100 g of dried peels.
Total Flavonoids
Total flavonoid content was determined by a colorimetric method. Briefly, 0.25 mL of optimal diluted sample was added into a tube containing 1 mL of double distilled water. Following this, 0.075 mL of 5% NaNO2, 0.075 mL of 10% AlCl3, and 0.5 mL of 1 M NaOH were added, in sequence, at 0, 5, and 6 min. Finally, the volume of reacting solution was adjusted to 2.5 mL with double distilled water. The absorbance of the solution at a wavelength of 510 nm was detected. Estimation of the flavonoid content was carried out using catechin as a standard and the results were expressed in terms of milligrams of catechin equivalents (CE) per 100 g of dried peels.
Flavonoids Composition
The contents of the flavanone glycosides and polymethoxy flavones were determined by high performance liquid chromatography (HPLC), according to a revised method of Wang et al.Citation[14] Briefly, 10 μL of diluted sample were separated on a hypersil C18 column (250 × 4.6 mm i.d., Thermo, Fisher Scientific, Waltham, MA, USA) at 35°C, with a controlled flow rate of 1 mL/min and set wavelengths of 284 and 332 nm. The mobile phase was composed of (a) 2% acetic acid and (b) 0.5% acetic acid-acetonitrile (50:50, v/v) and the gradient elution program was performed as follows: 0 min, 95:5; 10 min, 90:10; 40 min, 60:40; 55 min, 45:55; 60 min, 20:80; 65 min, 0:100 and held for 10 min. Identification of the specific flavanone glycosides and polymethoxy flavones was based on the retention times of the sample peaks to those of the authentic reference standards. The amount of each constituent in the citrus fruit peel extracts was estimated by its integrated datum.
DPPH Radical-Scavenging Capacity
The DPPH radical-scavenging capacity was measured using a microplate method.Citation[15] Briefly, 20 μL of serially diluted sample was reacted with 180 μL of 0.2 mM DPPH methanolic solution in the wells of a 96-well flat-bottomed plate for 5 min. The absorbance at wavelength of 540 nm was then measured by an enzyme-linked immunosorbent assay reader (EL800; BIO-TEK Instruments Inc., Winooski, VT, USA). The scavenging capacity of citrus fruit peel extract was expressed as the IC50, i.e., the concentration of the tested citrus fruit peel extract required to quench 50% of the DPPH radical present.
Oxygen Radical Absorbance Capacity
The oxygen radical absorbance capacity (ORAC) assay was performed as follow. First, 25 μL of diluted sample, trolox standard, and blank were pipetted into wells of a 96-well flat-bottomed plate. Then, 150 μL of fluorescein solution was added to each well and the plate was incubated at 37°C for 30 min in the dark. Next, 25 μL of 2,2-azobis (2-amidinopropane) dihydrochloride (AAPH) solution was added to the wells as a source of peroxyl radical, after which the solution's fluorescence readings were taken every 2 min for a total of 120 min with a microplate multimode detector (Zenyth 3100; Anthos Labtec Instruments Inc., Wals, Austria). Finally, the difference between the area under the fluorescence decay curve for each sample, standard and the corresponding area for the blank was determined to calculate the ORAC value. The results were expressed in terms of millimoles of trolox equivalents (TE) per 100 g of dried peels.
Superoxide Anion (O2 −)-Scavenging Activity
The O2 −-scavenging activity of sample extract was determined as follows. Briefly, 25 μL of different concentrations of sample solution, 62.5 μL of 300 μM NBT and 62.5 μL of 936 μM NADH were added separately into wells. Next, 62.5 μL of 30 μM phenazine methosulfate was added into each well, and the absorbance at 540 nm was read after incubation at ambient temperature for 5 min. The scavenging capacity (%) of the sample extracts to O2 − was expressed as [1 – (Asample – Asample blank)/(Acontrol – Acontrol blank)] × 100%, where Asample – Asample blank was the difference in the absorbance of a sample with and without phenazine methosulfate, respectively, and Acontrol – Acontrol blank was the difference in the absorbance of the PBS control with and without phenazine methosulfate.
NO-Scavenging Activity
The NO-scavenging activity was measured as follows. Serial diluted sample was reacted with 5 mM sodium nitroprusside in the wells of a 96-well flat-bottomed plate for 90 min. Then, an equal volume of Greiss reagent (1% of sulphanilamide and 0.1% of naphthylethylenediamine in 2.5% HPO3) was added to each well to measure the nitrite content. The scavenging capacity (%) of the sample extracts to NO was expressed as [1 – (Asample – Asample blank)/(Acontrol – Acontrol blank)] × 100%, where Asample – Asample blank is the difference in the absorbance of a sample with or without 5 mM sodium nitroprusside, and Acontrol – Acontrol blank is the difference in the absorbance of the PBS control with or without 5 mM sodium nitroprusside.
Peroxynitrite-Scavenging Capacity
The peroxynitrite-scavenging capacity of the citrus fruit peel was evaluated on the basis of the inhibition of peroxynitrite-induced nitrotyrosine formation in BSA.Citation[15] First, a diluted sample solution was mixed with 200 μg/mL of BSA in PBS. Peroxynitrite was then added, and the reaction solution was incubated for 30 min at 37°C. Next, the formation of 3-nitrotyrosine was determined by Immunoblot analysis. Briefly, 10 μL of reaction solution was loaded into, and separated on, a 10% SDS-polyacrylamide gel and then transferred to polyvinylidene fluoride filters. The filters were then blocked, probed with anti-3-nitrotyrosine antibodies, incubated with secondary antibody conjugated to alkaline phosphatase, and detected using an NBT/BCIP solution. Finally, the intensities of the bands on the filters were quantified with a software-supported photoimager (ImageMaster VDS; Amersham Pharmacia Biotech Co., Piscataway, NJ, USA).
Statistical Analysis
All results presented herein are expressed as mean ± SD for at least three independent tests for each citrus fruit peel extract. The significance of the differences between the treatments was analyzed by ANOVA, and followed by Duncan's multiple range test for multiple comparisons. The correlation between two variants was analyzed by application of the Pearson test. All of the statistical analyses were performed by means of SPSS software with the level of significant difference between compared data sets being set at p < 0.05 (SPSS for Windows, ver. 10.0; SPSS Inc., Chicago, IL, USA).
RESULTS
Total Phenolics and Flavonoids Content of Different Citrus Fruit Peels
The results of the total phenolics and flavonoids contents of different citrus fruit peels are shown in . The phenolics content of the citrus fruit peel varied greatly. The tonkan peel (1160 mg GAE/100 g dried peel) had the highest content of total phenolics, whereas the kumquat peel (450 mg GAE/100 g dried peel) had the lowest content. The difference was more than two-fold. Similarly, the flavonoid content of the citrus fruit peel varied greatly. The lemon peel (326 mg CE/100 g dried peel) had the highest content of total flavonoids, whereas the content of the kumquat peel (52 mg/100 g dried peel) was only one-sixth of that in the lemon peel.
Table 1 Total phenolics, total flavonoids, DPPH-scavenging activity, and oxygen radical absorbance capacity (ORAC) of methanolic extract of different citrus fruit peelsFootnote a
Flavonoid Composition of Different Citrus Fruit Peels
According to the results of HPLC analysis, four flavanone glycosides (hesperidin, naringin, neohesperidin, and narirutin) and three polymethoxy flavones (nobiletin, sinensetin, and tangeretin) have been identified in the extracts of citrus fruit peels. As shown in , the total amount of flavanone glycosides in the extracts of the tested citrus fruit peels varied greatly. The concentration of flavanone glycosides contained in the grapefruit peel was 5494 mg/100 g dried peel, which was the highest value of the tested citrus peel extracts. The kumquat peel (7 mg/100 g dried peel) had the lowest content of flavanone glycosides. More than 90% of the flavanone glycosides were hesperidin in the peels of the lemon, liucheng, ponkan, or tonkan. Naringin was the most dominant flavanone glycoside in the peels of the wenden, peiyu, or grapefruit. Additionally, narirutin was the main flavanone glycoside in the murcott peel.
Table 2 Composition of flavanone glycoside and polymethoxy flavone in different citrus fruit peelsFootnote a
As shown in , polymethoxy flavones were only found in the peels of oranges, mandarins, or their hybrids. The liucheng (30 mg/100 g dried peel) had the medium amount of polymethoxy flavones. However, the ponkan, tonkan, and murcott were rich in polymethoxy flavones. Except for ponkan, approximately 80% of the polymethoxy flavones was nobiletin, which was the most abundant compound, whereas sinensetin was the least abundant compound. In ponkan, 56% of the polymethoxy flavones was tangeretin, whereas 44% of the polymethoxy flavones was nobiletin. The distribution of flavonoids in different citrus fruit peels was in agreement with the results of previous studies.Citation[14,Citation16]
Antioxidant Capacities of Different Citrus Fruit Peels
The antioxidant capacities of the citrus fruit peels are presented in . The DPPH-scavenging capacity of the citrus fruit peel extract was presented as IC50. Of the nine citrus fruit peels tested, the tonkan, lemon, and peiyou had good DPPH radical scavenging activities, which had an IC50 value of less than 10 mg/mL. The kumquat had the lowest DPPH scavenging activity (IC50 = 38.5 mg/mL). The ORAC value of the citrus fruit peels also varied greatly. The grapefruit had the highest ORAC value (30.9 mmole TE/100 g dried peels) whereas the kumquat had the lowest ORAC value (10.0 mmole TE/100 g dried peels). The results of the antioxidant capacities based on the ORAC assay were: grapefruit > liucheng > tonkan = peiyou > wendun = lemon = ponkan > murcott > kumquat.
NO-Scavenging Capacities of Different Citrus Peels
The results of the NO-scavenging capacities of the citrus fruit peels are shown in . All of the citrus fruit peel extracts had the NO-scavenging capacity, and all exhibited a linear dose-response curve at concentrations less than 6.3 mg/mL. At a concentration of 3.1 mg/mL, the order of the NO-scavenging capacity was: grapefruit (54.2%) > peiyou (41.4%) > lemon (37.9%) = wendun (37.0%) = murcott (36.9%) = ponkan (36.2%) > liucheng (30.1%) = kumquat (29.9%) = ponkan (29.1%).
Figure 1 NO-scavenging capacity of methanolic extract of different citrus fruit peels. The values are expressed as means ± S.D. of triplicate tests. Means not sharing a common letter at the same tested concentration were significantly different (p < 0.05) when analyzed by ANOVA and Duncan's multiple range test. (Color figure available online.)
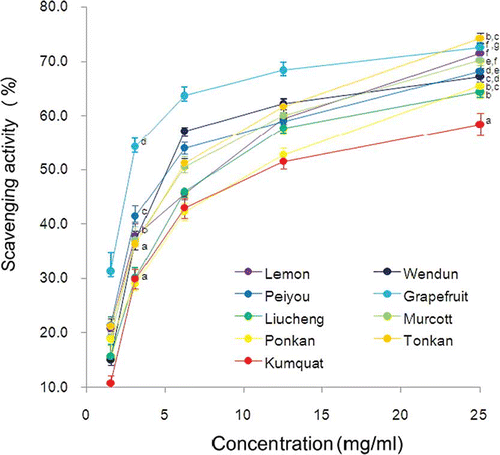
O2 −-Scavenging Capacities of Different Citrus Peels
The O2 −-scavenging capacities of the tested citrus fruit peels are shown in . All of the citrus fruit peel extracts showed a typical dose-response curve and exhibited a linear curve at concentrations from 1.3 to 0.3 mg/mL. Therefore, we compared the O2 −-scavenging capacities at concentrations of 1.3 mg/mL, and the order of the results was: lemon (32.1%) > peiyou (27.5%) = grapefruit (26.9%) = wendun (26.0%) > tonkan (21.7%) = liucheng (21.6%) > ponkan (17.6%) = murcott (15.9%) > kumquat (8.5%).
Figure 2 O2 −-scavenging capacity of methanolic extract of different citrus fruit peels. The values are expressed as means ± S.D. of triplicate tests. Means not sharing a common letter at the same tested concentration were significantly different (p < 0.05) when analyzed by ANOVA and Duncan's multiple range test. (Color figure available online.)
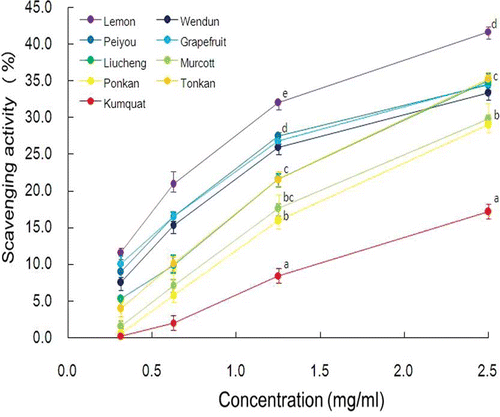
Inhibitory Capacities of Different Citrus Fruit Peels on Peroxynitrite-Mediated Nitrotyrosine Formation
As shown in , after addition of peroxynitrite to BSA, a significant amount of nitrotyrosine was formed. However, the peroxynitrite-induced nitrotyrosine formation under such conditions was attenuated by the addition of various citrus fruit peel extracts. At a concentration of 1 mg/mL, the formation of nitrotyrosine was almost completely inhibited by the peel extract of lemon, tonkan, or liucheng. Therefore, the inhibitory capacities of citrus fruit peels were further evaluated by using a lower concentration (0.5 mg/mL). The order of the results was: lemon (65.5%), tonkan (61.8%), liucheng (61.8%), ponkan (55.5%), grapefruit (21.1%), peiyou (16.8%), wendun (−3.0%), murcott (−6.0%), and kumquat (−6.0%).
Figure 3 Peroxynitrite-scavenging capacity of methanolic extract of different citrus fruit peels. The peroxynitrite-scavenging capacity was evaluated based on the inhibitory activity of peroxynitrite-induced nitrotyrosine formation in BSA. The values are expressed as means ± S.D. of triplicate tests. Means not sharing a common letter at the same tested concentration were significantly different (p < 0.05) when analyzed by ANOVA and Duncan's multiple range test. (Color figure available online.)
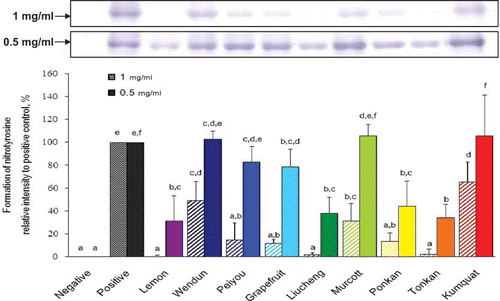
Correlations Among Phenolics, Flavonoids Content, Antioxidant Capacity, and Peroxynitrite-Scavenging Activity
The correlation coefficients among the total phenolics, antioxidant capacities, NO-scavenging activity, O2 −-scavenging activity, and peroxynitrite-scavenging activity were analyzed by Pearson test and the results are shown in . We found that the total phenolics content of a particular citrus fruit peel was highly correlated with not only the antioxidant capacities, but also the O2 − or peroxynitrite-scavenging activity. Additionally, total flavonoids was only highly correlated with O2 −-scavenging and moderately correlated with peroxynitrite-scavenging activities (). However, neither the total amount nor the individual amount of the detected flavanone glycosides of a particular citrus fruit peel was correlated with its peroxynitrite-scavenging activitiy.
Table 3 Correlation coefficients of total phenolics and flavonoids with antioxidant, radical scavenging, and peroxynitrite-scavenging capacities
DISCUSSION
Citrus peels, by-products of a juice processing plant, contain abundant bioactive compounds, such as phenolic acids, flavonoids, limonoids, and fiber. They are an economic resource and worth exploiting as functional food.Citation[17,Citation18] In order to increase the value of citrus fruit peels, more health promotion function should be investigated. In this study, we evaluated the peroxynitrite-scavenging abilities of several citrus fruit peels by measuring their inhibitory effects on peroxynitrite-mediated tyrosine nitration. Of the tested citrus fruit peels, the lemon, liucheng, and tonkan had the best scavenging effect. The ponkan, grapefruit, and peiyou had moderate scavenging activities, whereas the murcott, wendun, and kumquat had poor scavenging activities. Additionally, the peroxynitrite-scavenging abilities of the citrus fruit peels were highly correlated with the total phenolics (r = 0.92, p < 0.001), and moderately correlated with the total flavonoids (r = 0.65, p < 0.041). However, they were not correlated with the content of individual flavonoid analyzed. These results indicate that the inhibitory effects of citrus fruit peels on peroxynitrite-mediated tyrosine nitration were attributed to the combination effects of phenolic compounds. Flavanone glycosides and polymethoxy flavones are abundant and unique in citrus fruits, and have been documented to provide several healthy benefits including peroxynitrite-scavenging activity. However, the peroxynitrite-scavenging capacity of citrus fruit peels did not solely depend on them. Apparently, in addition to flavanone glycosides and polymethoxy flavones, many other antioxidant phenolics also contribute to peroxynitrite-scavenging capacity of citrus fruit peels.
Donating electron or acting as an alternative substance of nitration are two proposed mechanisms in which peroxynitrite-induced tyrosine nitration was inhibited by phenolic compounds.Citation[19] The oxidative products of catechol-containing flavonoids and the nitrated products of flavanones are formed when reacting with peroxynitrite.Citation[20] Although divergent presented in some cases, the structural features and electrical variables needed for high peroxynitrite-scavenging activity are identified. In most cases, they are crucial affecting factors on antioxidant activities.Citation[21] For instance, the peroxynitrite-scavenging activities of flavonoids are affected by structural features, such as the number of hydroxyl groups, a catechol group in the B-ring, a hydroxyl group at the 3-position, and the 2,3 double bond.Citation[22] Moreover, the peroxynitrite-scavenging activities of flavonoids can also be accurately predicted by calculating the enthalpy for tyrosyl radical repair by flavonoids and the energy of the highest occupied molecular orbital and net charge upon C3′ and C4′.Citation[23,Citation24] Thus, it is suggested that phenolics or flavonoids responsible for the antioxidant activity would also be expected to be responsible for the peroxynitrite-scavenging abilities. The high correlation between the total phenolics and the antioxidant or peroxynitrite-scavenging activities indicates that the results were in agreement with the above statements.
Additionally, citrus fruit peel extracts could suppress the inducible nitric oxide synthase (iNOS) expression and subsequent NO secretion and, therefore, would inhibit peroxynitrite formation by the way of preventing the formation of the parent molecule (NO). This inhibitory effect was primarily attributed to polymethoxyflavones, especially nobiletin.Citation[25,Citation26] Of the citrus fruit peels tested in this study, ponkan peel had the most abundant polymethoxyflavones content and, therefore, was proposed as good protector against peroxynitrite toxicity. This was in agreement with ours previous study.Citation[26]
McCartyCitation[27] reported that the peroxynitrite-scavenging ability of plant phenolic compounds, especially flavonoids, is important to the cardioprotective effects associated with high fruit and vegetable intakes. Although citrus fruit juice contains a variety of nutrients and phytochemicals and has numerous healthy benefits,Citation[28,Citation29] the exploitable functional components in citrus fruit peels could not be ignored. Citrus fruit peels have abundant phenolics content and polymethoxyflavones content, which are responsible for the peroxynitrite-scavenging activities of citrus fruit peels. We concluded that citrus fruit peels, such as ponkan peel, would be useful raw materials for creating new value-added functional products.
ACKNOWLEDGMENTS
The authors would like to thank the National Science Council (Taipei, Taiwan) for financially supporting this research under Contract No. NSC-97-2320-B-264-001-MY3.
REFERENCES
- Szabo , C. 2003 . Multiple pathway of peroxynitrite cytotoxicity . Toxicology Letters , 140–141 : 105 – 112 .
- Ischiropoulos , H. and Beckman , J.S. 2003 . Oxidative stress and nitration in neurodegeneration: Cause, effect, or association? . Journal of Clinical Investigation , 111 : 163 – 169 .
- Pennathur , S. , Bergt , C. , Shao , B. , Byun , J. , Kassim , S.Y. , Singh , P. , Green , P.S. , McDonald , T.O. , Brunzell , J. , Chait , A. , Oram , J.F. , O'Brien , K. , Geary , R.L. and Heinecke , J.W. 2004 . Human atherosclerotic intima and blood of patients with established coronary artery disease contain high density lipoprotein damaged by reactive nitrogen species . Journal of Biological Chemistry , 279 : 42977 – 42983 .
- Ricciardolo , F.L.M. , Stefano , A.D. , Sabatini , F. and Folkerts , G. 2006 . Reactive nitrogen species in the respiratory tract . European Journal of Pharmacology , 533 : 240 – 252 .
- Klotz , L.-O. and Sies , H. 2003 . Defense against peroxynitrite: Selenocompounds and flavonoids . Toxicology Letters , 140–141 : 125 – 132 .
- Fernandez-Lopez , J. , Fernandez-Gines , J.M. , Aleson-Carbonell , L. , Sendra , E. , Sayas-Barbera , E. and Perez-Alvarez , J.A. 2004 . Application of functional citrus by-products to meat products . Trends in Food Science & Technology , 15 : 176 – 185 .
- Murakami , A. , Nakamura , Y. , Ohto , Y. , Yano , M. , Koshiba , T. , Koshimizu , K. , Tokuda , H. , Nishino , H. and Ohigashi , H. 2000 . Suppressive effects of citrus fruits on free radical generation and nobiletin, an anti-inflammatory polymethoxyflavonoid . Biofactors , 12 : 187 – 192 .
- Tripoli , E. , Guardia , M.L. , Giammanco , S. , Majo , D.D. and Giammanco , M. 2007 . Citrus flavonoids: Molecular structure, biological activity and nutritional properties: A review . Food Chemistry , 104 : 466 – 479 .
- Schieber , A. , Stintzing , F.C. and Carle , R. 2001 . By-products of plant food processing as a source of functional compounds—Recent developments . Trends in Food Science and Technology , 12 : 401 – 413 .
- Ou , M. 1999 . “ Chen pi ” . In Regular Chinese Medicine Handbook , 508 – 509 . Taiwan : Warmth Publishing Ltd .
- Arteel , G.E. , Briviba , K. and Sies , H. 1999 . Protection against peroxynitrite . FEBS Letters , 445 : 226 – 230 .
- Lin , C.C. , Hung , P.F. and Ho , S.C. 2008 . Heat treatment enhances the NO-suppressing and peroxynitrite-intercepting activities of Kumquat's (Fortunella margarita Swingle) peel . Food Chemistry , 109 : 95 – 103 .
- Ho , S.C. and Lin , C.C. 2008 . Investigation of heat treating conditions for enhancing the anti-inflammatory activity of citrus fruit (Citrus reticulata) peels . Journal of Agricultural and Food Chemistry , 56 : 7976 – 7982 .
- Wang , Y.C. , Chuang , Y.C. and Hsu , H.W. 2008 . The flavonoid, carotenoid and pectin content in peels of citrus cultivated in Taiwan . Food Chemistry , 106 : 277 – 284 .
- Ho , S.C. , Tsai , T.H. , Tsai , P.J. and Lin , C.C. 2008 . Protective capacities of certain spices against peroxynitrite-mediated biomolecular damage . Food and Chemical Toxicology , 46 : 920 – 928 .
- Nogata , Y. , Sakamoto , K. , Shiratsuchi , H. , Ishii , T. , Yano , M. and Ohta , H. 2006 . Flavonoid composition of fruit tissues of citrus species . Bioscience, Biotechnology, and Biochemistry , 70 ( 1 ) : 178 – 192 .
- Bocco , A. , Cuvelier , M.E. , Richard , H. and Berset , C. 1998 . Antioxidant activity and phenolic composition of citrus peel and seed extract . Journal of Agricultural and Food Chemistry , 46 : 2123 – 2129 .
- Gorinstein , S. , Martín-Belloso , O. , Park , Y.S. , Haruenkit , R. , Lojek , A. , Ciz , M. , Capsi , A. , Libman , I. and Trakhtenberg , S. 2001 . Comparison of some biochemical characteristics of different citrus fruits . Food Chemistry , 74 : 309 – 315 .
- Kerry , N. and Rice-Evans , C. 1999 . Inhibition of peroxynitrite-mediated oxidation of dopamine by flavonoid and phenolic antioxidants and their structural relationships . Journal of Neurochemistry , 73 ( 1 ) : 247 – 253 .
- Pollard , S.E. , Kuhnle , G.G.C. , Vauzour , D. , Vafeiadou , K. , Tzounis , X. , Whiteman , M. , Rice-Evans , C. and Spencer , J.P.E. 2006 . The reaction of flavonoid metabolites with peroxynitrite . Biochemical and Biophysical Research Communications , 350 : 960 – 968 .
- Amić , D. , Davidović-Amić , D. , Beslo , D. , Rastija , V. , Lucić , B. and Trinajstić , N. 2007 . SAR and QSAR of the antioxidant activity of flavonoids . Current Medicinal Chemistry , 14 ( 7 ) : 827 – 845 .
- Santos , M.R. and Mira , L. 2004 . Protection by flavonoids against the peroxynitrite-mediated oxidation of dihydrorhodamine . Free Radical Research , 38 ( 9 ) : 1011 – 1018 .
- Sadeghipour , M. , Terreux , R. and Phipps , J. 2005 . Flavonoids and tyrosine nitration: Structure-activity relationship correlation with enthalpy of formation . Toxicology in Vitro , 19 : 155 – 165 .
- Calgarotto , A.K. , Miotto , S. , Honorio , K.M. , da Silva , A.B.F. , Marangoni , S. , Silva , J.L. , Comar Jr , M. , Oliveira , K.M.T. and da Silva , S.L. 2008 . A multivariate study on flavonoid compounds scavenging the peroxynitrite free radical . Journal of Molecular Structure: THEOCHEM , 808 : 25 – 33 .
- Choi , S.Y. , Ko , H.C. , Ko , S.Y. , Hwang , J.H. , Park , J.G. , Kang , S.H. , Han , S.H. , Yun , S.H. and Kim , S-J. 2007 . Correlation between flavonoid content and the NO production inhibitory activity of peel extracts from various citrus fruits . Biological and Pharmaceutical Bulletin , 30 ( 4 ) : 772 – 778 .
- Huang , Y.S. and Ho , S.C. 2010 . Polymethoxy flavones are responsible for the anti-inflammatory activity of citrus fruit peel . Food Chemistry , 119 : 868 – 873 .
- McCarty , M.F. 2008 . Scavenging of peroxynitrite-derived radicals by flavonoids may support endothelial NO synthase activity, contributing to the vascular protection associated with high fruit and vegetable intakes . Medical Hypothesis , 70 : 170 – 181 .
- Mothershaw , A.S. and Jaffer , T. 2004 . Antimicrobial activity of foods with different physico-chemical characteristics . International Journal of Food Properties , 7 ( 3 ) : 629 – 638 .
- Vitali , F. , Saija , A. , Artico , M. , Tita , B. and Franchitto , S. 2007 . Antiulcer potential of a standardized extract of red orange juice in the rat . International Journal of Food Properties , 10 ( 2 ) : 331 – 344 .
