Abstract
Alcalase was used in the present study to carry out an enzymatic hydrolysis of soybean protein isolate and a plastein reaction of the prepared hydrolysate in vitro, aiming to investigate the influence of the plastein reaction on the antioxidant properties of the modified hydrolysate. Soybean protein hydrolysate was prepared in a degree of hydrolysis of 14.0%, exhibited a scavenging activity of 43.6% on ABTS radical in vitro, and thus was used as the substrate of the plastein reaction to prepare the plastein-reaction-stressed hydrolysate. Response surface methodology was applied to select suitable reaction conditions as follows: enzyme addition level 1037 U/g peptides, substrate concentration 29.7% (w/v), reaction temperature 20.3°C. The stressed hydrolysate showed the highest scavenging activity on ABTS radical (about 47.9%) or maximal reaction extent when reaction time was 6 h. Three stressed hydrolysates with different reaction extents were prepared and evaluated for other antioxidant activities. Compared to the original hydrolysate, the stressed hydrolysate with lower reaction extent exhibited a similar (P > 0.05) scavenging activity on DPPH (or superoxide) radical and reducing power, but a significant higher activity (P < 0.05) on hydroxyl radical. The stressed hydrolysate with the highest reaction extent behaved as these investigated antioxidant properties were significantly higher (P < 0.05) than the original hydrolysate except for scavenging activity on DPPH radical. The results of the present study highlight that the alcalase-catalyzed plastein reaction appears to be capable of improving antioxidant properties of soybean protein hydrolysate.
INTRODUCTION
Reactive oxygen species (ROS) or free radicals play an important role in many degenerative diseases.[Citation1] Formation of ROS or free radicals, such as superoxide anion and hydroxyl radical, is an unavoidable consequence of respiration in aerobic organisms. ROS or free radicals are very unstable, and can react easily with the sensitive groups or substances in the body, leading to cell or tissue damage. Intake of antioxidants, including antioxidant proteins or peptides, have a positive effect on body health, as they can protect the body against damage by ROS or free radicals and play an important role in many diseases, such as cardiovascular diseases, diabetes mellitus, cancer, and Alzheimer's disease.Citation[2, Citation3] ROS or free radicals also induce oxidative reactions in foods (mainly on lipids and proteins), leading to the deterioration in flavor, texture, and color.[Citation4] Bioactive peptides derived from various food proteins by enzymatic hydrolysis have antioxidant properties, and have become a topic of great interest in recent studies.
Beyond their basic nutritional functions, food components are relevant to improve health state or reduce disease risk.[Citation5] Bioactive peptides have been found in many food sources,[Citation6] which are inactive within the sequences of parent proteins but could be released during gastrointestinal digestion or food processing.[Citation7] It was found that some food proteins and protein hydrolysates exhibit antioxidant activities through multiple pathways, such as inactivation of ROS or free radicals, chelation of pro-oxidative transition metals, reduction of hydroperoxides, and alteration of the physical properties of food systems.[Citation4] The antioxidant activities of food proteins can be increased in a number of ways, e.g., the Maillard reaction, while the protein hydrolysates obtained from hydrolytic reactions usually have stronger antioxidation than the intact proteins.[Citation4] Many works were conducted to determine the antioxidant activities in vitro of protein hydrolysates, including those from soybean proteins,Citation8–10 Citation Citation10] milk proteins,Citation[11, Citation12] and other proteins.Citation[13, Citation14] More importantly, plastein reaction was applied to treat some protein hydrolysates to enhance their antioxidation against metal-induced lipid oxidation,[Citation15] or to improve their scavenging activities on some radicals.[Citation16] Whether the plastein reaction can enhance the antioxidation of soybean protein hydrolysate remains to be an unknown issue.
In the present work, soybean protein isolate was hydrolyzed by alcalase, and then the prepared hydrolysate was subjected to alcalase-catalyzed plastein reaction to prepare a plastein-reaction-stressed soybean protein hydrolysate. With response surface methodology (RSM), alcalase addition level (E/S ratio), substrate concentration, and reaction temperature were optimized for the plastein reaction, and the decreased amount of free amino groups of the stressed hydrolysate on peptide basis was used as the response for condition selection. Some antioxidant properties of the stressed hydrolysate were evaluated to reveal the impact of the alcalase-catalyzed plastein reaction on these properties of the soybean hydrolysate.
MATERIALS AND METHODS
Protein, Protease, and Chemicals
Soybean protein isolate with crude protein content of 90.0% (w/w) were purchased from Harbin High Tech. Food Co. Ltd. (Harbin, China). 2,2-Diphenyl-1-picrylhydrazyl (DPPH) and 2,2′-azino-bis(3-ethyl-benzothiazoline-6-sulfonic acid) (ABTS) were purchased from Sigma-Aldrich Co. (St. Louis, MO, USA). Alcalase, with a practical activity of 100 kU/g, was purchased from Novozymes (China) Biotechnology Co. Ltd. (Tianjin, China). Other chemicals were of analytical grade. Water used was redistilled water.
Preparation of Soybean Protein Hydrolysate
Soybean protein isolate was dispensed in 200 mL of water to give an original protein concentration of 8% (w/v). The pH of the solution was adjusted to 8.0 by 2 mol/L NaOH. The hydrolysis was started by adding alcalase solution to the solution to give an approximate E/S ratio of 1 kU/g proteins. The mixture was kept at 50°C with stirring, and 20 mL of the sample solution was separated from the mixture after 0.5, 1, 2, 3, 4, 5, 6, and 7 h of hydrolysis, respectively. The separated solutions were heated at 100°C for 15 min to inactivate alcalase and adjusted to pH 4.5 by 2 mol/L HCl. The solutions were centrifuged at 10,000× g for 15 min. All collected supernatants were analyzed for their degree of hydrolysis (DH) and scavenging activity on ABTS radical. Based on analysis results, the hydrolysate with the highest scavenging activity on ABTS radical was bulk prepared, lyophilized, stored at −20°C, and used as the substrate for the plastein reaction.
Assays of Enzyme Activity, Protein or Peptide Content, and Degree of Hydrolysis
The activity of alcalase was assayed by a reference method.[Citation17] Nitrogen contents of the isolate and the hydrolysate were measured by the Kjeldahl procedure according to FIL-IDF 20B:1993,[Citation18] and multiplied by 6.25 to give the protein or peptide content. The content of free amino groups of the soybean protein isolate or the prepared hydrolysate was measured by o-phthaldialdehyde (OPA) assay.[Citation19] The OPA reagent was prepared by combining the following reagents to a final volume of 100 mL: 75 mL 0.2 mol/L sodium borate buffer (pH 9.5), 5 mL 400 g SDS/L, 80 mg OPA (in 1 mL methanol), and 0.4 mL β-mercaptoenthanol. The assay was conducted by adding 3 mL of the sample (or standard) solution to 3 mL OPA reagent. The absorbance of the mixed solution was measured at 335 nm with a spectrophotometer (UV-2401PC, Shimadzu, Kyoto, Japan) and taken after 6 min. L-leucine standard solution was prepared by dissolving 0.3 g L-leucine in 1 mol/L HCl and diluting to 0.6 mg/mL with water. Then, a serial of diluted solutions (0–30 μg/mL) were prepared by further dilution and used for the determination of the standard curve.
The DH of the prepared hydrolysate was determined by assaying the amount of free amino groups of the hydrolysate by the OPA method and then calculated by using Eq. (1) given by Adler-Nissen:[Citation20]
where h is the number of broken peptide bonds per unit weight of the hydrolysate and htot is the total number of bonds per unit weight of the protein, which equals 8.2 meq/g proteins.
Optimization of Plastein Reaction Conditions of Soybean Protein Hydrolysate
Some conditions of the plastein reaction for soybean protein hydrolysate were selected by employing the RSM with a central composite design. In the experimental design, reaction time was set at 6 h, while alcalase addition level (E/S ratio) (U/g peptides) (X 1), substrate concentration (w/v) (X 2), and reaction temperature (°C) (X 3) were chosen as independent variables and set at five levels (). An experimental combination consisting of 20 runs was applied in the present study.
Table 1 Factors and coding levels used in response surface analysis
For all experiments, the lyophilized hydrolysate was reconstituted in water at the designed concentrations, together with the designed alcalase addition. The reaction mixtures were kept at the investigated temperatures for 6 h with continuous agitation, and then heated at 100°C for 15 min to inactivate the enzyme. The mixtures were cooled to room temperature and diluted properly to analyze the content of free amino groups. The decreased amount of free amino groups of the stressed hydrolysate was calculated by subtracting the content of free amino groups of the stressed hydrolysate after the plastein reaction from that of the original hydrolysate before the plastein reaction, expressed as μmol − NH2/g peptides, and used as the response to select suitable reaction conditions.
The second-order polynomial coefficients were calculated and analyzed by using Design Expert software Version 7.0 (Stat-Ease Inc., Minneapolis, MN, USA). A second-order polynomial model (Eq. 2) including all interaction terms was used to predict the response (Y):
where Y is the dependent variable (the response); β 0, β i , β ii , and β ij are coefficients estimated by the model, and xi and xj are levels of the independent variables. The equation was expressed in the form of three-dimensional surface plots to show the relationship between the response and the levels of each variable and to deduce the optimal conditions. The combination of the optimized variables was used to give the predicted maximal response. Finally, three practical experiments were conducted to verify the validity of the model.
Assay of Antioxidant Activity In Vitro
Scavenging activity of the analysis sample (soybean protein isolate, the prepared or the stressed hydrolysate) on DPPH radical was determined by a reported method[Citation21] with some modifications at ambient temperature. The sample dissolved in ethanol (1–20 mg/mL) was mixed with 1 mL of DPPH radical ethanol solution (0.2 mmol/L). The mixture was shaken vigorously and left to stand for 30 min in the dark before measuring the absorbance at 517 nm against a blank in the spectrophotometer. The ethanol was used as the control. The scavenging activity of the sample was thus calculated by using Eq. (3) below.
The scavenging activity on ABTS radical of the analysis sample was determined as per the method[Citation6] with some modifications. The ABTS radical solution was produced by mixing ABTS solution (7 mmol/L) with potassium persulfate solution (2.45 mmol/L) and allowing the mixture to stand in the dark at ambient temperature for 12–16 h before use. In the assay, the radical solution was diluted with 5 mmol/L phosphate buffer (pH 7.4) to an absorbance of 0.7 ± 0.02 at 743 nm. Sample solution in the amount of 50 μL was mixed with 5 mL of the diluted radical solution. The absorbance was taken in the spectrophotometer at 30°C exactly 1 min after initial mixing and up to 6 min. The phosphate buffer was used as the control. The scavenging activity of the analysis sample was also calculated with Eq. (3):
where AS and AC are the absorbance for the sample and control, respectively.
Reducing power of the analysis sample was measured as a reported method.[Citation22] An aliquot of 1 mL sample solution was mixed with 2.5 mL of 0.2 mol/L phosphate buffer (pH 6.6) and 2.5 mL of 1% (w/w) potassium ferricyanide. The mixture was incubated at 50°C for 30 min, and 2.5 mL of 10% (w/w) trichloroacetic acid was added to stop the reaction. The mixture was centrifuged at 1650× g for 10 min. The separated supernatant of 2.5 mL was mixed with 2.5 mL of water and 0.5 mL of 0.1% (w/w) ferric chloride solution. After a 10-min reaction time at ambient temperature, the absorbance of the resulting solution was measured at 700 nm, expressed as the reducing power of the sample. Higher absorbance of the mixture indicated higher reducing power of the sample.
Scavenging activity on hydroxyl radical (·OH) of the analysis sample was assayed by using a reported method[Citation23] with some modifications. 1,10-Phenanthroline (PA) or FeSO4 was dissolved in phosphate buffer (0.15 mol/L, pH 7.4) to give a fixed concentration of 0.75 mmol/L. The PA solution of 2 mL was mixed with the FeSO4 solution of 2 mL, and then 1 mL H2O2 solution (0.01%) and 1 mL sample solution were added. The mixture was incubated at 37°C for 1 h, and the absorbance was measured at 536 nm. The assay results were used to calculate the activity in Eq. (4) as follows:
where AS , AC , and AB are the absorbance of the sample solution; control solution containing PA, FeSO4, and H2O2; and blank solution containing PA and FeSO4, respectively.
Scavenging activity on superoxide radical (·O− 2) of the analysis sample was evaluated by a reference method.[Citation24] The sample solution of 1.0 mL was mixed with 1.8 mL of 50 mmol/L Tris-HCl buffer (pH 8.2). The mixture was incubated at 25°C for 10 min, and then 0.1 mL of 10 mmol/L pyrogallol (in 10 mmol/L HCl) was added. The absorbance of the mixed solution at 320 nm was measured up to 4 min. The oxidation rate of pyrogallol for the sample was calculated as the slope of the absorbance line (ΔAS ). The oxidation rate of pyrogallol for the control was measured with 1.0 mL water (ΔAC ). The scavenging activity of the sample was thus calculated as [(ΔAC – ΔAS )/ΔAC ] × 100%.
Statistical Analysis
All data were collected from three independent trials and expressed as means ± standard deviation (SD). Differences between the mean values of multiple groups were analyzed by one-way analysis of variance (ANOVA) with Duncan's multiple comparison test. All of the tests were considered statistically significant at P < 0.05. SPSS version 13.0 (SPSS Inc., Chicago, IL, USA) and Microsoft Excel version 2003 software (Microsoft Corporation, Redmond, WA, USA) were used to analyze or report the data.
RESULTS AND DISCUSSION
Preparation of Soybean Protein Hydrolysate by Alcalase
To select a suitable hydrolysis time, the DH and scavenging activity on ABTS radical in vitro of the eight prepared soybean protein hydrolysates during 7-h hydrolysis periods were evaluated (). The DH of the prepared hydrolysate was increased from 6.6 to 14.4% as the hydrolysis time was prolonged from 0.5 to 7 h. The scavenging activity on ABTS radical of the prepared hydrolysate increased from 39.8 to 44.5% as the increase of DH. The hydrolysate prepared by 6 h hydrolysis had a DH of 14% and exhibited a scavenging activity of 43.6% on ABTS radical at 2 mg/mL. A hydrolysis time longer than 6 h could not enhance the activity of the hydrolysate significantly (P > 0.05). Hydrolysis time of 6 h was a reasonable selection to prepare a soybean protein hydrolysate as the substrate of the plastein reaction.
Figure 1 Effects of hydrolysis time on the degree of hydrolysis (DH) and scavenging activity on ABTS radical of the prepared soybean protein hydrolysate. The graph chart was for DH while the column chart was for scavenging activity. Peptide content used in the activity evaluation was 2 mg/mL. (Color figure available online.)
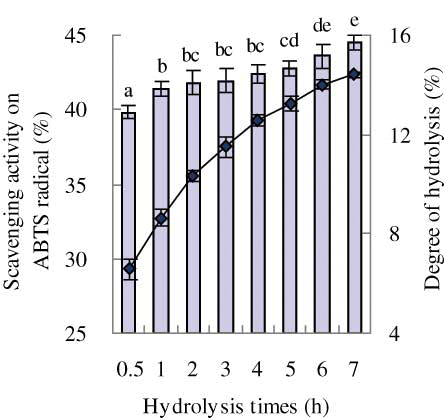
It was reported that as the DH of peanut protein hydrolysate increased from 10 to 20%, its scavenging activity on DPPH radical was enhanced from 21 to 51%; however, further increase in the DH did not increase the activity significantly.[Citation25] In another study, hydrolysis time (1–5 h) also behaved impact on the scavenging activity of a casein hydrolysate prepared by papain on ABTS or DPPH radical, e.g., hydrolysis time of 2 h gave the hydrolysate the highest activity.[Citation10] The two results supported the present result, i.e., longer hydrolysis time did not lead the prepared hydrolysate a further higher scavenging activity. However, this result is a contrast to a reported result,[Citation26] in which the antioxidant activity of porcine plasma protein hydrolysate was increased as the DH was in the range from 0 to 17.6%, or to another result of Li et al.,[Citation27] in which the scavenging activity on DPPH radical for porcine collagen hydrolysate was enhanced as the increase of DH.
Suitable Conditions for Plastein Reaction of Soybean Protein Hydrolysate
Alcalase addition, substrate concentration and reaction temperature were optimized for the plastein reaction of the prepared hydrolysate by RSM. After removal of those insignificant factors, a quadratic model (EquationEq. 5) was obtained by using the Design Expert 7.0 software (Stat-Ease Inc., Minneapolis, MN, USA):
To assess the effects of the three conditions on the decreased amount of free amino groups of the stressed hydrolysate, three-dimensional response surface plots () were generated from the equation by keeping one variable at its zero level and changing the other two variables with different combinations. A detailed discussion about these impacts is not carried out here. Three optimal conditions were obtained by using the software. The optimal alcalase addition, substrate concentration, and reaction temperature were 1037 U/g peptides, 29.7% (w/v), and 20.3°C, respectively. With these selected conditions, the expected response was 177 μmol/g peptides while the actual one was 171 μmol/g peptides (mean of three trials). This result indicates that the established model (EquationEq. 5) could be used to predict the practical result.
Figure 2 Response surface graphs for the decreased amount of free amino groups of the plastein-reaction-stressed hydrolysate as a function of: (a) E/S ratio and substrate concentration (reaction temperature at its central level); (b) substrate concentration and reaction temperature (E/S ratio at its central level); and (c) E/S ratio and reaction temperature (substrate concentration at its central level). (Color figure available online.)
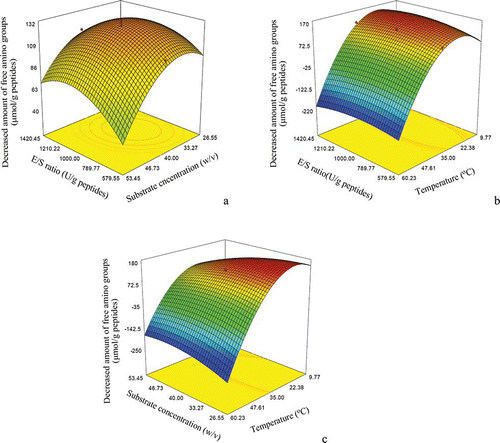
Table 2 Scavenging activity on ABTS radical of the plastein-reaction-stressed hydrolysates (PRSH) prepared with different reaction times
It was reported that plastein products were at a maximum when the substrate concentration was in the region of 20–40 or 30–50% (w/w)Citation[28, Citation29] or when the reaction temperature was increased from 10 to 50°C.[Citation28] Williams et al.[Citation30] found that plastein yield increased over the substrate concentration ranging from 11 to 43%, while plastein formation was higher at 65°C. Ono et al.[Citation15] found that the plastein reaction carried out at 55°C and 30% substrate concentration gave the highest yield. Compared to these reported results, the present result shared similarity in substrate concentration but in a lower temperature. Plastein reaction is an exothermic reaction,[Citation31] a lower reaction temperature is thus reasonable.
Antioxidant Activity of Plastein-Reaction-Stressed Soybean Protein Hydrolysate
With the optimized reaction conditions, eight stressed hydrolysates were prepared with reaction time of 1–8 h. Their decreased amount of free amino groups or scavenging activity on ABTS radical was evaluated and is listed in , with soybean protein isolate and the original hydrolysate as two controls. Unsurprisingly, soybean protein isolate showed the lowest activity than all hydrolysate evaluated (P < 0.05). Two stressed hydrolysates with higher reaction extent (i.e., higher decreased amount of free amino groups) showed higher activity than the original hydrolysate (PRSH 5 or PRSH 6 vs. SPH) significantly (P < 0.05). This fact indicates that the plastein reaction could enhance the antioxidant activity of the original hydrolysate. However, longer reaction time than 6 h gave the stressed hydrolysate a lower reaction extent and activity (e.g., PRSH 6 vs. PRSH 7 or PRSH 8). This might be due to the occurrence of hydrolysis in the reaction system, as the decreased amount of free amino groups of PRSH 6, PRSH 7, or PRSH 8 was 169, 150, or 143 μmol/g peptides, respectively (i.e., the occurred hydrolysis exhibited some adverse impact on the activity of the stressed hydrolysate).
To reveal the impact of the plastein reaction on other antioxidant activities of the stressed hydrolysate, the PRSH 1, 3, and 6, together with the two controls, were evaluated for their scavenging activities on DPPH, hydroxyl, or superoxide radical and reducing power. The results are given in Soybean protein isolate had the lowest properties measured (P < 0.05). Compared to the original hydrolysate, the PRSH 1 and PRSH 3 showed similar activity on DPPH or superoxide radical and reducing power (P > 0.05), but higher activity on hydroxyl radical (P < 0.05). The PRSH 6 exhibited higher activity on hydroxyl or superoxide radical and reducing power than the original hydrolysate (P < 0.05) but similar activity on DPPH radical (P > 0.05).
Table 3 Comparison of some antioxidant properties of soybean protein isolate (SPI), soybean protein hydrolysate (SPH), and plastein-reaction-stressed hydrolysate (PRSH).Footnote*
The present study indicates that alcalase-catalyzed plastein reaction totally enhanced the antioxidant activity of the stressed hydrolysate except for its activity on DPPH radical. It was reported that the papain-catalyzed plasteins reaction could enhance the scavenging activity on DPPH or ABTS radical of casein hydrolysate.[Citation16] Plastein reaction is also capable of improving the antioxidant properties of squid hepatopancreas hydrolysate,[Citation15] or the ACE inhibition in vitro of soybean protein[Citation32] or caseinCitation33–35 Citation Citation35] hydrolysate. The present result shows that alcalase-catalyzed plastein reaction is applicable to prepare soybean protein hydrolysate with higher antioxidant activity. The mechanism accounting for the improved antioxidant activity of the stressed hydrolysate remains unknown. More detailed studies are needed in later work.
CONCLUSIONS
Hydrolysis of soybean protein isolate by alcalase to a degree of hydrolysis of 14% led the obtained hydrolysate higher scavenging activity on ABTS radical. Modification of the prepared hydrolysate with alcalase-catalyzed plastein reaction under the optimal conditions could further improve some antioxidant properties of the stressed hydrolysate. Compared to the original hydrolysate, the stressed hydrolysate with lower reaction extent showed better scavenging activity on hydroxyl radical, but an unchanged scavenging activity on DPPH or superoxide radical and reducing power. The stressed hydrolysate with the highest reaction extent exhibited better scavenging activity on hydroxyl or superoxide radical and reducing power, but an unchanged scavenging activity on DPPH radical. Hydrolysis of soybean protein isolate by alcalase followed by alcalase-catalyzed plastein reaction is capable of preparing soybean protein hydrolysate with much better antioxidant properties.
ACKNOWLEDGMENTS
This work was funded by the Innovative Research Team of Higher Education of Heilongjiang Province (No. 2010td11). The authors also wish to thank the anonymous reviewers and the editors for their valuable work or suggestion to this article.
REFERENCES
- Beckman , K.B. and Ames , B.N. 1998 . The free radical theory of aging matures . Physiological Reviews , 78 : 547 – 581 .
- Aruoma , O.I. 1998 . Free radicals, oxidative stress, and antioxidants in human health . Journal of the American Oil Chemists’ Society , 75 : 199 – 212 .
- Valko , M. , Leibfritz , D. , Moncol , J. , Cronin , M.T.D. , Mazur , M. and Telser , J. 2007 . Free radicals and antioxidants in normal physiological functions and human disease . International Journal of Biochemistry and Cell Biology , 39 : 44 – 84 .
- Elisa , R.J. , Kellerby , S.S. and Decker , E.A. 2008 . Antioxidant activity of proteins and peptides . Critical Reviews in Food Science and Nutrition , 48 : 430 – 441 .
- Arvanitoyannis , I.S. and van Houwelingen-Koukaliaroglou , M. 2005 . Functional foods: A survey of health claims, pros and cons, and current legislation . Critical Reviews in Food Science and Nutrition , 45 : 385 – 404 .
- Pihlanto , A. , Akkanen , S. and Korhonen , H.J. 2008 . ACE-inhibitory and antioxidant properties of potato (Solanum tuberosum) . Food Chemistry , 109 : 104 – 112 .
- Littoralis , S. , Vercruysse , L. , Smagghe , G. , Beckers , T. and Camp , J.V. 2009 . Antioxidative and ACE inhibitory activities in enzymatic hydrolysates of the cotton leafworm . Food Chemistry , 114 : 38 – 43 .
- Amadou , I. , Le , G.W. , Shi , Y.H. and Jin , S. 2011 . Reducing, radical scavenging, and chelation properties of fermented soy protein meal hydrolysate by Lactobacillus plantarum LP6 . International Journal of Food Properties , 14 : 654 – 665 .
- Beermann , C. , Euler , M. , Herzberg , J. and Stahl , B. 2009 . Anti-oxidative capacity of enzymatically released peptides from soybean protein isolate . European Food Research and Technology , 229 : 637 – 644 .
- Zhang , L. , Li , J.R. and Zhou , K.Q. 2010 . Chelating and radical scavenging activities of soy protein hydrolysates prepared from microbial proteases and their effect on meat lipid peroxidation . Bioresource Technology , 101 : 2084 – 2089 .
- Pihlanto , A. 2006 . Antioxidative peptides derived from milk proteins . International Dairy Journal , 16 : 1306 – 1314 .
- Rossini , K. , Noreña , C.P.Z. , Cladera-Olivera , F. and Brandelli , A. 2009 . Casein peptides with inhibitory activity on lipid oxidation in beef homogenates and mechanically deboned poultry meat . LWT–Food Science and Technology , 42 : 862 – 867 .
- Sun , Q. and Luo , Y. 2011 . Porcine hemoglobin hydrolysate prepared with pepsin: Antioxidant activities and their mechanisms . International Journal of Food Properties , 14 : 840 – 853 .
- Nagai , T. , Nagashima , T. , Abe , A. and Suzuki , N. 2006 . Antioxidative activities and angiotensin I-converting enzyme inhibition of extracts prepared from chum salmon (Oncorhynchus Keta) cartilage and skin . International Journal of Food Properties , 9 : 813 – 822 .
- Ono , S. , Kasai , D. , Sugano , T. , Ohba , K. and Takahashi , K. 2004 . Production of water soluble antioxidative plastein from squid hepatopancreas . Journal of Oleo Science , 53 : 267 – 273 .
- Zhao , X.H. , Wu , D. and Li , T.J. 2010 . Preparation and radical scavenging activity of papain-catalyzed casein plasteins . Dairy Science and Technology (LE LAIT) , 90 : 521 – 535 .
- Sarath , G. , De La Motte , R.S. and Wagner , F.W. 1989 . “ Protease assay methods ” . In Proteolytic Enzymes, A Practical Approach , Edited by: Beynon , R.J. and Bond , J.S. 25 – 55 . Oxford , UK : IRL Press .
- International Dairy Federation (IDF) . 1993 . Milk. Determination of the nitrogen (Kjeldahl method) and calculation of the crude protein content. IDF Standard 20B , Brussels , , Belgium : International Dairy Federation .
- Hernández , M.J.M. , Domingo , E.B. , Camañas , R.M.V. and Alvarez-Coque , M.C.G. 1999 . Evaluation of the proteolysis degree with o-phthalaldehyde/N-acetyl-L-cysteine reagent . Fresenius’ Journal of Analytical Chemistry , 338 : 62 – 65 .
- Adler-Nissen , J. 1979 . Determination of the degree of hydrolysis of food protein hydrolysates by trinitrobenzenesulfonic acid . Journal of Agricultural and Food Chemistry , 27 : 1256 – 1261 .
- Nsimba , R.Y. , Kikuzaki , H. and Konishi , Y. 2008 . Antioxidant activity of various extracts and fractions of Chenopodium quinoa and Amaranthus spp . seeds. Food Chemistry , 106 : 760 – 766 .
- Yildirim , A. , Mavi , A. and Kara , A.A. 2001 . Determination of antioxidant and antimicrobial activities of Rumex crispus L . extracts. Journal of Agricultural and Food Chemistry , 49 : 4083 – 4089 .
- Li , Y.H. , Jiang , B. , Zhang , T. , Mu , W.M. and Liu , J. 2008 . Antioxidant and free radical-scavenging activities of chickpea protein hydrolysate (CPH) . Food Chemistry , 106 : 444 – 450 .
- Tang , X.Y. , He , Z.Y. , Dai , Y.F. , Xiong , Y.L. , Xie , M.Y. and Chen , J. 2010 . Peptide fractionation and free radical scavenging activity of zein hydrolysate . Journal of Agricultural and Food Chemistry , 58 : 587 – 593 .
- Jamdar , S.N. , Rajalakshmi , V. , Pednekar , M.D. , Juan , F. , Yardi , V. and Sharma , A. 2010 . Influence of degree of hydrolysis on functional properties, antioxidant activity and ACE inhibitory activity of peanut protein hydrolysates . Food Chemistry , 121 : 178 – 184 .
- Liu , Q. , Kong , B. , Xiong , Y.L. and Xia , X. 2010 . Antioxidant activity and functional properties of porcine plasma protein hydrolysate as influenced by the degree of hydrolysis . Food Chemistry , 118 : 403 – 410 .
- Li , B. , Chen , F. , Wang , X. , Ji , B. and Wu , Y. 2007 . Isolation and identification of antioxidative peptides from porcine collagen hydrolysate by consecutive chromatography and electrospray ionization-mass spectrometry . Food Chemistry , 102 : 1135 – 1143 .
- Sukan , G. and Andrews , A.T. 1982 . Application of the plastein reaction to caseins and to skim milk powder . I. Protein hydrolysis and plastein formation. Journal of Dairy Research , 49 : 265 – 278 .
- Mao , X.Y. , Ni , J.R. , Sun , W.L. , Hao , P.P. and Fan , L. 2007 . Value-added utilization of yak milk casein for the production of angiotensin-I-converting enzyme inhibitory peptides . Food Chemistry , 103 : 1282 – 1287 .
- William , R.J. , Brownsell , V.L. and Andrews , A.T. 2001 . Application of the plastein reaction to mycoprotein: I . Plastein synthesis. Food Chemistry , 72 : 329 – 335 .
- Fujimaki , M. , Kato , M. , Aria , S. and Yamashita , M. 1971 . Application of microbial proteinase to soybean and other materials to improve acceptability . Journal of Applied Bacteriology , 34 : 119 – 131 .
- Gao , B. and Zhao , X.H. 2012 . Modification of soybean protein hydrolysates by alcalase-catalyzed plastein reaction and the ACE-inhibitory activity of the modified product in vitro . International Journal of Food Properties , 15 : 982 – 996 .
- Zhao , X.H. and Li , Y.Y. 2009 . An approach to improve ACE-inhibitory activity of casein hydrolysates with plastein reaction catalyzed by alcalase . European Food Research and Technology , 228 : 1015 – 1021 .
- Li , Y.Y. , Li , T.J. and Zhao , X.H. 2010 . Preparation of alcalase-catalyzed casein plasteins in the presence of proline addition and the ACE-inhibitory activity of the plasteins in vitro . European Food Research and Technology , 231 : 197 – 207 .
- Xu , W. , Li , T.J. and Zhao , X.H. 2013 . Coupled neutrase-catalyzed plastein reaction mediated the ACE-inhibitory activity in vitro of casein hydrolysates prepared by alcalase . International Journal of Food Properties , 16 : 429 – 443 .
