Abstract
The objectives of this study were to determine phenolic content, antioxidant activity of methanolic extracts from different parts of Cordia evolutior (leaf, bark, and fruit), and nutrition composition. The leaf extract showed the highest total phenolic content (25.40 ± 0.34 mg GAE/g extract) and total flavonoid content (69.70 ± 3.37mg RE/g extract) accompanied with the best antioxidant activity through all antioxidant assays. The fruit proximate compositions of crude protein, ash, carbohydrate, fiber, and fat were analyzed. Macro-nutrient contents were found to be higher in the fruit when compared to micronutrients. The analysis also showed the presence of almost all of the essential and non-essential amino acids. Linolenic acid content was higher than stearic acid among the fatty acids in the fruit.
INTRODUCTION
Living organisms require an ample amount of oxygen for their metabolism and energy production. However, free radicals are produced during the energy production processCitation[1] as the unavoidable consequence of respiration in aerobic organisms. Free radicals are unstable species that react rapidly and destructively with biomolecules, such as protein, lipid, DNA, and RNA, in the body. Uncontrolled generation of free radicals is associated with lipid and protein peroxidation, resulting in cell structural damage, tissue injury, or gene mutation, and ultimately leads to the development of various health disorders, such as Alzheimer's disease, cancer, atherosclerosis, diabetes mellitus, hypertension, and ageing.Citation[2] These protective effects are considered to be related to the various antioxidants contained in fruits and vegetables.Citation[3] On the other hand, lipid auto-oxidation, which is initiated by free radicals, also results in food quality deterioration.Citation[4] Antioxidants play an important role in defending the body against free radicals damage. Antioxidants refer to a group of compounds that are able to delay or inhibit the oxidation of lipids or other biomolecules and, thus, prevent or repair the damage of the body cells that is caused by oxygen.Citation[5, Citation6] They work by preventing the formation of new free radical species, converting existing free radicals into less harmful molecules and preventing radical-chained reactions.Citation[7] For instance, phenolic compounds, such as quercetin and ellagic acid, are good antioxidants that are able to protect body cells from injuries caused by reactive oxygen and nitrogen species.Citation[8]
Cordia evolutior belongs to the family Cordiaceae. The fruit is edible, which is traditionally used by the tribes in the Anamalai hills, Coimbatore district, Western Ghats, Tamil Nadu.Citation[9] The plant bark and leaves are bitter, acrid, thermogeneic, anthelmintic, digestive, carminative, diuretic, anaphrodisiac, depurative, vulnerary, and stomachic. They are useful in cephalalgia, gastropathy, anorexia, flatulence, helminthiasis, strangury, indolent ulcers, leprosy, skin diseases, hysteria, and epilepsy. The main objectives of the study were to determine the phenolic content and antioxidant activities of methanolic extracts from different parts of C. evolutior. Therefore, the edible part of the fruit was evaluated by its nutrition composition.
MATERIALS AND METHODS
The chemicals used in this study were of analytical reagent grade and include: methanol, chloroform, Tween 20, and trichloroacetic acid; ammonium thiocyanate (99.99%) and linoleic acid; gallic acid, β-carotene (Type I synthetic, 95%) and (+) α-tocopherol, iron (II) chloride-80 mesh 98%, ethylenediaminetetra acetic acid (EDTA), 1,1-diphenyl-2-picrylhydrazyl (DPPH), and Lascorbic acid 99%; Folin-Ciocalteu phenol reagent, hydrochloric acid (37%), aluminium trichloride (AlCl3), and dimethyl sulphoxide (DMSO) (Merck, Darmstadt, Germany); thiobarbituric acid and buffer solution (phosphates) pH 7.00 ± 0.02, 20°C.
Sample Preparation
Cordia evolutior (Clarke) Gamble plant was collected from Anamalai Hills, Coimbatore District, Tamil Nadu. The collected plant material was identified and their authenticity was confirmed by comparing the voucher specimen at the herbarium of Botanical Survey of India, Southern Circle Coimbatore, Tamilnadu. C. evolutior plants were cleaned and separated into three different parts, namely, leaf, bark, and fruit. All parts were individually freeze-dried (Model Number: LMF–25, Shanghai Lyomac Mechanical Technology Co., Ltd., Shanghai, China (Mainland).
Methanol Extraction
Different parts of C. evolutior were, respectively, pulverized into fine powder using a willy mill and mixed with methanol at the ratio of 1:10 (w/v). Then these mixtures were manually swirled for 15 min and left in a sonicator (Power sonic 505, HwaShin Technology Co., Seoul, Korea) for 60 min. After that, these mixtures were individually filtered through Whatman filter paper No. 1, and the entire extraction process was repeated twice on the residue obtained from the filtration process. The filtrates were individually pooled and methanol was removed from the filtrates under reduced pressure (Rotavapor R210, Buchi, Postfach, Flawil, Switzerland). Finally, C. evolutior extracts were cooled in a dessicator for 30 min before the yield of each extract was calculated. The methanolic extracts from different parts of C. evolutior were kept at −80°C prior to further analyses.
Total Phenolic Content (TPC)
TPC of C. evolutior extracts were determined using Folin-Ciocalteu assay.Citation[10] Briefly, 100 mg of C. evolutior methanolic extracts were individually dissolved in 10 mL of methanol. Then, 0.1 mL of these solutions was mixed with 2.5 mL of 10-fold diluted Folin-Ciocalteau reagent, and 2.0 mL of 7.5% sodium carbonate (Na2CO3). After incubation at 40°C for 30 min, the absorbance of the reaction mixtures was measured at 760 nm by using a spectrophotometer (Pharmaspec UV-1700, Shimadzu, Kyoto, Japan). The equation of the gallic acid calibration curve was Y = 8.2313, X − 0.078 (where X was concentration of garlic acid equivalents (GAE) expressed as milligrams GAE per gram of dried extract and Y was measured absorbance), and the correlation coefficient was R 2 = 0.9971.
Total Flavonoid Content (TFC)
TFC was determined by the aluminium calorimetric methodCitation[11] using Rutin as a standard. Briefly, the test samples were individually dissolved in DMSO. Then, the sample solution (150 μL) was mixed with 150 μl of 2% AlCl3. After 10 min of incubation at ambient temperature, the absorbance of the supernatant was measured at 435 nm by using a spectrophotometer (Pharmaspec UV-1700, Shimadzu, Kyoto, Japan). Rutin was chosen as a standard (the concentration range: 0.005 to 0.1 mg/mL) and the total flavonoid content was expressed as milligram RE per g of dry extracts. The absorbance at 415 nm = 0.0097 rutin (mg/mL) + 0.0127, R 2 = 0.9995.
DPPH Scavenging Activity
DPPH scavenging activity of C. evolutior extracts was determined according to the method described by Singh et al.,Citation[12] with slight modifications. In brief, 0.1 mL C. evolutior extract at various concentrations was added, respectively, to 0.49 mL of methanol and 0.39 mL of DPPH methanolic solution (4 mg/100 mL). Then, the mixtures were vortexed vigorously and allowed to stand in the dark for 60 min. Finally, the absorbance of these mixtures was measured by using a spectrophotometer (Pharmaspec UV-1700, Shimadzu, Kyoto, Japan) at 515 nm. The sample concentration providing 50% of radical scavenging activity (IC50) was obtained through interpolation of linear regression analysis. The lower IC50 indicates higher radical scavenging activity and vice versa. Ascorbic acid and α-tocopherol were used as standards.
Hydroxyl Radical Scavenging Activity
Hydroxyl radical scavenging activity of C. evolutior methanolic extracts was determined by using an electron spin resonance spectrometer (JEOL-JES-FA100, Tokyo, Japan). Hydroxyl radical was generated through a Fenton reaction, with 5,5-dimethyl N-oxide pyroline (DMPO) as the trapping agent. The reaction mixture contained 40 μL of DMPO (400 mM), 37.5 μL of FeSO4 (0.4 mM), 112.5 μL of EDTA (0.1 mM), 60 μL of the sample or blank, and 150 μL of H2O2 (2.0 mM). The electron spin resonance (ESR) measurement was conducted 1 min after preparing each reaction mixture at room temperature. The condition of ESR measurement was as follows: sweeping field, 336.45 ± 5 mT; microwave power, 8 mw; mod width, 0.1 mT; sweep time, 2 min; time constant, 0.1 s; and amplitude, 160. Dimethyl sulphoxide (DMSO) was used as the standard in this experiment and hydroxyl radical scavenging activity of C. evolutior extracts were expressed in gram DMSO equivalent per gram of sample (g DMSOE/g sample).
β-Carotene Bleaching Activity
β-Carotene bleaching activity of C. evolutior extracts was determined according to the method of Wettasinghe and Shahidi.Citation[13] In brief, 3 mL of β-carotene solution (5 mg β-carotene/50 mL chloroform) were added to 40 mg of linoleic acid and 400 mg of Tween 20. Then, the mixture was mixed well and dried under a stream of nitrogen. Immediately, 100 mL of distilled water were added to the dried mixture to form a β-carotene-linoleic acid emulsion. In order to determine the β-carotene bleaching activity of the extract, 1.5 mL of emulsion were respectively added to 20 μL of C. evolutior extracts. After that, these mixtures were incubated in a water bath at 50°C for 60 min. Finally, the absorbances of the reaction mixtures were read at 470 nm by using a UV–visible spectrophotometer (Pharmaspec UV-1700, Shimadzu, Kyoto, Japan). Alpha-tocopherol was used as a standard in this experiment. Antioxidant activity (AA%) of C. evolutior extracts were calculated by using the following equation:
Total Antioxidant Activity Assay
Ferric thiocyanate (FTC) test
A FTC test on C. evolutior extracts was conducted according to the method described by Kikuzaki and Nakatani.Citation[14] In this study, 4 mg of C. evolutior extracts were individually dissolved in 4 mL of methanol. Then, the extract solutions were respectively mixed with linoleic acid (4.1 mL), 0.05 M phosphate buffer pH 7.0 (8 mL), and distilled water (3.9 mL). These mixtures were then kept in screw-cap containers at 40°C in the dark. In order to determine the FTC values, 0.1 mL of these mixtures was respectively added into 9.7 mL of 75% ethanol and 0.1 mL of 30% ammonium thiocyanate. Precisely 3 min after the addition of 0.1 mL of ferrous chloride solution (in 3.5% HCl) to the reaction mixture, the absorbance of the samples was read at 500 nm by using a spectrophotometer (Pharmaspec UV-1700, Shimadzu, Kyoto, Japan). This procedure was repeated every 24 h until the control sample reached its maximum absorbance value. Ascorbic acid and α-tocopherol were used as standard antioxidants in this test.
Thiobarbituric acid (TBA) test
A TBA testCitation[15] was conducted instantly after the control sample from the FTC test reached its maximum absorbance value. In brief, 1.0 mL of 20% aqueous trichloroacetic acid and 2.0 mL of 0.67% aqueous thiobarbituric acid were added to 2 mL of sample solutions acquired from the FTC test. The mixtures were then placed in a boiling water bath for 10 min. After cooling under running tap water, the mixtures were centrifuged at 3000 rpm for 30 min. Finally, the absorbance of supernatants at 532 nm was measured by using a spectrophotometer (Pharmaspec UV-1700, Shimadzu, Kyoto, Japan).
Proximate Composition and Minerals
The proximate composition of C. evolutior fruit was determined by AOAC.Citation[16] Moisture, crude fat, protein, dietary fiber, and ash were determined according to the Association of Official Analytical Chemists standard method. The protein content of the samples was calculated using the factor N × 6.25. Carbohydrate was estimated by subtracting the sum of moisture, protein, fat, fiber, and ash content from 100 g. Calcium, potassium, and sodium were estimated using a flame photometer (EEI 100, GmbH, Hamburg, Germany). Iron and magnesium were determined by use of an absorption spectrophotometer (Baird Alpha 2AA, Baird Corporation, Bedford, UK). Gross energy was determined using bomb calorimetry. The minerals of C. evolutior fruits were measured by wet digestion method. Ashes of C. evolutior were added to HCl:H2O (1:3) and dried in a water bath. Sample solutions were subsequently analyzed by an inductively coupled plasma atomic emission spectroscope (ICP-AES, Thermo Jarrell Ash, Franklin, MA, USA).
Amino Acid Analysis
Amino acids were determined as per the method of Ishida et al.Citation[17] First, 100 mg of finely homogenized sample was taken in a borosil test tube. Then, 10 mL 6 N HCl was added into the test tube. The tubes were sealed after filling with nitrogen gas and the contents of the tube were digested by keeping at 120°C for 24 h in an oven. The test tubes were cooled and then the contents were filtered using Whatmann No. 1 filter paper. The test tubes were rinsed with distilled water and then filtered. The filtrate was evaporated in a vaccum flash evaporator. Deionized water was added into the tubes and the evaporation was allowed to continue until the contents are acid free. The amino acids were dissolved in 0.05 N HCl. Samples were filtered through a membrane filter of 0.45 μM, and 20 μL of this was injected into an amino acid analyzer (Shimadzu LC-10AS HPLC, McKinley Scientific, Sparta, NJ, USA) equipped with a cation exchange column packed with a strongly acidic cation exchange resin, i.e., styrene di-vinyl benzene co-polymer with sulphonic group. The mobile phase of the system consists of two buffers: buffer A and buffer B. A gradient system was followed for the effective separation of amino acids. The oven temperature was maintained at 60°C. The total run was programmed for 60 min. The amino acid analysis was done with a non-switching flow method and fluorescence detection after post-column derivatization with ortho-phthaladehyde or o-phthalaldehyde. In the case of proline and hydroxyl proline, imino group is converted to amino group with hypochlorite. Amino acid standard was also run to calculate the concentration of amino acids in the sample. The amount of each amino acid is expressed as g/100 g protein. The essential amino acids score was calculated with reference to the FAO/WHO reference amino acid pattern:
Analysis of Fatty Acids
Fatty acid compositions of C. evolutior fruit was determined by the AOAC.Citation[18] For lipid extraction, 50 g of fruits were homogenized in 150 mL of chloroform:methanol (1:2, v/v) mixture and reacted at 100°C for 30 min filling N2, and then filtrated through Whatman No. 1 filter paper. Extracted lipid from each sample was saponified in 0.5 N NaOH methanolic solution and esterified in BF3-methanol. Fatty acids were determined using a gas chromatography (Varian star 3600; Varian Inc., Walnutcreek, CA, USA) equipped with a flame ionization detector and Omagawax 205 fused-silica bond capillary column (30 m × 0.32 mm, i.d. × 0.25 μm film thickness). The temperatures of the oven, injection port, and detector were 140, 250, and 260°C, respectively, and nitrogen flow rate was 50 mL/min.
Statistical Analyses
Data was reported as mean ± standard deviation from triplicate determination. Analysis of variance (ANOVA) accompanied with the least significant difference (LSD) and Tukey tests (SPSS for Windows, Version 15, SPSS Inc., Chicago, IL, USA) were conducted to identify the significant difference between samples (p < 0.05).
Table 1 Extraction yield, total phenolic content, and total flavonoid content of C. evolutior extracts (n = 3)
RESULTS AND DISCUSSION
Extraction Yield, Total Phenolic Content, and Total Flavonoid Content
Table 1 presents the yield, total phenolic content, and total flavonoid content of C. evolutior methanolic extracts. The yield of the extracts varied from 13.66 to 23.78%. Among all tested extracts, the highest and the lowest yields were respectively obtained from the bark and fruit of C. evolutior (p < 0.05). The yield of methanolic extracts from different parts of C. evolutior is presented in the following order: bark > leaf > fruit (p < 0.05). The content of extractable phenolic compounds in the C. evolutior extracts was determined through a linear gallic acid standard curve (y = 8.2313x + 0.078; r 2 = 0.9971). The total phenolic content of C. evolutior extracts varied from 2.85 to 25.40 mg GAE/g extract. The highest content of total phenolic compounds was detected in the C. evolutior leaf extract (25.40 mg GAE/g extract), whereas the lowest content was measured in the fruit extract (2.85 mg GAE/g extract) (p < 0.05). Total phenolic content of C. evolutior extracts is arranged in the following descending order: leaf > bark > fruit (p < 0.05). This finding is in agreement with some previous studies, which reported that total phenolic content of leaf extract was higher than other parts of the plant for Beta vulgaris (blood turnip), Petroselinum crispum (parsley), and Coriandrum sativum (Chinese parsley).Citation[19, Citation20] Higher amounts of phenolics in leaflets in comparison to that of roots may be attributed to the presence or absence of light that affects the phenolic contents of organs. Generally, there is a rise in total phenolics in plants grown in the sunny situations relative to the shady ones, but it can be seen at the intra-individual level by comparing plant parts exposed to different amounts of light.Citation[21, Citation22] This suggests that leaf might be the part that is rich in phenolic compounds in many plants. Several studies have revealed that the phenolic content in the plants are associated with their antioxidant activities,Citation[23] probably due to their redox properties, which allow them to act as reducing agents, hydrogen donors, and singlet oxygen quenchers.Citation[24]
Flavonoids are the most common and widely distributed group of plant phenolic compounds, which usually are very effective antioxidants.Citation[25] In this study, the total flavonoid content of methanolic extracts from different parts of C. evolutior was evaluated by aluminium colorimetric assay. The total flavonoid content of C. evolutior extracts was varied considerably from 2.52 to 69.70 μg RE/g extract. The data presented in indicates that the highest flavonoid content of 69.70 μg RE/g extract was observed in the extract of leaf and the lowest content was observed in the extract of the fruit (2.52 μg RE/g extract) (p < 0.05). Total flavonoid content of C. evolutior extracts is arranged in the following sequence: leaf > bark > fruit (p < 0.05). The total flavonoid content of C. evolutior extracts is well in correspondence to the total phenolic content (r = 0.9836). This indicates that the flavonoids are the major phenolic compounds present in C. evolutior plant.
Antiradical Activity
Table 2 shows DPPH and hydroxyl radicals scavenging activity of C. evolutior methanolic extracts. In general, IC50 values of all tested samples through a DPPH scavenging activity test were ranging from 1.40 to 26.41 mg/mL and the DPPH scavenging activity is arranged in the following descending order: ascorbic acid (IC50 = 0.02 mg/mL) > α-tocopherol (IC50 = 0.06 mg/mL) > leaf > bark > fruit (p < 0.05). In this study, DPPH scavenging activity of C. evolutior extracts shows a similar trend with the result of total phenolic content (r = 0.9228) and total flavonoid content (r = 0.8478), indicating that DPPH radical scavenging activity of C. evolutior extracts is highly related to the amount of phenolic compounds, especially flavonoids that are present in the extracts.
Meanwhile, hydroxyl radical scavenging activities of C. evolutior extracts were ranging from 38.37 to 145.90 g DMSOE/g extract (). Among all tested extracts, leaf extract exhibited the strongest hydroxyl radical scavenging activity (145.90 g DMSOE/g extract), while fruit extract showed the least antiradical activity (38.37 g DMSOE/g extract) (p < 0.05). Hydroxyl scavenging activity of C. evolutior extracts is presented in the following descending order: leaf > bark > fruit (p < 0.05). A correlation test shows that hydroxyl radical scavenging activity of C. evolutior extracts is well correlated with its total phenolic (r = 0.9171) and total flavonoid (r = 0.9512) contents, supporting the former statement on the contribution of phenolic compounds (particularly flavonoids) in antiradical activity of C. evolutior extracts.
Table 2 DPPH and hydroxyl radicals scavenging activity of C. evolutior methanolic extracts (n = 3)
β-Carotene Bleaching (BCB) Activity
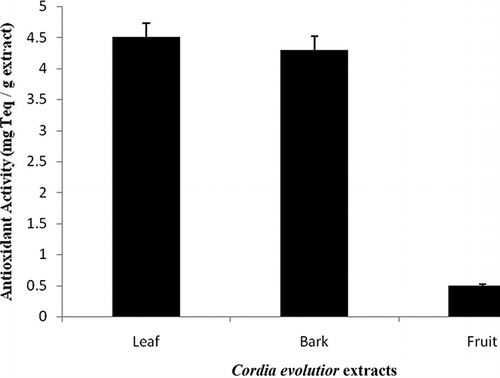
Antioxidant activity of C. evolutior extracts as measured by bleaching of β-carotene was determined through the interpolation of a linear α-tocopherol standard curve (y = 16.784x + 3.1533; r 2 = 0.9708) and expressed in mg alpha-tocopherol equivalent (Teq)/g extract. In the BCB assay, linoleic acid produces hydroperoxides during incubation at 50°C. The presence of hydroperoxides cause rapid discoloration of β-carotene.Citation[26] However, hydroperoxides formed in this system can be neutralized by the antioxidants from the extracts.
A variation in antioxidant activity of C. evolutior extracts ranging from 0.51 to 4.43 mg Teq/g extract was observed. shows that the C. evolutior leaf extract again exhibited the highest antioxidant activity (4.43 mg Teq/g extract) through BCB assay, while fruit extract showed the least antioxidant activity (0.51 mg Teq/g extract) towards the bleaching of β-carotene. Antioxidant activity of C. evolutior extracts through BCB assay is arranged in the following order: leaf > bark > fruit (p < 0.05). This result is in agreement with the previous study by Elzaawely et al.,Citation[27] who reported that the leaf extract of Alpinia zerumbet exhibited higher inhibitory activity towards β-carotene oxidation than other parts of the plant. On the other hand, bark extract of C. evolutior also showed good antioxidant activity in reducing the oxidation of β-carotene in this study (p < 0.05). As discussed previously, high antioxidant activity of C. evolutior leaf and bark extracts might be due to its high phenolic content, likewise flavonoids, in particular.
Total Antioxidant Activity: Ferric Reducing Antioxidant Power (FRAP) and TBA Tests
Figure 2 shows the hydroperoxides inhibitory activity of C. evolutior extracts through FTC test. As shown in , almost all C. evolutior extracts (except for fruit) significantly retarded the formation of hydroperoxides in the linoleic acid emulsion system throughout the incubation period as compared to the control sample (p < 0.05). From the third day onward, the absorbance value of the control was higher (p < 0.05) than other samples (except for fruit) and reached its maximum absorbance on the ninth day of incubation. The overall inhibitory activity of C. evolutior extracts against hydroperoxides formation can be established in the following descending order: ascorbic acid > leaf > bark > fruit > α-tocopherol > control. The fruit extract showed greater absorbance value (p < 0.05) than the control sample at the end point of the incubation period (ninth day), indicating that C. evolutior fruit extract might possess pro-oxidative properties that enhance the autoxidation of linoleic acid in the emulsion system and increase the generation of reactive substances.
Figure 2 Hydroperoxides inhibitory activity of C. evolutior extracts through ferric thiocyanate test.
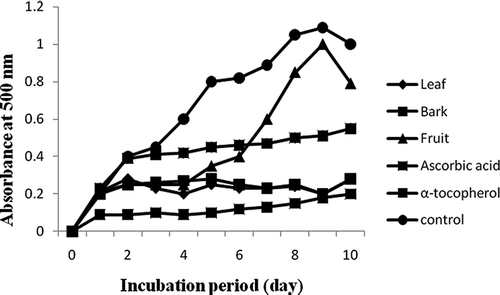
The C. evolutior leaf extract exhibited the highest hydroperoxides inhibitory activity that was superior to ascorbic acid, α-tocopherol, as well as other tested C. evolutior extracts (p < 0.05). This result suggested that the leaf extract might contain primary antioxidant compounds, which are able to react aggressively with free radicals, particularly hydroxyl radicals, thereby terminating the radical-chained reaction and retard the formation of hydroperoxides.Citation[28] After the control sample reached its maximum absorbance value in the FTC test, a TBA test was subsequently conducted on the samples. This test measures the thiobarbituric acid reactive substances content at a later stage of lipid oxidation, involving the quantification of the secondary products formed from lipid oxidation. In this test, low absorbance value indicates higher thiobarbituric acid reactive substances inhibitory activity. shows that the fruit extract exhibited the weakest thiobarbituric acid reactive substances inhibitory activity, while the leaf extract possessed the strongest activity (p < 0.05). The strength of thiobarbituric acid reactive substances inhibitory activity of leaf and bark extracts was similar to ascorbic acid (p > 0.05) but superior to α-tocopherol (p < 0.05).
Figure 3 Thiobarbituric acid reactive substances inhibitory activity of C. evolutior extracts measured by thiobarbituric acid test.
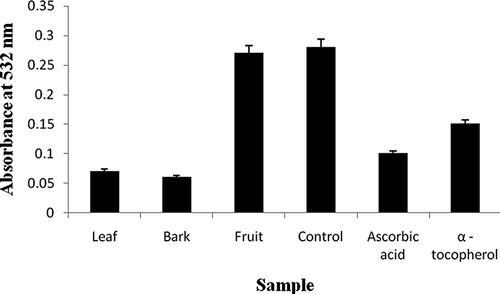
Thiobarbituric acid reactive substances inhibitory activity of all tested samples is presented in the following descending order: leaf > bark > ascorbic acid > α-tocopherol > fruit > control (p < 0.05). The trend of thiobarbituric acid reactive substances inhibitory activity of C. evolutior extracts is rather similar to the trend of the FTC test in this study. This suggests that reduction of thiobarbituric acid reactive substances content in leaf and bark samples might due to the lower hydroperoxides accumulation in the respective samples, previously. Besides, secondary antioxidant compounds that might present in these extracts may also contribute to the inhibition of hydroperoxides decomposition in these samples.
Proximate Composition
Proximate compositions of C. evolutior fruit are shown in . The crude protein was found to be high (23.28%) compared to fat (3.86%) ash content (10.47%), carbohydrate (11.32%) and fiber (5.56%). Plant food that provides more than 12% of its calorific value from protein are considered to be a good source of protein. Furthermore, adults, pregnant and lactating mothers required 34–56 g, 13–19 g, and 71 g of protein daily, respectively.Citation[29] In accordance with these, the fruit selected for the present study, C. evolutior fruit, might serve as an efficient source of protein-rich food.
Table 3 Proximate composition, minerals, and fatty acids of the C. evolutio r fruit
Minerals
The mineral contents of C. evolutior fruit are shown in . It has been found that the fruit was rich in Ca, K, and Mg. The values for the Ca, K, and Mg were 1,774, 1,322, and 361 mg/100 g, respectively. Calcium has been reported to be effective in the building of skeletal structures and muscle functioning, while magnesium is important in the ionic balance and enzyme co-factors.Citation[30] It has been shown that C. evolutior fruit also had some minerals, such as Ca, K, and Mg, which were beneficial to the human body so that they were thought to be used as food materials useful in health.
Aminoacid Analysis
The aminoacid composition of the C. evolutior fruit sample along with the essential amino acid requirements pattern suggested by FAO/WHO is shown in . The amino acid profile of the sample revealed that the proteins of the fruit contained adequate levels of amino acid as compared with FAO/WHO. The amount of amino acids, histidine (6.8 g/100 g protein), leucine (9.7 g/100 g protein), iso leucine (7.8 g/100 g protein), phenylalanine + tyrocine (9.6 g/100 g protein), threonine (7.2 g/100 g protein), tryptophan (1.8 g/100 g protein), and valine (8.2 g/100 g protein), were found to be higher, whereas the amino acid lysine (1.2 g/100 g protein), methonine + cysteine (g/100 g protein), recorded a lower level but appreciable content than the reference pattern of FAO/WHO. Amino acids in the form of proteins make up the greatest portion of our body weight. Apart from this, cysteine was found to have the ability to quench DPPH radicals.Citation[31] Arginine plays vital roles in nutrition and metabolism. It is classified as an essential amino acid in infants and young children and as a conditionally essential amino acid in adults at times of trauma or disease. The chemical score and amino acid index are widely used for screening potential protein foods. Amino acid deficiency can be met by consuming large amounts of legumes, or by taking a mixture of legumes, or by employing the complimentarily that exists between high sulphur amino acid cereals and legumes.
Table 4 Amino acid profile of C. evolutio r fruit
Fatty Acids
Fatty acids compositions of C. evolutior fruit are shown in . A total of 11 fatty acids ranging from C8 to C22 were identified. In C. evolutior fruit, the higher content of unsaturated fatty acids, such as linoleic acid (30%) and linolenic acid (33%), rather than that of saturated fatty acids, such as palmitic acid (11%) and steric acid (4%), were recorded. The fatty acid composition of a diet alters the composition of membrane phospholipids, which in turn changes membrane functions.Citation[32] The plant contains unsaturated fatty acids of physiologic and nutritional significance, such as oleic and linoleic acids, which could be contributory to the nutritional needs of the consumers.
CONCLUSION
This study clearly demonstrated that high antioxidant activity was observed in the leaf and bark extracts of Cordia evolutior as compared to other tested extracts. Many plant-based foods are good sources of phytochemical antioxidants and exhibit cardio-health promotion properties. Phytochemical antioxidants from fruits and leaves can significantly inhibit the development of cardiovascular disease. The C. evolutior fruit proximate compositions of crude protein, carbohydrate, and fiber contents were presented at a better level. Macro-nutrient contents were found to be higher in the fruit when compared to micronutrients. The fruit nutrition composition findings lead to use as a dietary supplement or as a functional ingredient in nutraceutical and pharmaceutical products.
REFERENCES
- Packer , L. 1999 . “ Free radicals ” . In The Antioxidant Miracle: Your Complete Plan for Total Health and Healing , Edited by: Packer , L. and Colman , C . 16 – 17 . Canada : John Wiley & Sons Inc .
- Mantle , D. , Eddeb , F. and Pickering , A. 2000 . Comparison of relative antioxidant activities of British medicinal plant species in vitro . Journal of Ethnopharmacology , 72 : 47 – 51 .
- Zin , Z.M. , Hamid , A.A. , Osman , A. , Saari , N. and Misran , A. 2007 . Isolation and identification of antioxidative compound from fruit of mengkudu (Morinda citrifolia L.) . International Journal of Food Properties , 10 : 363 – 373 .
- Kanner , J. 1994 . Oxidative processes in meat and meat products . Quality implications. Meat Science , 36 : 169 – 189 .
- Shahidi , F. and Naczk , M. 2004 . Phenolics in food and nutraceuticals , Boca Raton , FL : CRC Press .
- Tachakittirungrod , S. , Okonogi , S. and Chowwanapoonpohn , S. 2007 . Study on antioxidant activity of certain plants in Thailand: Mechanism of antioxidant action of guava leaf extract . Food Chemistry , 103 : 381 – 388 .
- Rodriguez , J. , Olea-Azar , C. , Cavieres , C. , Norambuena , E. , Delgado-Castro , T. and Soto-Delgado , J. 2007 . Antioxidant properties and free radical-scavenging reactivity of a family of hydroxynaphthalenones and dihydroxyanthracenones . Bioorganic and Medicinal Chemistry , 15 : 7058 – 7065 .
- Sroka , Z. and Cisowski , W. 2003 . Hydrogen peroxide scavenging, antioxidant and anti-radical activity of some phenolic acids . Food and Chemical Toxicology , 41 : 753 – 758 .
- Ramachandran , VS. 2007 . Wild edible plants of the Anamalais, Coimbatore district, western Ghats, Tamil Nadu . Indian Journal of Traditional Knowledge , 6 : 173 – 176 .
- Singleton , V.L. , Orthofer , R. and Lamuela-Raventos , R.M. 1999 . Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin–Ciocalteu reagent . Methods in Enzymology , 299 : 152 – 178 .
- Lin , J.-Y. and Tang , C.-Y. 2007 . Determination of total phenolic and flavonoid contents in selected fruits and vegetables, as well as their stimulatory effects on mouse splenocyte proliferation . Food Chemistry , 101 : 140 – 147 .
- Singh , RP , Murthy , KNC. and Jayaprakasha , GK. 2002 . Studies on the antioxidant activity of pomegranate (Punica granatum) peel and seed extracts using in vitro models . Journal of Agricultural and Food Chemistry , 50 : 81 – 86 .
- Wettasinghe , M. and Shahidi , F. 1997 . Antioxidant activity of preformed cooked cured-meat pigment in a β-carotene/linoleate model system . Food Chemistry , 58 : 203 – 207 .
- Kikuzaki , H. and Nakatani , N. 1993 . Antioxidant effects of some ginger constituents . Journal of Food Science , 58 : 1407 – 1410 .
- Mackeen , M.M. , Ali , A.M. , Lajis , N.H. , Kawazu , K. , Hassan , Z. and Amran , M. 2000 . Antimicrobial, antioxidant, antitumor-promoting and cytotoxic activities of different plant part extracts of Garcinia atroviridis Griff ex. T. Anders . Journal of Ethnopharmacology , 72 : 395 – 402 .
- AOAC . 1995 . Official Methods of Analysis , 16th , 1094 Arlington , VA : Association of Official Analytical Chemists .
- Ishida , Y. , Fugita , T. and Asai , K. 1981 . New detection and separation method for aminoacid by high performance liquid chromatography . Journal of Chromatography , 204 : 143 – 148 .
- AOAC . 1990 . “ Method 969 ” . In Official Methods of Analysis , 15th , 33 Arlington , VA : Association of Official Analytical Chemists .
- Pyo , Y.H. , Lee , T.C. , Logendra , L. and Rosen , R.T. 2004 . Antioxidant activity and phenolic compounds of Swiss chard (Beta vulgaris subspecies cycla) extracts . Food Chemistry , 85 : 19 – 26 .
- Wong , P.Y.Y. and Kitts , D.D. 2006 . Studies on the dual antioxidant and antibacterial properties of parsley (Petroselinum crispum) and cilantro (Coriandrum sativum) extracts . Food Chemistry , 97 : 505 – 515 .
- Mole , S. and Waterman , P.G. 1987 . “ Tannins as antifeedants to mammalian herbivores: Still an open question? ” . In Allelochemicals: Role in Agriculture and Forestry , Edited by: Waller , G.R. 572 – 587 . Washington , DC : American Chemical Society .
- Mole , S. , Ross , J.A.M. and Waterman , P.G. 1988 . Light induced variation in phenolic levels in foliage of rain forest plants . I. Chemical changes. Journal of Chemical Ecology , 14 : 1 – 21 .
- Omken , B. , Sigva , H.O. , Mutlu , S. , Doganlar , S. , Yemenicioglu , A. and Frary , A. 2009 . Total antioxidant activity and total phenolic contents in different Turkish eggplant (Solanum melongena L.) cultivars . International Journal of Food Properties , 12 : 616 – 624 .
- Chang , S.T. , Wu , J.H. , Wang , SY. , Kang , P.L. , Yang , N.S. and Shyur , L.F. 2001 . Antioxidant activity of extracts from Acacia confusa bark and heartwood . Journal of Agriculture and Food Chemistry , 49 : 3420 – 3424 .
- Yanishlieva-Maslarova , N.V. 2001 . “ Inhibiting oxidation ” . In Antioxidants in Food: Practical Applications , Edited by: Pokorny , J. , Yanishlieva , N. and Gordon , M.H . 22 – 70 . Cambridge : Woodhead Publishing Limited .
- Wettasinghe , M. and Shahidi , F. 1999 . Antioxidant and free radical-scavenging properties of ethanolic extracts of defatted borage (Borago officinallis L.) seeds . Food Chemistry , 67 : 399 – 414 .
- Elzaawely , A.A. , Xuan , T.D. and Tawata , S. 2007 . Essential oils, kava pyrones and phenolic compounds from leaves and rhizomes of Alpinia zerumbet (Pers.) and their antioxidant activity . Food Chemistry , 103 : 486 – 494 .
- Ismail , H.I. , Chan , K.W. , Mariod , A.A. and Ismail , M. 2010 . Phenolic content and antioxidant activity of cantaloupe (Cucumis melo) methanolic extracts . Food Chemistry , 119 : 643 – 647 .
- Anonymous . 2002 . Dietary reference intake for energy, carbohydrate, fibre, fat, fatty acids, cholesterol, protein and amino acid (micronutrients) , Food and Nutrition Board, Institute of Medicine, National Academy of Sciences, Allahabad, India .
- FAO . 1990 . Food and Agriculture Year Book, Statistic Series No. 94 , Rome : Food and Agriculture Organization .
- Yokozawa , T. , Chen , C.P. , Dong , E. , Tanaka , T. , Nonaka , G.I. and Nishioka , I. 1998 . Study on the inhibitory effect of tannins and flavonoids against DPPH radical . Journal of Food and Agricultural Chemistry , 56 : 213 – 222 .
- Field , C.J. , Angel , A. and Clandinin , M.T. 1985 . Relationship of diet to fatty acid composition of human adipose tissue structural and stored lipids . Asian Journal of Clinical Nutrition , 42 : 1206 – 1220 .