Abstract
The main antioxidant compounds (lycopene, β-carotene, total phenolic, and vitamin C) and total antioxidant activity in eight dried tomatoes marketed in Brazil were determined. The total antioxidant activity was determined by the DPPH radical scavenging method and the β-carotene/linoleic acid system in both hydrophilic and hydrophobic extracts. The contents of the antioxidant compounds varied significantly among the products analyzed. The hydrophobic extracts showed higher antioxidant activity with the β-carotene/linoleic acid system while the hydrophilic extracts showed higher antioxidant activity with the DPPH method. Despite differences found, the dried tomatoes can be considered products with high contents of antioxidant compounds with high antioxidant activity.
INTRODUCTION
Tomatoes are one of the most produced and consumed fruits and vegetables in the world. In recent decades, tomatoes and their derivatives have received increased attention due to their benefits to human health. Various studies have shown an inverse association between the consumption of tomatoes and tomato derivatives and the risk of developing chronic non-transmissible diseases (CNTD), such as several types of cancer and cardiovascular diseases.[ Citation 1 ,Citation 2 ] The beneficial action of tomatoes and their derivatives is attributed to the high antioxidant potential of these fruits. Tomatoes and tomato products are considered good sources of antioxidant substances, including phenolic compounds, ascorbic acid, and carotenoids (mainly lycopene). The concentrations of tomato antioxidant compounds substantially vary due to the influence of several factors, such as maturation stage, cultivar, climate, processing conditions, and storage.[ Citation 3 − Citation 5 ]
Lycopene, the main antioxidant compound present in tomatoes, is an apolar pigment without vitamin A activity that is formed by a highly unsaturated acyclic chain with 11 conjugated double bonds and 2 non-conjugated bonds.[ Citation 6 ,Citation 7 ] Among known carotenoids, lycopene is considered to be the carotenoid that possesses the highest antioxidant capacity, and the ingestion of lycopene-rich foods has been associated with a reduction of the incidence of cardiovascular diseases and several types of cancer, including cancers of the digestive tract, lung, and prostate.[ Citation 8 ,Citation 9 ] Tomatoes and tomato derivatives are the main source of lycopene in human diets, and they are considered to be functional foods by many researchers due to their high lycopene content of tomatoes.[ Citation 10 ,Citation 11 ]
Tomato is an important source of vitamin C and phenolic compounds in the human diet.[ Citation 12 ] Despite showing only moderate content of vitamin C and phenolic compounds, tomato, due to its high consumption, is the main source of food phenolic and the third largest contributor with vitamin C in the diet of Americans.[ Citation 13 ,Citation 14 ] Phenolic compounds are potent antioxidants that act in the reactions of lipid peroxidation and reduction of singlet oxygen.[ Citation 15 ] In addition, exert antioxidant activity synergistically with carotenoids.[ Citation 16 ] In tomatoes, the phenolic compounds are present mainly in conjugated form, and quercetin, kaenferol, naringenin, rutin, and chlorogenic acid present in the phenolic highest concentration.[ Citation 4 ] Vitamin C is a water soluble substance that exerts several functions in the human body. Among them, participates in the hydroxylation of proline and lysine, required to biosynthesis of collagen, increases the absorption of nonheme iron,[ Citation 12 ] acts in the conversion of cholesterol into bile acids, and is able to donate electrons to neutralize free radicals.[ Citation 17 ] The antioxidant effect of vitamin C has been linked to the prevention of chronic diseases, such as some cancers (stomach, lung, and liver) and coronary heart disease.[ Citation 18 ,Citation 19 ]
Besides being consumed in the fresh form in salads and other preparations, tomatoes are widely consumed in processed forms, including sauces, juices, and purées. More recently, tomatoes have been consumed in dehydrated forms, such as dried tomatoes. The consumption of dried tomatoes as an ingredient in pizzas and pastas or as an appetizer is growing in Brazil.[ Citation 3 ] Dried tomatoes can be obtained through different drying techniques under different time, temperature, light exposure, and oxygen conditions. Therefore, dried tomato products available in the retail market have significant differences in their content of antioxidant compounds and, consequently, antioxidant potential.[ Citation 1 ] The benefits of dried tomatoes on human health vary among available retail products. There is still little information regarding the chemical characteristics and antioxidant potential of preserved dried tomatoes available in retail markets. Thus, the objective of this work was to evaluate the content of antioxidant compounds and total antioxidant activity in vitro of preserved dried tomatoes available in retail markets.
MATERIALS AND METHODS
Sample Collection and Preparation
The preserved (in oil) dried tomatoes were obtained at the central market of Belo Horizonte, Minas Gerais. Eight products were selected with three samples of each product from the same production lot. The products were designated with letters A, B, C, D, E, F, G, and H. The preserved dried tomatoes were placed in sieves (1 mm) for 10 min to drain the excess oil present in the preserves. Each sample was of dried tomato then triturated for 1 min in a microprocessor to obtain a homogeneous mass, which was then used in the chemical analyses. All analyses were performed in triplicate.
Moisture
Moisture was determined by gravimetric method in accordance with the rules of the Association of Official Analytical Chemists.[ Citation 20 ]
Lycopene and β-Carotene Levels
Lycopene and β-carotene levels were determined according to the method proposed by Nagata and Yamashita.[ Citation 12 ] The carotenoids were extracted using a mixture of acetone and hexane (4:6). The extracts were subjected to readings at different wavelengths (453, 505, 645, and 663 nm) using a spectrophotometer. The lycopene and β-carotene concentrations of the samples were calculated according to the following equations: lycopene (mg/100 mL) = 0.0458.A663 + 0.204.A645 + 0.372.A505 – 0.0806.A453 and β-carotene (mg/100 mL) = 0.216.A663 – 1.22.A645 – 0.304.A505 + 0.452.A453. The results were transformed to be expressed as μg.g−1.
Vitamin C
The ascorbic acid content was determined by the colorimeter method with 2,4 dinitrophenylhydrazine proposed by Strohecker and Henning.[ Citation 22 ] The reading was performed using a Beckman 640 B spectrophotometer with a computerized system, and the results were expressed as mg.100 g−1 of dried tomato pulp.
Phenolic Compounds
The total phenolic content in the hydrophilic extract was determined using the method proposed by Waterhouse,[ Citation 23 ] using the Folin-Ciocalteu reagent. Briefly, 0.5 mL of the sample extracts was added to tubes containing 2.5 mL of 10% (v/v) Folin-Ciocalteu solution. Two milliliters of a 4% (v/v) sodium carbonate solution was then added. The tubes were agitated and incubated for 120 min in the dark. The blue color produced by the reduction of the Folin-Ciocalteu reagent by the phenolic was spectrophotometrically measured at 750 nm. The phenolic content of the samples was calculated using the equation of the straight line obtained from the established gallic acid standard curve. The results were expressed as milligrams of gallic acid equivalent per 100 grams of sample (mg GAE.100 g−1 dried tomato pulp).
Antioxidant Activity
The total antioxidant activity of the dried tomatoes was determined using two different methods as follows: (1) the 2,2-diphenyl-1-picrylhydrazyl (DPPH) radical scavenging method and (2) the linoleic acid/β-carotene system. For the determination of the total antioxidant activity (TAA), two extracts corresponding to the hydrophilic and hydrophobic portion of the samples were prepared. To obtain the hydrophilic extract, 5 g of the homogenized sample was weighed, and 40 mL of 50% methyl alcohol was then added to the sample. The mixture was homogenized and incubated for 1 h at room temperature. After this period, the mixture was centrifuged at 23.723× g for 17 min. The supernatant was collected, and 40 mL of 70% acetone was added to the residue. The sample was then incubated for 1 h followed by centrifugation at 23.713× g for 17 min. The supernatant was collected and added to the first supernatant, and distilled water was added to the mixture to reach a final volume of 100 mL. To obtain the hydrophobic extract, 40 mL of ethyl ether was added to the hydrophilic extract residue. The mixture was homogenized and incubated for 1 h at room temperature followed by centrifugation at 23.713× g for 17 min, and the supernatant was collected. This procedure was repeated, and ethyl ether was added to reach a final volume of 100 mL.
The measurement of TAA by the DPPH radical scavenging method was conducted according to the methodology proposed by Rufino et al.[ Citation 24 ] with adaptations. Sample extracts (0.1 mL) or standard antioxidants (6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid, Trolox; 0.1 mL) were added at a concentration of 0.2 mg.mL−1 to 3.9 mL of the DPPH solution. After 30 min, readings at 515 nm were taken using a spectrophotometer, and the results were expressed as a free radical scavenging percentage (% FRS) according to the following equation: % FRS = (Ac - Am) * 100/Ac, where Ac is the absorbance of the control (0.1 mL of a solution containing 50% methanol and 70% acetone + 3.9 mL of DPPH solution) and Am is the absorbance of the sample.
To determine TAA by the β-carotene/linoleic acid system, the procedures proposed by Rufino et al.[ Citation 25 ] were adopted. Extracts (0.4 mL) were added to 5 mL of the system solution (β-carotene + linoleic acid + oxygenated water), and readings at 470 nm were taken after 2 min and 120 min using a spectrophotometer. The results were expressed as the inhibition percentage of β-carotene oxidation (%I) according to the following equation: %I = (Ac - Am) * 100/Ac, where Ac is the initial absorbance of the control—final absorbance of the control and Am is the initial absorbance of the sample—final absorbance of the sample. The Trolox, which is an antioxidant compound analogous to vitamin E, but hydrophilic, was used as a reference antioxidant at a concentration of 0.2 mg.mL−1. All of the chemical analyses were performed in triplicate.
Statistical Analysis
This study was conducted in a completely randomized design (CRD) with three replicates. In all, 8 treatments and 24 samples were used. Sisvar 5.0 software was used to analyze the data. The data were subjected to analysis of variance (ANOVA) and Tukey's test at 5% probability.
RESULTS AND DISCUSSION
The average moisture and antioxidant compound contents in the wet (fresh dried tomato) and dry matter (dehydrated dried tomato) of the dried tomato products are presented in . The moisture content varied considerably among the analyzed products. Products G and B have the highest and lowest moisture content, respectively.
Table 1 Average moisture and antioxidant compound contents in the wet matter (WM) and dry matter (DM) of preserved dried tomatoes
Considering the wet matter of preserved dried tomatoes, which is the form that consumers acquire and consume this product, the lycopene content was higher in products B and E and lower in product G. Product G had a high moisture content, which contributes to reduce lycopene concentration. The highest β-carotene content was found in products A and B, and the lowest β-carotene content was found in product F (). The difference in moisture between the products only partly explains the variation in the content of lycopene and other antioxidants analyzed in this study. The product A, for example, had one of the lowest moisture contents and still had one of the lowest lycopene contents. Moreover, in the dry matter was also found significant difference in the content of antioxidant compounds, which can be associated with other factors (). The products analyzed in this study were purchased from the retail market of Brazil. The producers do not provide information on the cultivar and origin of the tomatoes, tempo, and temperature drying, if was used osmotic dehydration as a pretreatment and stage of ripeness of tomatoes. All these factors may be associated with variation in the level of antioxidants and total antioxidant activity found in dried tomatoes analyzed in this study. The variation of lycopene and β-carotene contents in tomato products has also been observed by others. Markovic et al.[ Citation 26 ] observed extensive variation in the lycopene content of several tomato products (juice, purée, ketchup, pasta, and canned tomatoes) acquired in markets of Zagreb, Croatia. Baranska et al.[ Citation 27 ] found lycopene content variations of 2.62–60.40 mg.100 g−1 in fresh tomatoes, 18.80–100.87 mg.100 g−1 in ketchups, and 50.35–128.60 mg.100 g−1 in tomato puree. The results of the present study were in agreement with the values observed by Toor and Savage,[ Citation 28 ] who evaluated lycopene contents in dehydrated tomatoes of three cultivars, with moisture contents ranging between 78.2 and 84.1%, and they found significant differences in the lycopene contents among the cultivars with lycopene contents varying from 66.1 to 130.8 μg.g−1. Muratore et al.[ Citation 29 ] reported high lycopene (230.5 to 297.7 μg.g−1) and β-carotene (101.8 to 167.7 μg.g−1) contents in dehydrated cherry tomato under different temperatures and with a final moisture content ranging between 58 and 61%. The largest lycopene and β-carotene contents were observed in tomatoes subjected to dehydration at a low temperature (40°C). Several factors, such as light, oxygen, and high temperatures (>100°C), favored reduced carotenoid contents.[ Citation 30 ]
The total phenolic contents significantly varied among the studied products. Products E and H had the highest and lowest phenolic contents, respectively (). Toor and Savage[ Citation 28 ] found phenolic content values between 44.6 and 74.3 mg.100 g−1 in tomatoes semi-dehydrated; these results are lower than the phenolic contents found in the present study. This may be partly explained by the lower moisture content of the dried tomatoes analyzed in this study. To produce the dried tomatoes, the tomato is subjected to the thermal treatment, which favors the liberation of phenolic compounds and lycopene from the cellular matrix. Thus, phenolic compound and lycopene are more bioavailable in tomatoes subjected to thermal processing, such as dried tomato, which improves the nutritional value and functional properties of the tomatoes.[ Citation 31 ]
The vitamin C content () was highest in products C and E and lowest in products D, G, and H. Tomatoes are recognized as a good vitamin C source. However, vitamin C contents can substantially vary among different varieties. Guil-Guerrero and Rebolloso-Fuentes[ Citation 32 ] found vitamin C contents between 39 and 263 mg.100 g−1 in eight tomato varieties. Toor and Savage[ Citation 28 ] reported ascorbic acid contents similar to that of most of the dried tomato products in the present study, and they found vitamin C contents between 35.3 and 46.5 mg.100 g−1 in tomatoes dehydrated at 42°C for 19 h, with a final moisture content of approximately 80%. Muratore et al.[ Citation 29 ] reported vitamin C contents (between 43.9 and 150.9 mg.100 g−1) in dehydrated cherry tomato under different temperatures (moisture contents ranging between 58 and 61%) that on average were greater than the contents found in the present study. George et al.[ Citation 14 ] observed an 80% reduction in the vitamin C content of thermally processed tomatoes. Together, these results demonstrate the wide variation of vitamin C contents in processed tomato products. Temperature and air are factors that most affect the retention of vitamin C in tomato products subjected to heating, such as dried tomatoes.[ Citation 14 ,Citation 31 ] Thus, the variation of vitamin C contents in the products analyzed in this study may be associated with the use of different processing conditions and raw materials having of various initial vitamin C contents. However, the vitamin C content in the dried tomato products in this study allowed them to be considered good sources of vitamin C.
The presence of phenolic compounds, ascorbic acid, and lycopene places tomatoes among the foods with high antioxidant potential.[ Citation 9 ] However, the various techniques used to dehydrate tomatoes can significantly affect the concentration of their antioxidant components and antioxidant activity.[ Citation 33 ] The differences in the composition of the products analyzed may be the result of several of the following factors: the use of different cultivars by the producers; the origin of the tomato (cultivation site); the use of osmotic dehydration as a pretreatment; and the drying conditions, such as time and temperature, used by the producers. The drying conditions (time, temperature, light, and oxygen) of the products analyzed in the present study were not known. However, the results indicated a lack of standardization of the techniques used to obtain the dried tomatoes and/or use of the tomatoes of different cultivars and origin, which resulted in products with different moisture levels and antioxidant compounds. These differences cause difficulties for nutritionists when quantifying the product's contribution to human diets. Considering the results presented in , it is recommended that producers add the content of antioxidant compounds to the nutritional labeling of the product so that consumers can consider such information when purchasing the product.
The total antioxidant activity (TAA) of the hydrophilic and hydrophobic extracts of the preserved dried tomatoes as measured by the DPPH free radical scavenging method is shown in . In general, the hydrophilic extracts had higher capacities to sequester DPPH radicals than the hydrophobic extracts (). A similar situation for hydrophilic and hydrophobic extracts of tomatoes and/or tomato products has been observed by others.[ Citation 9 ,Citation 34 ] In contrast, it has been reported that hydrophobic extracts have a higher capacity than hydrophilic extracts to sequester DPPH radicals.[ Citation 35 ]
Figure 1 Total antioxidant activity (TAA) of the hydrophilic and hydrophobic extracts of the preserved dried tomatoes as measured by the DPPH free radical scavenging method. The extract concentration was 1.25 mg.mL−1. Columns with the same shading and same letters do not differ among themselves according to Tukey's test at 5% probability. Lowercase letters compare the TAA values of the hydrophilic extracts, and the uppercase letters compare the TAA values of the hydrophobic extracts.
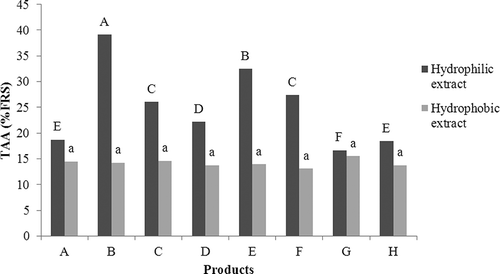
There was no significant difference (p < 0.05) among the antioxidant activities of the hydrophobic extracts of the analyzed products. The free radical scavenging percentage (%FRS) of the hydrophobic extracts (rich in carotenoids) varied from 13.13 to 15.56%. However, the antioxidant activities of the hydrophilic extracts (rich in phenolic compounds and ascorbic acid) presented significant differences, with product B presenting the highest antioxidant activity (%FRS = 39.15%) and product G presenting the lowest antioxidant activity (%FRS = 16.66%) (). Trolox, an antioxidant compound similar to vitamin E but hydrophilic in nature, was used as a reference antioxidant. Trolox at a concentration of 0.2 mg.mL−1 presented an average %FRS of 94.67%.
The total antioxidant activity of a given food item is the result of the activities of each antioxidant component of that food item. Furthermore, the antioxidant components of a food item can interact among themselves to produce synergistic or inhibitory effects.[ Citation 13 ,Citation 35 ] Thus, the total antioxidant activity of a food item can be higher or lower than the sum of the antioxidant activity of each compound when separately considered. Another important aspect of food item antioxidant activity is that it substantially varies with the concentrations of the extracts and the polarity of the solvents used for the extraction of the antioxidant compounds.[ Citation 36 ]
In this work, products A, G, and H had lower antioxidant activity in their hydrophilic extracts, and these products also had lower phenolic compound levels. Phenolic compounds are potent antioxidant agents, and the antioxidant activity observed in various plants is directly related to the presence of these compounds.[ Citation 37 ] Heating can induce the formation of compounds, such as melanoidins in the Maillard reaction, and these compounds can have some antioxidant activity, contributing to the TAA of the final product.[ Citation 28 ]
The TAA of the hydrophilic and hydrophobic extracts of the preserved dried tomatoes evaluated by the β-carotene/linoleic acid system is shown in . Unlike the results observed using the DPPH method, the hydrophobic extracts had higher TAA values than the hydrophilic extracts as measured by the β-carotene/linoleic acid system. The hydrophobic extracts of all of the products had high TAA values with inhibition percentages ranging between 72.5 and 86.65%. In the β-carotene/linoleic acid system, the standard antioxidant, Trolox, had an average inhibition percentage (%I) of 79.93%. Products F, G, and H had inhibition percentages similar to the standard antioxidant percentage, which indicated that the concentration of the extract was approximately six times higher than the concentration of Trolox. The hydrophobic extract of dried tomatoes is rich in apolar compounds, such as β-carotene and lycopene. According to Basuny et al.,[ Citation 38 ] lycopene is highly efficient in neutralizing the radicals that result from lipid peroxidation, which protects the cell membranes and lipoproteins.
Figure 2 Total antioxidant activity (TAA) of the hydrophilic and hydrophobic extracts of the preserved dried tomatoes as measured by the β-carotene/linoleic acid method. The extract concentration was 1.25 mg.mL−1. Columns with the same shading and same letters do not differ among themselves according to Tukey's test at 5% probability. The lowercase letters compare the TAA of the hydrophilic extracts, and the uppercase letters compare the TAA of the hydrophobic extracts.
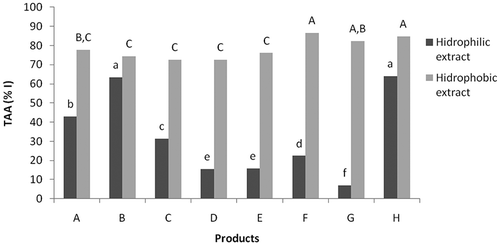
The results of the present study were in agreement with findings reported by Shen et al.,[ Citation 16 ] who found high inhibition of lipid peroxidation in tomato extracts with high lycopene levels compared to extracts with high content of phenolic compounds or ascorbic acid. These authors also reported inhibition percentages of lipid peroxidation that were equal to 76.1% for extracts rich in lycopene and 56.9% for extracts containing phenolic compounds at a concentration of 0.5 mg.mL−1.
The hydrophilic extracts of the products had different antioxidant activities. While the extracts of products B and H presented a high inhibition percentage of lipid peroxidation (>60%), the hydrophilic extracts of products D, E, F, and G presented a low inhibition percentage of lipid oxidation (<22%). According to Lavelli et al.,[ Citation 39 ] tomato processing increases the antioxidant activity of the lipophilic fraction and reduces the antioxidant activity of the hydrophilic fraction in relation to fresh tomatoes.
According to Martinez-Valverde et al.,[ Citation 4 ] the antioxidant activity of tomatoes depends on the solvent used for extraction and the analytical method. Furthermore, no isolated antioxidant component can explain the total antioxidant activity of tomatoes, which is due to the synergistic effects among the antioxidants present.
Dried tomatoes are produced without the removal of the exocarp, which is the fraction that proportionally possesses the highest lycopene content and antioxidant activity of the tomato.[ Citation 28 ] Moreover, heat processing may cause an increased release of lycopene from the cell matrix. Thus, dried tomatoes can have higher antioxidant potential than tomatoes fresh.
Several methods have been used to determine the antioxidant activity in the foods. However, there is no method that is universal for all plant and food extracts. The existence of various methods to evaluate antioxidant activity causes difficulty in the selection of the most appropriate methodology for a specific study. Furthermore, different assay conditions (concentration, pH, oxidation time, temperature, and oxygenation) hinder the interpretation and comparison of the obtained results.[ Citation 40 ]
Thus, it should be emphasized that in vitro tests allow a good evaluation of the antioxidant potential of foods. However, it is necessary to test these foods in the human diet for to evaluate antioxidant effect in the blood and cellular level.
CONCLUSIONS
The products analyzed in this study can be considered good sources of antioxidant compounds because they contain significant amounts of vitamin C, phenolics, and carotenoids, especially lycopene. The obtained data indicated that there is a lack of standardization in dried tomato processing, which generates products with different characteristics, including humidity levels, antioxidant compounds, and antioxidant activities. The total antioxidant activity was dependent on the solvent and method used. The hydrophilic extracts presented higher TAA values than the hydrophobic extracts in the DPPH radical scavenging method, and the hydrophobic extracts had higher TAA values than the hydrophilic extracts in the β-carotene/linoleic acid system.
The dried tomatoes had high antioxidant potential when considering the total antioxidant activity of the hydrophobic extract as measured by the β-carotene/linoleic acid method.
ACKNOWLEDGMENTS
The authors wish to thank the National Council for Scientific and Technological Development (CNPq) for their financial support.
REFERENCES
- Oriozola-Serrano , I. , Soliva-Fortuny , E. , Hernández-Jover , T. and Martín-Belloso , O. 2009 . Carotenoid and phenolic profile of tomato juices processed by high intensity pulsed electric fields compared with conventional thermal treatments . Food Chemistry , 112 : 258 – 266 .
- Ordonez-Santos , L.E. , Vazquez-Oderiz , M.L. and Romero-Rodriguez , M.A. 2011 . Micronutrient contents in organic and conventional tomatoes (Solanum lycopersicum L.) . International Journal of Food Science and Technology , 46 : 1561 – 1568 .
- Bugianese , R. , Salucci , M. , Leonardi , C. , Ferracane , R. , Catasta , G. , Azzini , E. and Maiani , G. 2004 . Effect of domestic cooking on human bioavailability of naringenin, chlorogenic acid, lycopene and carotene in cherry tomatoes . European Journal of Nutrition , 43 : 360 – 366 .
- Martinez-Valverde , I. , Periago , M.J. , Provan , G. and Chesson , A. 2002 . Phenolic compounds, lycopene and antioxidant activity in commercial varieties of tomato (Lycopersicum esculentum) . Journal Science Food Agriculture , 82 : 323 – 330 .
- Herken , E.M. and Guzel , S. 2010 . Total antioxidant capacity and total phenol contents of selected commercial fruit juices in Turkey . International Journal of Food Properties , 13 : 1373 – 1379 .
- Roldán-Gutiérrez , J.M. and Castro , M.D.L. 2007 . Lycopene: The need for better methods for characterization and determination . Trends in Analytical Chemistry , 26 : 163 – 170 .
- Heredia , A. , Peinado , I. , Barrera , C. and Andrés Grau , A. 2009 . Influence of process variables on colour changes, carotenoids retention and cellular tissue alteration of cherry tomato during osmotic dehydration . Journal of Food Composition and Analysis , 22 : 285 – 294 .
- Chang , C.H. and Liu , Y.C. 2007 . Study on lycopene and antioxidant contents variations in tomatoes under air-drying process . Journal of Food Science , 72 : 532 – 540 .
- Djuric , Z. and Powell , L.C. 2001 . Antioxidant capacity of lycopene-containing foods . International Journal of Food Sciences and Nutrition , 52 : 143 – 149 .
- Abushita , A.A. , Hebshi , E.A. , Daood , H.G. and Biacs , P.A. 1997 . Determination of antioxidant vitamins in tomatoes . Food Chemistry , 60 : 207 – 212 .
- Sahlin , E. , Savage , G.P. and Lister , C.E. 2004 . Investigation of the antioxidant properties of tomatoes after processing . Journal of Food Composition and Analysis , 17 : 635 – 647 .
- Fantini , A.P. , Canniatti-Brazaca , S.G. , Souza , M.C. and Mansi , D.N. 2008 . Disponibilidade de ferro em misturas de alimentos com adição de alimentos com alto teor de vitamina C e de cisteína . Ciência e Tecnologia de Alimentos , 28 : 435 – 439 .
- Sanchez-Moreno , C. , Plaza , L. , Ancos , B. and Cano , M.P. 2006 . Nutritional characterisation of commercial traditional pasteurised tomato juices: carotenoids, vitamin C and radical-scavenging capacity . Food Chemistry , 98 : 749 – 756 .
- George , S. , Tourniaire , F. , Gautier , H. , Goupy , P. , Rock , E. and Caris-Veyrat , C. Changes in the contents of carotenoids, phenolic compounds and vitamin C during technical processing and lyophilisation of red and yellow tomatoes . Food Chemistry , 2011 ( 124 ) 1603 – 1611 .
- Duarte-Almeida , J.M. , Santos , R.J. , Genovese , M.I. and Lajolo , F.M. 2006 . Avaliação da atividade antioxidante utilizando sistema β-Caroteno/ácido linoléico e método de seqüestro de radicais DPPH• . Ciência e Tecnologia de Alimentos , 26 : 446 – 452 .
- Shen , Y.C. , Chen , S.L. and Wang , C.K. 2007 . Contribution of tomato phenolics to antioxidation and down-regulation of blood lipids . Journal of Agricultural and Food Chemistry , 55 : 6475 – 6480 .
- Das , S. , Das , N. and Srivastava , L.M. 2006 . Role of ascorbic acid on in vitro oxidation of low-density lipoprotein derived from hypercholesterolemic patients . Clinica Chimica Acta , 372 : 202 – 205 .
- Du , G. , Li , M. , Ma , F. and Liang , D. 2009 . Antioxidant capacity and the relationship with polyphenol and vitamin C in Actinidia fruits . Food Chemistry , 113 : 557 – 562 .
- Leong , L.P. and Shui , G. 2002 . An investigation of antioxidant capacity of fruits in Singapore markets . Food Chemistry , 76 : 69 – 75 .
- Association of Official Analytical Chemists . 1998; 1094 p . Official Methods of the Association of the Agricultural Chemists , AOAC : Washington .
- Nagata , M. and Yamashita , I. 1992 . Simple method for simultaneous determination of chlorophyll and carotenoids in tomato fruit . Nippon Shokuhin Kogyo Gakkaishi , 39 : 925 – 928 .
- Strohecker , R.L. and Henning , H.M. 1967 . Analisis de Vitaminas: Métodos Comprobados , 428 p Madri : Paz Montalvo .
- Waterhouse , A.L. 2002 . “ Polyphenolics: Determination of total phenolics ” . In Current Protocols in Food Analytical Chemistry Edited by: Wrolstad , R.E. and John , Wiley . 11 – 18 . New York
- Rufino , M.S.M. , Alves , R.E. , Brito , E.S. , Morais , S.M. , Sampaio , C.G. , Pérez-Jiménez , J. and Saura-Calixto , F.D. 2007 . Metodologia científica: Determinação da atividade antioxidante total em frutas pela captura do radical livre DPPH . EMBRAPA Comunicado Técnico , 127 : 1 – 4 .
- Rufino , M.S.M. , Alves , R.E. , Brito , E.S. , Filho , J.M. and Moreira , A.V.B. 2006 . Metodologia científica: Determinação da atividade antioxidante total em frutas no sistema β-caroteno/Ácido Linoléico . EMBRAPA Comunicado Técnico , 126 : 1 – 4 .
- Markovic , K. , Hruskar , M. and Vahcic , N. 2006 . Lycopene content of tomato products and their contribution to the lycopene intake of Croatians . Nutrition Research , 26 : 556 – 560 .
- Baranska , M. , Tze , W.S. and Shulz , H. 2006 . Determination of lycopene and β-carotene content in tomato fruits and related products: Comparison of FT-Raman, ATR-IR, and NIR spectroscopy . Analytical Chemistry , 78 : 8456 – 8461 .
- Toor , R.K. and Savage , G.P. 2006 . Effect of semi-drying on the antioxidant components of tomatoes . Food Chemistry , 94 : 90 – 97 .
- Muratore , G. , Rizzo , V. , Licciardello , F. and Maccarone , E. 2008 . Partial dehydration of cherry tomato at different temperature and nutritional quality of the products . Food Chemistry , 111 ( 4 ) : 887 – 891 .
- Colle , I.J.P. , Lemmens , L. , Tolesa , G.N. , Vanbuggenhout , S. , De Leeschouwer , K. , Van Loey , A.M. and Hendrickx , M.E. 2010 . Lycopene degradation and isomerization kinetics during thermal processing of an olive oil/tomato emulsion . Journal of Agricultural and Food Chemistry , 58 : 12784 – 12789 .
- Dewanto , V. , Wu , X. , Adom , K.K. and Liu , R.H. 2002 . Thermal processing enhances the nutritional value of tomatoes by increasing total antioxidant activity . Journal of Agricultural and Food Chemistry , 50 : 3010 – 3014 .
- Guil-Guerrero , J.L. and Rebolloso-Fuentes , M.M. 2009 . Nutrient composition and antioxidant activity of eight tomato (Lycopersicon esculentum) varieties . Journal of Food Composition and Analysis , 22 : 123 – 129 .
- Yahia , E. , Soto-Zamorra , G. , Brencht , J.K. and Gardea , A. 2007 . Postharvest hot air treatment effects on the antioxidant system in stored mature-green tomatoes . Postharvest Biology and Technology , 44 : 107 – 115 .
- Larrosa , M. , Espín , J.C. and Tomás-Baberon , F.A. 2003 . Antioxidant capacity of tomato juice functionalised with enzymatically synthesized hydroxytyrosol . Journal Science Food Agricultural , 83 : 658 – 666 .
- Ishida , B.K. and Chapman , M.H. 2004 . A comparison of carotenoid content and total antioxidant activity in catsup from several commercial sources in the United States . Journal of Agricultural and Food Chemistry , 52 : 8017 – 8020 .
- Kuskoski , E.M. , Asuero , A.G. , Troncoso , A.M. , Mancini-Filho , J. and Feet , R. 2005 . Aplicación de diversos métodos químicos para determinar actividad antioxidant en pulpa de frutos . Ciência e Tecnologia de Alimentos , 25 : 726 – 732 .
- Fang , Z. , Zhang , Y. , Lu , Y. , Ma , G. , Chen , J. , Liu , D. and Ye , X. 2009 . Phenolic compounds and antioxidant capacities of bayberry juices . Food Chemistry , 113 : 884 – 888 .
- Basuny , A.M. , Gaafar , A.M. and Arafat , S.M. 2009 . Tomato lycopene is a natural antioxidant and can alleviate hypercholesterolemia . African Journal of Biotechnology , 8 : 6627 – 6633 .
- Lavelli , V. , Peri , C. and Rizzolo , A. 2000 . Antioxidant activity of tomato products as studied by model reaction using xanthine oxidase, myeloperoxidase, and koper-induced lipid peroxidation . Journal of Agricultural and Food Chemistry , 48 : 1442 – 1448 .
- Niki , E. 2002 . Antioxidant activity: Are we measuring it correctly? . Nutrition , 16 : 524 – 525 .
