Abstract
Leaves and stem bark composition from Morus species (M. alba var. alba, M. alba var. rosa and M. rubra) was evaluated for its antioxidant and antimicrobial activities in order to enhance its therapeutic uses. Total phenolics and flavonoids contents were estimated in hydromethanolic and aqueous extracts. Results showed highest content in M. rubra leaves aqueous extract (1129 mg gallic acid equivalent/100 g dry weight and 816 mg gallic acid equivalent/100 g dry weight, respectively). Using 2,2-diphenyl-1-picrylhydrazyl assay, the highest antioxidant activity was obtained in aqueous stem barks extracts. M. alba var. alba has an IC50 of 2.84 mg/ml and the IC50 value of M. rubra was the highest (4.78 mg/ml). ABTS.+ (2,2'-azinobis-(3-ethylbenzothiazoline-6-sulfonic acid)) method and reducing power assay were used to confirm the results from the 2,2-diphenyl-1-picrylhydrazyl test. All extracts expressed considerable free radical-scavenging properties. Hydromethanolic and aqueous extracts were tested against Staphylococcus aureus, Enterococcus feacalis, Staphylococcus epidermis, Escherichia coli, and Salmonella Typhimurium bacteria. Hydromethanolic stem bark extracts have the highest antimicrobial activities, and it may be a good antimicrobial agent for human gastrointestinal infections. This plant could be used as an additive to foods and also as a possible source to obtain new and effective herbal medicines to treat infections of multi-drug resistant strains of microorganisms.
INTRODUCTION
Phytotherapy is applied worldwide for certain pathologies. However, in Africa, it is an important tool for treating people not only when other medicines are not available, but also because it is a cheap way for the treatments to be done. Morus is one of the medicinal plants used in Tunisia for different purposes, for example, as an anti-diabetic, an anti-hypertensive, an anti-inflammatory, and a vermifuge. Until now, the compounds and the pharmacological pathways of the therapeutic activity of these Tunisian mulberry extracts are unclear, but the relevance of this knowledge is huge, especially in techniques that allowed doctors to have their scientific base and quality control.
About 100 species of Morus have been described, but Morus taxonomy is complicated and there were many groups using different names. Many species are generally accepted, such as Morus alba (white mulberry; eastern Asia), Morus rubra (red mulberry; eastern North America), Morus nigra (black mulberry; southwest Asia), Morus mesozygia (African mulberry; southern and central Africa), etc. The mulberry plant is a monoecious or dioecious plant, growing up to 10–12 m high. It is widely distributed in India, China, Japan, North Africa, South Europe, etc. It possesses a number of pharmaceutical properties useful in treating many serious diseases like diabetes mellitus, atherosclerosis, hyperlipidemia, hypertension, etc. The Chinese Pharmacopoeia lists leaves, root bark, branches, and fruits as ingredients in medicinal preparations, but other parts, including the sap and wood ash, were also widely used.
Mulberry leaves are used as anti-hyperglycemic nutraceutical food for patients with diabetes mellitus, and they prevented throat infections, irritations and inflammations. In Japan, the mulberry leaf was used as tea and its consumption has been increasing.[Citation1] The stem bark was consumed as purgative, vermifuge and anti-diabetic,[Citation2] and the root barks have anti-helmentic and cathartic properties.[Citation3] Mulberry is with limited requirements, drought resistance ability, and capable of growing in different soil types. Hence, mulberry is widespread throughout Tunisia. Currently, mulberry crops have regressed despite its therapeutic and nutritional virtues (vitamin C, vitamin E, anthocyanins). It can easily be used in traditional medicine and thus treats some diseases (such as diabetes type 2) without resorting to drugs.
The use of plants for medicinal purposes has been practiced for many centuries by all populations. Subsequently, as a source of pharmacologically active compounds, particularly in the search for drugs for many diseases, plants continue to be used with phytotherapeutic activities and other industrial applications. One important activity for some plants is free radical-scavenging power, which is also crucial for the food industry.[Citation4] In order to evaluate the therapeutic potential of the mulberry tree, the present study was conducted to compare the phenolic composition, antioxidant, and antimicrobial activities of leaves and stem bark from three Tunisian Morus species: Morus alba var. alba, Morus alba var. rosa, and Morus rubra.
MATERIALS AND METHODS
Plant Material
Leaves and stem bark of Morus alba var. alba, Morus alba var. rosa, and Morus rubra L. were harvested during May 2010 from mulberry trees in Gabes Province (southern Tunisia: 33° 40′ N, 10° 15′ E). Plant material was collected, immediately put in aluminum foil on ice, and stored at –20°C in darkness in the laboratory upon arrival. Then, it was washed with distilled water and lyophilized. The dried materials were ground into powder with a blender, sieved through an 80-mesh sieve, and stored in an air-tight container at –20°C until use.
Extract Preparation
Approximately 3 g of lyophilized leaves and stem barks of Morus alba var. alba, Morus alba var. rosa, and Morus rubra L. were extracted with 100 ml 50/50 water/methanol (hydromethanolic extract) in a water bath at 80°C for 15 min. The same amount (3 g) of lyophilized leaves and stem bark was extracted with water. Respectively, hydromethanolic and aqueous phases were filtered and concentrated under vacuum to give the crude extracts that were resolved in 4 ml of the extract solvent and stored at –20°C prior to experimentation.
Determination of Total Phenolics and Flavonoids Content
Quantification of total phenolic content was determined using Folin-Ciocalteau method reported by Elfalleh et al.[Citation5] Briefly, the samples (0.1 ml) were adjusted to 1 ml with distilled water and then mixed with 0.5 ml Folin-Ciocalteau reagent and 4 ml sodium carbonate solution (Na2CO3, 1 M). The tubes were vortexed and incubated at 45°C for 15 min. Afterward, absorption was measured at 760 nm. All measurements were performed in triplicate. The total phenolic contents of the extracts were expressed as mg gallic acid equivalents (GAE) per 100 g dry weight (DW; mg GAE/100 g DW).
Total flavonoid amounts in extracts were measured spectrophotometrically.[Citation6,Citation7] The method is based on the formation of a complex flavonoid–aluminum, having the maximum absorbance at 430 nm. The samples (0.1 ml) were adjusted to 1 ml with distilled water and then mixed with 0.5 ml of 2% methanolic aluminium chloride solution. After 15 min at room temperature, the absorbance was measured at 430 nm. Total flavonoid contents were calculated as rutin equivalent (RE) from a calibration curve (mg RE/100 g DW).
Antioxidant Activities
2,2-Diphenyl-1-picrylhydrazyl (DPPH) radical-scavenging activity
The DPPH radical-scavenging activity of the extracts, which were dissolved in methanol, was determined according to the modified method reported by Elfalleh et al.[Citation5] Several concentrations from original extract were prepared (0.5, 1, 2, 4, 6, and 7 mg/ml). Each solution was mixed with 1 ml DPPH solution (250 μM) and the mixture was shaken vigorously and left to stand for 30 min in the dark. Remaining DPPH amount, which is characterized by a decrease in absorption and a bleaching of its purple color, was measured at 517 nm using a spectrophotometer (Spectro UV–VIS Auto, Labomed Inc., CA, USA). The radical-scavenging activity was calculated as percent inhibition from the following equation:
ABTS Radical-Scavenging Activity
The total antioxidant activity (TAA) values were estimated by the Trolox equivalent antioxidant capacity (TEAC) test.[Citation8] In this test, the relative capacity of antioxidants was measured to scavenge the ABTS.+ (2,2'-azinobis-(3-ethylbenzothiazoline-6-sulfonic acid)) radical compared to the antioxidant potency of Trolox is used as a standard. ABTS.+ was generated by oxidation of ABTS with potassium persulfate. The ABTS.+ radical generated by mixing 7 mM ABTS solution with 2.45 mM K2S2O8 in the dark for 24 h, at room temperature. Before usage, the ABTS.+ solution was diluted with ethanol to get an absorbance of 0.700 ± 0.020 at 734 nm. Twenty-five μl of antioxidant sample or Trolox standard was added to 1 ml of the diluted ABTS.+ solution. The reaction mixture was vortexed for 20 s and then the absorbance was recorded at 734 nm at 5 min. The final TEAC value of the antioxidant compound was calculated by comparing ABTS.+ decolourisation with Trolox, which gives a useful indication of the antioxidant potential of the plant extracts. Measurement was performed in triplicate, and results are expressed as means ± standard deviations.
Reducing Power Assay
The reducing power was determined according to the method of Chu et al.[Citation9] Various concentrations of hydromethanolic and aqueous extracts of leaves and stem barks (2.5 ml) were mixed with 2.5 ml of 0.2 M sodium phosphate buffer (pH 6.6) and 2.5 ml of 1% potassium ferricyanide. The mixture was incubated at 50°C for 20 min. Afterward, 2.5 ml of 10% trichloroacetic acid (w/v) was added, and the mixture was centrifuged at 650 rpm for 10 min; 2.5 ml of supernatant was mixed with 2.5 ml deionised water and 0.5 ml of 0.1% of ferric chloride, and the absorbance was measured at 700 nm. Increased absorbance of the reaction mixture indicated increased reducing power. The assays were carried out in triplicate, and the results are expressed as mean values ± standard deviations.
Screening for Antibacterial Activity
Screening for antibacterial activity was carried out using 24 h cultures of Staphylococcus aureus ATCC 25923, Enterococcus feacalis ATCC 29212, Staphylococcus epidermis CIP 06510, Escherichia coli ATCC 35218, and Salmonella Typhimurium ATCC 14028. Activity of the above-mentioned extracts was tested separately using disc diffusion method,[Citation10] which is one of the most used susceptibility testing methods worldwide. The medium (Müeller–Hinton Agar Medium) was sterilized by autoclaving at 120°C. About 30 ml of the medium was transferred aseptically into each sterilized Petri plate. The plates were left at room temperature for solidification. The extracts were freshly reconstituted with suitable solvents (methanol/water [v/v] and distilled water) and tested at various concentrations. Standard discs (6 mm diameter) were impregnated with 12.5 μl of different concentrations of plant extracts and placed on Müeller–Hinton Agar Medium already inoculated with 100 μl of bacterial suspension. Methanol and distilled water were used as negative controls. Standard disc (6 mm diameter) with ampicillin (10 μg/ml) and nalidixic acid (30 μg/ml) were used as a positive controls for antibacterial activity. Plates were incubated at 37°C for 24 h. Antimicrobial activity was evaluated by measuring the zone of inhibition against the tested bacteria. All tests were performed in triplicate.
Statistical and Chemometric Methods
All tests were carried out in triplicate, and the results were presented as means ± SD. Differences at p < 0.05 were considered statistically significant. Cultivar values for each compound were compared to the mean of all cultivars by calculating a confidence interval. An analysis of variance (ANOVA) was used to compare mulberry species.
RESULTS AND DISCUSSION
Total Phenolic and Flavonoid Contents
Hydromethanolic and aqueous extracts were evaluated for their total and flavonoids amounts. Indeed, many authors suggested that the maximum antioxidant yield was obtained with methanol compared to other solvents.[Citation11,Citation12] shows the amount of total phenolics and total flavonoids from the different extracts of leaves and stem barks. In the hydromethanolic leaf extracts, total phenolics contents ranged from 345.20 (M. alba var. rosa) to 631.53 mg GAE/100 g DW basis (M. rubra). In aqueous extract, the amounts varied between 759 GAE/100 g DW in M. alba var. alba and 1129 mg GAE/100 g DW in M. rubra. For the stem barks, hydromethanolic and aqueous extracts of M. alba var. alba revealed the highest total phenolics (303.73 and 807 mg GAE/100 g DW, respectively). In leaves, the highest total flavonoid contents were detected in M. rubra (398.33 and 816 RE/100 g DW for hydromethanolic and aqueous extract, respectively). For the stem bark, the highest flavonoid amounts were quantified in hydromethanolic and aqueous extracts of M. alba var. alba (173 mg RE/100 g DW and 450 mg GAE/100 g DW in methanolic and aqueous extract, respectively).
Table 1 Total phenolics and flavonoids content in Morus alba var. alba, Morus alba var. rosa, and Morus rubra extracts
These results revealed a higher total phenolic in mulberry leaf extracts compared to stem bark extracts. Furthermore, important total phenolic and flavonoid contents were obtained with water extracts derived from leaf portions. Our results differ from other authors who reported that methanol was found to be the most effective solvent in extraction of total phenolics from Morus indica leaves compared to acetone and water.[Citation13] Zhishen et al.[Citation14] suggested that the flavonoid content of M. alba leaves, collected from different Japanese regions, was 2645 mg RE/100 g DW; this value is significantly higher than that observed in the present study. This difference may be due to many factors such as drought, pollution, UV light, and pathogen attacks, as suggested by many authors.[Citation15,Citation16] Indeed, climate change can increase the production of phenolic compounds.[Citation17]
On the other hand, total phenolic content in M. nigra and M. alba stems, collected in spring, revealed respective levels ranging from 500 to 1200 mg GAE/100 g and from 500 to 800 mg GAE/100 g fresh weight.[Citation18] This confirms the preference of leaves over stem barks by herbal medicine practitioners. In addition, the total phenolic and flavonoid contents in M. alba fruits were, respectively, 181 mg GAE/100 g and 29 mg RE/100 g.[Citation19] Therefore, it is noted that the contents of flavonoids in the studied leaves and stem barks are more important than in fruits. This implied that bioactive compounds from leaves and stem barks might be potential natural sources for the development of antioxidant function in dietary food.
Methanol is commonly used in the laboratory; however, water has been used to prepare decoctions. Results obtained with water show that different studied parts of mulberry contained a good quantities of natural antioxidants (). This can support the use of mulberry decoctions by the elderly as natural anti-diabetics, anti-hypertensives, anti-inflammatories, and vermifuges. These results encourage further study of the possibility of improving the extraction of natural antioxidants using water and avoiding organic solvents. Indeed, a suitable extracting procedure should be developed and improved to recover more natural antioxidants for possible application in health-promoting supplements and for the pharmaceutical and food industries.
Antioxidant Activity
For a long time, natural products were simply the drugs available, and in modern medicine, it is the main element displaying industrial drugs activities. Numerous studies have suggested that phenolic compounds are responsible for the antioxidant activity in plants.[Citation20] This activity has been attributed to the redox properties, which act as a reducing agent, in addition to acting as a hydrogen donor, singlet oxygen quencher, and metal chelator.[Citation21] In the present study, three methods were used to evaluate the antioxidant capacities of leaves and stem bark from Morus species. The free radical-scavenging activity was determined by DPPH and ABTS tests and these two methods were confirmed by reducing power assay.
Table 2 Antioxidant effect (IC50) of Morus alba var. alba, Morus alba var. rosa, and Morus rubra extracts in DPPH radical scavenging and ABTS+ radical scavenging assays
DPPH-ABTS Radical-Scavenging Activities
The radical-scavenging activity on DPPH was expressed as IC50. This value was the concentration of the extract required to inhibit 50% of the initial DPPH free radical. The IC50 of all extracts is shown in . Leaf and stem bark extracts possess obviously different antioxidant activities. In hydromethanolic leaf extracts, the IC50 values varied between 3.62 and 6.95 mg/ml for M. rubra and M. alba var. rosa, respectively. IC50 of aqueous leaves extracts ranged from 3.55 mg/ml in M. rubra and 5.59 mg/ml in M. alba var. alba. Hydromethanolic stem bark extracts showed the lowest IC50 (3.20 mg/ml) in M. alba var. alba, and the highest value (5.39 mg/ml) was observed in M. rubra. The same result was observed in stem bark aqueous extract. M. alba var. alba show an IC50 of 2.84 mg/ml, and the IC50 value of M. rubra was 4.78 mg/ml.
Arabshahi-Delouee and Urooj[Citation13] reported that M. alba and M. rubra leaves showed IC50 values of 119.16 and 94.66 μg/ml respectively. They showed that methanolic Morus indica leaf extract was found to be the most active radical scavenger with an IC50 value of 79.53 μg/ml. In other studies, mulberry green tea revealed a concentration higher than 100 μg/ml, 12% of DPPH radical.[Citation22] DPPH radical-scavenging activity (IC50) of M. alba and M. nigra stem extracts ranged, respectively, from 80 to 100 μg/ml and from 80 to 90 μg/ml.[Citation18] Higher scavenging activity has been reported in pomegranate juice (Punica granatum L.), where IC50 values varied between 15.98 and 23.98 μg/ml.[Citation5]
The results of DPPH radical-scavenging assay revealed that the extracts, by hydrogen and/or electron donation, might prevent reactive radical species from reaching biomolecules such as lipoproteins, polyunsaturated fatty acids (PUFA), DNA, amino acids, proteins, and sugars in susceptible biological and food systems.[Citation23] The ABTS free radical assay can be used to measure the antioxidant activity of a broad diversity of substances, e.g., both aqueous phase radicals and lipid peroxyl radicals.[Citation24] In this experiment, the ABTS method was used to confirm the results from the DPPH test since it is based on a similar antioxidant mechanism. The scavenging activity of the extracts on free radical ABTS, generated by potassium persulfate, was compared with a standard amount of Trolox. The results, calculated as TEAC, are shown in . All extracts expressed a free radical-scavenging property, but in different degrees.
Hydromethanolic leaves and stem bark extracts show TEAC values ranged, respectively, from 0.84 mmol/100 g DW in M. alba var. rosa to 1.48 mmol/100 g DW in M. rubra, and from 1.29 mmol/100 g DW in M. alba var. rosa to 1.80 mmol/100 g DW in M. alba var. alba. Aqueous leaves and stem bark extracts show TEAC values between 1.32 mmol/100 g DW in M. alba var. rosa and 1.72 mmol/100 g DW in M. rubra and between 0.96 mmol/100 g DW in M. rubra to 1.88 mmol/100 g DW in M. alba var. rosa, respectively. The lowest TEAC value, which indicated the weakest antioxidant activity, was obtained from hydromethanolic M. alba var. rosa leaf extract. This was in agreement with DPPH assay and total phenolics contents.
As a result, the ability of extracts to scavenge different free radicals in different systems shows that they may be useful phytotherapeutic agents for treating radical-related pathological damage. Through their important antioxidant composition, mulberry leaves can be considered as protective against oxidation of low-density lipoprotein (LDL), and therefore against atherosclerosis.[Citation25] However, the stem barks may be used as an effective and safe antioxidant source.
To explain the variation in the antioxidant activities measurements among different assays, different results to diverse mechanisms of action can be reported. ABTS is a method based on reduction of the 2,2-azinobis-(3-ethylbenzothiazoline sulphonate) radical, and DPPH is a method based on the scavenging of the DPPH radical, although both ABTS.+ and DPPH have been widely used to measure the antioxidant capacities of natural extracts, based on their ability to reduce the radical cation. The ABTS.+ reactions with antioxidants present in the test sample occur rapidly and can be assessed by following the decrease of absorbance sample at 700 nm for 6 min, much shorter than that of DPPH assay (30 min in the present study). Moreover, the DPPH assay determines the decrease absorbance sample at 517 nm, and the colored compounds such as anthocyanins and carotenoids present in the test sample may have the spectra that overlap with DPPH at 517 nm and thus interfere with the measurements.[Citation26]
Reducing Power Assay
presented the reducing power of hydrometanolic and aqueous extracts of leaves and stem bark as a function of their concentration. In this assay, the yellow color of the test solution changes to green and blue, depending on the reducing power of each compound. The presence of reducers (i.e., antioxidants) causes the reduction of the FeCitation3 +/ferricyanide complex to the ferrous form. Therefore, measuring the formation of Perl's Prussian blue at λmax 700 nm can monitor the FeCitation2 + concentration. The reducing power of leaves and stem bark aqueous and hydromethanolic extracts increased with concentration. For leaf extracts, it was showed that the strongest reducing power was observed with water. However, hydromethanolic stem bark extracts showed higher effect than the aqueous extract. Aqueous and hydromethanolic extracts from M. alba var. alba leaves showed high reducing power. Hydromethanolic M. rubra stem bark extracts showed the best reducing power. Aqueous and hydromethanolic M. alba var. alba and M. alba var. rosa showed similar effects.
Figure 1 Reducing power from Morus species of (a) methanolic leaves extracts (mg/ml); (b) aqueous leaves extracts (mg/ml); (c) methanolic steam bark extracts (mg/ml); (d) aqueous stem bark extracts (mg/ml). Each value is expressed as mean ± standard deviation (n = 3).
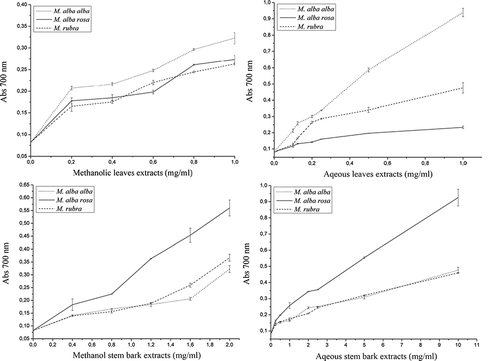
The antioxidant activity of phenolics is mainly due to their redox properties, which allow them to act as reducing agents, hydrogen donators, and singlet oxygen quenchers. In addition, they have a metal chelation potential.[Citation27] The extracts derived from this plant may offer a safe and natural source of antioxidants that may be used as additives to foods or consumed directly as therapeutic drugs and functional foods.
Antimicrobial Activity
Over the past decade, interest in drugs derived from higher plants, especially the phytotherapeutic ones, has increased greatly. It is estimated that about 25% of all modern medicines are directly or indirectly derived from higher plants.[Citation28] In some particular cases, such as antitumoral and antimicrobial drugs, about 60% of the medicines currently available on the market, and most of those in the late stages of clinical trials, are derived from natural products, mainly from higher plants.[Citation29] The stem bark and leaf extracts were screened for their antimicrobial properties against Staphylococcus aureus ATCC 25923, Enterococcus feacalis ATCC 29212, Staphylococcus epidermis CIP 06510, Escherichia coli ATCC 35218, and Salmonella Typhimurium ATCC 14028. Their antimicrobial activity was expressed by determining the diameter of the inhibition zone. Despite all the extracts revealed antimicrobial activity, they inhibited growth to variable extents, depending on the bacterium in question. As summarized in , hydromethanolic and aqueous extracts of stem bark and leaves showed antibacterial activity against both Gram-positive bacteria (Staphylococcus aureus, Enterococcus feacalis, and Staphylococcus epidermis) and Gram-negative bacteria (Escherichia coli and Salmonella Typhimurium).
Table 3 Antibacterial activity of methanol and aqueous extracts of Morus alba var. alba, Morus alba var. rosa, and Morus rubra as determined by disc diffusion method
Hydromethanolic stem bark extracts possess significantly more important antibacterial qualities than do hydromethanolic and aqueous leaf extracts. However, no antibacterial activity was observed from hydromethanolic and aqueous extracts of M. alba var. rosa and M. rubra leaves (). This might have resulted from the lack of solubility of the active constituents in aqueous and hydromethanolic solutions. Lack of activity can thus only be proven by using large doses. Alternatively, if the active principle is present in high enough quantities, there could be other constituents exerting antagonistic effects, or negating the positive effects of the bioactive agents.[Citation30] With no antibacterial activity, extracts may be active against other bacterial species that were not tested.[Citation31]
In fact, aqueous extract of M. alba var. alba leaves showed an antibacterial effect against only Salmonella Typhimurium and Staphylococcus epidermis, and its hydromethanolic extract exerted the effect against Staphylococcus aureus. From the stem bark aqueous extract, only this of M. rubra showed an antibacterial activity against Salmonella Typhimurium. Successful prediction of botanical compounds from plant material is largely dependent on the type of solvent used in the extraction procedure. The traditional healers or practitioners make use of water primarily as a solvent, but these studies showed that hydromethanolic extracts of these plants were certainly much better and more powerful. This may be due to the better solubility of active components in organic solvent.[Citation32] These observations can be rationalized in terms of the polarity of the compounds being extracted by each solvent and, in addition to their intrinsic bioactivity, by their ability to dissolve or diffuse in the different media used in the assay.
The selectivity obtained for mulberry leaves is clearly different from that of mulberry stem bark, which revealed a broad spectrum of antimicrobial activity. The bark inhibited the growth of several species of pathogenic microorganisms, representing Gram-positive (Staphylococcus aureus, Enterococcus feacalis, and Staphylococcus epidermis) and Gram-negative (Escherichia coli and Salmonella Typhimurium) bacteria. Hydromethanolic M. rubra stem bark extract revealed an antibacterial effect against the five studied bacteria, although their antioxidant activity is lowest. Its antibacterial activity can be due to the presence of antibacterial compounds (such arylbenzofuran and its derivatives) with important amounts than others stem bark and leaves. Stem bark of Morus mezozygia contained moracin T, moracin U, moracin C, and artocarpecin, which were able to inhibit the growth of Staphylococcus aureus LMP805, Streptococcus faecalis LMP806, Escherichia coli LMP701, and Salmonella Typhimurium LMP706.[Citation33]
As shown in , Salmonella Typhimurium was the most easily inhibited of all the bacteria exposed to stem bark extracts. These results are important due to the fact that Salmonella Typhimurium can produce several types of enterotoxins that cause gastroenteritis, which is a major food-borne disease in most countries. It was clearly stated that plants contained microbial inhibitors (i.e., flavonoids) soluble in aqueous methanol, and the flavonoid aglycones were more active than their glycosidic forms naturally present in plants.[Citation34,Citation35] Quercetin and other related compounds acted essentially by enzyme inhibition of DNA gyrase.[Citation36] The microorganism Escherichia coli, which is already known to be multi-resistant to drugs, were also resistant to the plant extracts tested. It was susceptible only to hydromethanolic M. rubra stem bark extract and showed 8 mm of inhibition zone. The resistance of this bacterium toward leaves and stem bark extracts can be related to lipopolysaccharides in their outer membrane.[Citation37] This observation, therefore, supports the notion that, in general, the Gram negative bacteria are more resistant than the Gram-positive ones.[Citation38]
CONCLUSION
In conclusion, the present study demonstrates that aqueous mulberry leaves and stem bark extracts may constitute a good source of polyphenols. These results encourage the use of leaves and stem bark as nutritional supplement and could be useful in the prevention of diseases in which free radicals are implicated. Moreover, the results demonstrate that stem bark may be a good antimicrobial agent against bacteria responsible for human gastrointestinal infections. This plant material could be a possible source to obtain new and effective herbal medicines to treat infections caused by multi-drug resistant strains of microorganisms from community as well as hospital settings. However, it is necessary to determine the toxicity of the active constituents, their side effects, and pharmaco-kinetic properties.
REFERENCES
- Deshmukh , S.V. , Pathak , N.V. and Takalikar , D.A. 1993 . Nutritional effect of mulberry (Morus alba) leaves as sole ration of adult rabbits . World Rabbit Science , 1 : 67 – 69 .
- Singh , K.P. and Ghosh , P. 1992 . Indian Silk , 31 ( 16 ) Mulberry cultivation under agroforestry and land management.–18
- Shivkumar , G.R. , Raman , K.A. , Magadum , S.B. and Datta , R.K. 1995 . Effect of phyto ecdysteroids on larval and economic parameters of silk worm, Bombyx morix L. Indian Journal of Sericulture , 34 46–49
- Madsen , H.L. and Bertelsen , G. 1995 . Spices as antioxidants . Trends in Food Science and Technology , 6 : 271 – 277 .
- Elfalleh , W. , Nasri , N. , Marzougui , N. , Thabti , I. , M'rabet , A. , Yahya , Y. , Lachiheb , B. , Guasmi , F. and Ferchichi , A. 2009 . Physico-chemical properties and DPPH-ABTS scavenging activity of some local pomegranate (Punica granatum) ecotypes . International Journal of Food Science and Nutrition , 60 : 197 – 210 .
- Kumazawa , S. , Hamasaka , T. and Nakayama , T. 2004 . Antioxidant activity of propolis of various geographic origins . Food Chemistry , 84 : 329 – 339 .
- Woisky , R.G. and Salatino , A. 1998 . Analysis of propolis: Some parameters and procedures for chemical quality control . Journal of Agricultural Research , 37 : 99 – 105 .
- Re , R. , Pellegrini , N. , Proteggente , A. , Pannala , A. , Yang , M. and Rice-Evans , C. 1992 . Antioxidant activity applying an improved ABTS radical cation decolorizationassay . Free Radical Biology and Medicine , 26 : 1231 – 1237 .
- Chu , Y.H. , Chang , C.L. and Hsu , H.F. 2000 . Flavonoid content of several vegetables and their antioxidant activity . Journal of the Science of Food and Agriculture , 80 : 561 – 566 .
- National Committee for Clinical Laboratory Standards (NCCLS). Performance standards for antimicrobial disk susceptibility test , 6th 1997 Wayne , PA Ed;. Approved Standard M2-A6
- Hayouni , E.A. , Abdrabba , M. , Bouix , M. and Hamdi , M. 2007 . The effects of solvents and extraction method on the phenolic contents and biological activities in vitro of Tunisian Quercus coccifera and Juniperus phoenicea L . fruit extracts. Food Chemistry , 105 : 1126 – 1134 .
- Wong , L.F. 2006 . “ Antioxidant and antimicrobial activities of ” . In Alpinia species , 150 Malaysia : Monash University . B.Sc. Hons. Thesisp.
- Arabshahi-Delouee , S. and Urooj , A. 2007 . Antioxidant properties of various solvent extracts of mulberry (Morus indica L.) . leaves. Food Chemistry , 102 : 1233 – 1240 .
- Zhishen , J. , Mengcheng , T. and Jianming , W. 1999 . The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals . Food Chemistry , 64 : 555 – 559 .
- Paliyath , G. and Fletcher , R.A. 1995 . Paclobutrazoltreatment alters peroxidase and catalase activities in heat-stressed maize coleoptiles . Physiology and Molecular Biology of Plants , 1 : 171 – 178 .
- Paliyath , G. , Pinhero , R.G. , Rao , M.V. , Murr , D.P. and Fletcher , R.-A. 1997 . Changes in activities of antioxidant enzymes and their relationship to genetic and paclobutrazol-induced chilling tolerance in maize seedlings . Plant Physiology , 114 : 695 – 704 .
- Nozolillo , C. , Isabelle , P. and Das , G. 1990 . Seasonal changes in phenolics constituents of jack pine seedlings (Pinus banksiana) in relation to the purpling phenomenon . Canadian Journal of Botany , 68 : 2010 – 2017 .
- Sývacý , A. and Sökmen , M. 2004 . Seasonal changes in antioxidant activity, total phenolic and anthocyanin constituent of the stems of two Morus species: Morus alba L . and Morus nigra L. Plant Growth Regulation , 44 : 251 – 256 .
- Ercisli , S. and Orhan , E. 2007 . Chemical composition of white (Morus alba), red (Morus rubra) and black (Morus nigra) mulberry fruits . Food Chemistry , 103 : 1380 – 1384 .
- Kaur , C. and Kapoor , H.C. 2001 . Antioxidants in fruits and vegetables-the millennium's health . International Journal of Food Science and Technology , 36 : 703 – 725 .
- Rice-Evans , C.A. , Miller , N.J. and Paganga , G. 1997 . Antioxidant properties of phenolic compounds . Trends in Plant Sciences , 4 : 302 – 309 .
- Rangkadilok , N. , Sitthimonchai , S. , Worasuttayangkurn , L. , Mahidol , C. and Satayavivad , Ruchirawat, M. 2007 . J. Evaluation of free radical scavenging and antityrosinase activities of standardized longan fruit extract . Food and Chemical Toxicology , 45 : 328 – 336 .
- Halliwell , B. , Aeschbach , R. , Loliger , J. and Aruoma , O.I. 1995 . The characterization of antioxidants . Food and Chemical Toxicology , 33 : 601 – 617 .
- Robert , R.E. , Pellegrini , M. , Proteggente , A. , Pannala , A. , Yang , M. and Rice-Evans , C.A. 1999 . Antioxidant activity applying an improved ABTS radical cation decolorization assay . Free Radical Biology and Medicine , 26 : 1231 – 1237 .
- Enkhmaa , B. , Shiwaku , K. , Katsube , T. , Kitajima , T. , Anuurad , E. , Yamasaki , M. and Yosuke , Y. Mulberry . 2005 . (Morus alba L.) leaves and their major flavonol quercetin 3-(6-malonylglucoside) attenuate atherosclerotic lesion development in LDL receptor–deficient mice . Journal of Nutrition , 135 : 729 – 734 .
- Arnao , M.B. 2000 . Some methodological problems in the determination of antioxidant activity using chromogen radicals: a practical case . Trends in Food Science and Technology , 11 : 419 – 421 .
- Rice-Evans , C.A. , Miller , N.J. , Bolwell , P.G. , Bramley , P.M. and Pridham , J.B. 1995 . The relative antioxidant activities of plant derived polyphenolic flavonoids . Free Radical Research , 22 : 375 – 383 .
- Shu , Y.Z. 1998 . Recent natural products based drug development: A pharmaceutical industry perspective . Journal of Natural Products , 61 : 1053 – 1071 .
- Cragg , G.M. , Newman , D.J. and Snader , K.M. 1997 . Natural products in drug discovery and development . Journal of Natural Products , 60 : 52 – 60 .
- Jager , A.K. , Hutchings , A. and van Staden , J. 1996 . Screening of Zulu medicinal plants for prostaglandinsynthesis inhibitors . Journal of Ethnopharmacology , 52 : 95 – 100 .
- Shale , T.L. , Strik , W.A. and Van Staden , J. 1999 . Screening of plants used by southern African traditional healers in the treatment of dysmenorrhoea for prostaglandin-synthesis inhibitors and uterine relaxing activity . Journal of Ethnopharmacology , 64 : 9 – 14 .
- de Boer , H.J. , Kool , A. , Broberg , A. , Mziray , W.R. , Hedberg , I. and Levenfors , J.J. 2005 . Antifungal and antibacterial activity of some herbal remedies from Tanzania . Journal of Ethnopharmacology , 96 : 461 – 469 .
- Kuete , V. , Fozing , D.C. , Kapche , W.F.G.D. , Mbaveng , A.T. , Kuiate , J.R. , Ngadjui , B.T. and Abegaz , B.M. 2009 . Antimicrobial activity of the methanolic extract and compounds from Morus mesozygia stem bark . Journal of Ethnopharmacology , 124 : 551 – 555 .
- Otshudi , A.L. , Foriers , A. , Vercruysse , A. , Van Zeebroeck , A. and Lauwers , S. 1999 . In vitro antimicrobial activity of six medicinal plants traditionally used for the treatment of dysentery and diarrhoea in Democratic Republic of Congo (DRC) . Phytomedicine , 7 : 167 – 172 .
- Auha , J. , Remes , S. , Heinonen , M. , Hopia , A. , Kahkonen , M. , Kujala , T. , Pihlaja , K. , Vuorela , H. and Vuorela , P. 2000 . Antimicrobial effects of Finnish plant extracts containing flavonoids and other phenolic compounds . International Journal of Food Microbiology , 56 : 3 – 12 .
- Cushnie , T.P.T. and Lamb , A.J. 2005 . Antimicrobial activity of flavonoids . International Journal of Antimicrobial Agents , 26 : 343 – 356 .
- Gao , Y. , Belkum , M.J.V. and Stiles , M. 1999 . The outer membrane of Gram-negative bacteria inhibits antibacterial activity of Brochocin-C . Applied Environmental Microbiology , 65 : 4329 – 4333 .
- Afolayan , A.J. 2003 . Extracts from the shoots of Arctotis arctotoides inhibit the growth of bacteria and fungi . Pharmaceutical Biology , 4 : 22 – 25 .
