Abstract
In order to recover and utilize peanut protein resource, peanut meal was fermented using Bacillus subtilis to prepare antioxidant peptides in this study. The antioxidant activities of the peanut peptides were evaluated via six in vitro tests, namely, 1,1-diphenyl-2-picrylhydrazyl radical scavenging activity, 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulphonic acid) scavenging activity, inhibition of linoleic acid autoxidation, reducing power, and metal chelating capacity. The peanut peptides showed 63.28% of scavenging effect on 1,1-diphenyl-2-picrylhydrazyl radical, 70.67% of scavenging effect on 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulphonic acid) and 39.32% of superoxide anion radical scavenging activity at 1.0 mg/ml, and 62.69% of inhibition on linoleic acid autooxidation at 0.8 mg/ml. Besides, the peanut peptides exhibited 32.8% of reducing power and 60.44% of Fe2+-chelating capacity at 2 mg/ml. Then the peanut peptides were desalted with macroporous resin; the fraction eluted with 75% ethanol showed the highest scavenging activity on 1,1-diphenyl-2-picrylhydrazyl radical. This fraction was subject to further purification using gel filtration chromatography and reverse phase-high performance liquid chromatography. At last, a peptide was gained, and it was identified to be Tyr-Pro with matrix-assisted laser desorption/ionization time-of-flight/time-of-flight mass spectrometry. The results indicated that it is feasible to prepare natural antioxidants from peanut meal using B. subtilis fermentation.
INTRODUCTION
Free radicals are generally very reactive molecules possessing one or more unpaired electrons. Free radicals are produced in living cells by normal metabolism and by exogenous sources, such as carcinogenic compounds and ionizing radiations.[Citation1] Free radicals are unstable species because they have unpaired electrons and seek stability through electron pairing with biological macromolecules. The proteins, lipids, and DNA of healthy human cells are good sources for these pairing electrons.[Citation2] Oxidation of biomolecules initiated by free radicals can lead to mutagenic changes, tissue damage, and cell death.[Citation3] They play a significant pathological role in cancer, emphysema, cirrhosis, atherosclerosis, arthritis, and various other degenerative diseases.[Citation4] Besides, lipid peroxidation caused by free radicals in the food system can lead to development of unpleasant rancid or off flavors as well as nutritional loss and formation of potentially toxic end-products.[Citation5] Consumption of these potentially toxic products can give rise to several diseases.[Citation6]
This brings about the need for synthetic and natural antioxidants, which delay or inhibit the oxidation of lipid or other molecules by inhibiting the initiation of propagation of oxidizing chain reactions. Synthetic antioxidants, such as butylated hydroxyanisole (BHA), butylated hydroxytoluene (BHT), and tert-butylhydroquinone (TBHQ), have been widely used in the food industry to prevent lipid oxidation, because they are effective and inexpensive. However, use of synthetic antioxidants brings consumers some health concerns.[Citation7] In the areas of human nutrition and biochemistry, natural antioxidants from food resources have been the focus of growing interest for their potential health benefits with no or little side effects, and there has also been an increasing tendency among the public to choose natural rather than synthetic antioxidants.[Citation8]
Many kinds of food proteins hydrolysates, including capelin protein hydrolysate, egg-yolk protein hydrolysate, Alaska pollack frame protein hydrolysate, and hoki frame protein hydrolysate, have been reported to possess antioxidants.[Citation9 − Citation12] Protein hydrolysates are important sources of antioxidants, and some potent antioxidative and radical-scavenging peptide sequences have been identified from the hydrolysis of various proteins.[Citation13] Peanut is one of the most important oilseeds in the world, ranking second with respect to planting area after rapeseed in oil plants.[Citation14] China and India are the largest producers of peanut, which accounts for 46.5% (19 million tons per annum) of total peanut production worldwide.[Citation15] In China, over 50% of peanut production is used for edible oil and the rest is consumed in other ways. Because of a large proportion of peanut consumed in peanut oil, thousands of tons of accessory product, peanut meal, which contains 50–55% high value protein,[Citation15] is generated every year. Conventional industrial processing of peanut oil involves pressing and solvent extraction, and these two methods are mainly used to produce edible oil.[Citation14] Due to severe denaturation of protein or residual solvent, the peanut meal is often discarded, or used as animal feeds or fertilizers.[Citation13,Citation14] This is a huge waste of protein resources, especially for poor countries and developing countries in which protein resources are in great deficit.
Bacillus subtilis, known also as the hay bacillus or grass bacillus, is a Gram-positive, catalase-positive bacterium commonly found in soil. B. subtilis is an ancient starter culture used widely for soybean foods. Traditional Bacillus-fermented soybean foods are known as a good source of protein and have been produced in different countries, such as India (kinema), China (douchi), Thailand (thua nao), and Japan (natto).[Citation16] The exact mechanism underlying the antioxidant activity of peptides has not fully been understood, yet various studies have indicated that they are inhibitors of lipid peroxidation, scavengers of free radicals, and chelators of transition metal ions.[Citation8] It is believed that antioxidant properties of the peptides are related to their amino acid composition, structure, hydrophobicity, and their correct positioning in peptide sequence.[Citation17,Citation18] However, further research is still needed to explore the structure-function relationship of antioxidant peptides.
Previous methods of preparing antioxidant peptides are to extract proteins from some materials, and then the extracted proteins were hydrolyzed using several proteases to obtain antioxidant peptides.[Citation10,Citation19] However, in this study, the peanut meal was directly fermented using B. subtilis to produce antioxidant peptides in order to know whether the peanut meal may represent a new source of antioxidant peptides. Then the antioxidant activities of the peanut peptides were determined. Furthermore, the purification and identification of the antioxidant peptides was determined using consecutive chromatography and MALDI-TOF/TOF MS.
MATERIALS AND METHODS
Materials and Chemicals
Peanut meal was kindly provided by Luhua Group, Shandong Province, China. The peanut meal was ground to a fine powder in a mill. The powder, which passed through a 50-mesh sieve, was stored at 4°C in a sealed plastic bag until use. DPPH·(1,1-diphenyl-2-picrylhydrazyl) and ferrozine were obtained from Sigma-Aldrich. All other chemicals used were analytical grade and made in China.
Microorganisms, Culture Condition, and Inoculum
A freeze-dried culture of B. subtilis was purchased from the China Center of Industrial Culture Collection (CICC). The B. subtilis strain, which showed high protease activity, was rejuvenated and maintained on a nutrient agar slope at 4°C. The inoculum was prepared by adding a loopful of cells to 100 ml of sterile nutrient broth (NB) medium, which contained 5 gL−1 beef extract, 30 gL−1 peptone, 5 gL−1 NaCl, and 1 gL−1 glucose at pH 7.2 (this medium is recommended by CICC), and then the nutrient broth was incubated in an air bath shaker for 24 h at 30°C. The cells from actively growing B. subtilis were harvested by centrifugation at 2680 g for 20 min at room temperature and resuspended in sterilized saline. After adjusting to a concentration of 108 colony forming units (cfu)/ml, the cell suspension was used to inoculate peanut meal for fermentation.
Preparation of Peanut Peptides
Peanut meal (40 g), distilled water (800 ml), NaCl (4 g), and glucose (0.8 g) were placed into each of several 1000-ml-capacity screw-capped bottles, the pH value was adjusted by adding 0.1 M NaOH solution to 8.0, and then sterilized at 115°C for 30 min in an autoclave. After cooling to room temperature, the peanut meal was inoculated quickly with B. subtilis inoculum prepared as done in the earlier section. The peanut meal was inoculated using B. subtilis inoculum at 2% (v/v) inoculation, and then the inoculated peanut meal was incubated in an air bath shaker at 30°C at 94 g of agitation for fermentation. The fermentation time was set for 49.5 h. The fermented peanut meal was boiled for 10 min in an autoclave to passivate the enzyme activity and then centrifuged at 2680 g for 20 min. Supernatant was filtered through a 0.45-μm membrane under vacuum. The filtrates were concentrated at 40°C using a rotary evaporator and freeze-dried. Prior to use, the raw peanut peptides were kept at 4°C.
DPPH Radical Scavenging Assay
The DPPH· radical-scavenging capacity of peanut peptides was determined according to the method described by Turkoglu et al.,[Citation20] with minor modification. Briefly, various concentrations of peanut peptide samples (2 ml) in distilled water were added to 2 ml of 0.2 mM DPPH · in ethanol. After a 30-min reaction at room temperature in the dark, the absorbance was measured against a blank control at 517 nm. Pure ethanol was used to calibrate the spectrophotometer. Inhibition rate (I%) on DPPH radical was calculated in the following formulation:
ABTS·+ Radical Scavenging Assay
ABTS · + (2,2′-azino-bis(3-ethylbenzothiazoline-6-sulphonic acid)) radical scavenging capacity of peanut peptides was determined by ABTS radical cation decolorization assay according to the method of Thana et al.[Citation21] ABTS was dissolved in water to a 7 mM concentration. ABTS radical cation (ABTS · +) was produced by reacting ABTS stock solution with 2.45 mM potassium persulfate (final concentration). Next, the mixture was left to stand in the dark at room temperature for 12–16 h before use. The radical was stable in this form for more than 2 days when stored in the dark at room temperature. The absorbance of ABTS · + at 734 nm was adjusted to 0.70 ± 0.02. For the spectrophotometer assay, 3 ml of the ABTS · + solution and 100 μl of sample solution were mixed and measured immediately after 6 min at 734 nm (absorbance did not change significantly up to 10 min). Inhibition rate (I%) on ABTS · + was calculated according to the following equation:
Superoxide Anion Scavenging Activity
The superoxide anion radical-scavenging effect was determined according to the method described by Xiao et al.[Citation22] The superoxide anion was produced by ammonium persulfate-N, N, N,′ N,′-tetramethylethylenediamine (AP-TEMED). One milliliter of solution A (48 mL of 1 M HCl, 36.6 g of Tris, and 0.23 mL of TEMED/100 mL) and 4 mL of solution B (AP, 0.14 g/100 mL) were mixed and reacted for 1 min, then 1 mL of peanut peptides sample at various concentrations was blended with the mixture above and vortexed. After this, 2 mL of the mixture above and 0.4 mL of 10 mM hydroxylammonium chloride were blended thoroughly and reacted at 25°C for 20 min. Finally, the mixture was blended with 2 mL of 17 mM 4-aminobenzenesulfonic acid and 2 mL of 7 mM α-aminonaphthaalene, and left to stand at 25°C for 20 min once again. The mixture was extracted with the same volume n-butanol, the n-butanol transferred, and read at 530 nm on a spectrophotometer. I% (inhibition percent) on superoxide anion was calculated per the following equation:
Inhibition of Linoleic Acid Autooxidation
The antioxidant activities of peanut peptides were determined by the method of Osawa and Namiki[Citation23] with some modifications. Two milliliters of the sample (0.4, 0.6, and 1.0 mg/ml, respectively), 2 ml of 2.5% (v/v) linoleic acid prepared with pure ethanol, 4 ml of 0.05 M phosphate buffer (pH 7.0), and 2 ml of distilled water were placed into a glass tube sealed tightly with a silicon rubber cap and kept at 40°C in the dark for 1 week. The degree of linoleic acid oxidation was measured by the method described by Chen et al.[Citation24] The sample solution (0.5 ml) incubated in the linoleic acid model system described above was mixed with 3.5 ml of 75% ethanol, 0.5 ml of 30% ammonium thiocyanate, and 0.5 ml 20 mM ferrous chloride dissolved in 1 M HCl. The solution was mixed thoroughly, and 3 min later, the absorbance was read at 500 nm. BHT was also assayed at the same concentration as control.
Reducing Power
The reducing power of peanut peptides was assayed according to the method described by Yildirim et al.,[Citation25] with a slight modification. An aliquot of 2 ml of peanut peptides sample was mixed with 2.5 ml of 0.2 M phosphate buffer (pH 6.6) and 2.5 ml of 1% (w/v) potassium ferricyanide. The mixtures were incubated at 50°C for 20 min. After incubation, 2.5 ml of 10% (w/v) trichloroacetic acid was added and the reaction mixtures were centrifuged at 2010 g for 10 min. Finally, 2 ml of the supernatant solution was mixed with 1 ml of distilled water and 0.5 ml of 0.1% (w/v) ferric chloride solution. After 10 min of reaction time, the absorbance of the solution was measured at 700 nm. Higher absorbance of the reaction mixture indicated higher reducing power. Reduced glutathione (GSH) was used as a reference.
Metal Chelating Capacity
The metal chelating capacity on ferrous ions was determined according to the method described by Giménez et al.,[Citation26] with a slight modification. A test sample of 3 ml was mixed with 50 μl of 2 mM FeCl2·4H2O, 750 μl of distilled water, and 200 μl of 5 mM ferrozine. The mixture was vortexed and allowed to stand at room temperature for 10 min prior to measuring the absorbance at 562 nm. Ethylene diamine tetraacetic acid (EDTA) was used as the control. The chelating ability was calculated in the equation given below:
Desalination of Peanut Peptides
Desalination of peanut peptides was carried out according to the method described by Zhang et al.,[Citation27] with some modifications. Macroporous resin DA201-C (Jiangsu Chemical Industry Factory, Wuxi, China) was used as the adsorbent. The macroporous resins were extensively washed with plenty of distilled water to remove salts and impurities. Prior to the adsorption experiments, adsorbents were washed with anhydrous ethanol, followed with distilled water, and then dried in a vacuum at a temperature of 60°C. The desalination tests of peanut peptides were performed as follows: 1.0 g of pretreated dry adsorbent was introduced into a 250-ml Erlenmeyer flask. First, the dried resins were swelled by using anhydrous ethanol and washed with distilled water until there was no ethanol and the water was blotted up. Then, 100 ml of 10 mg/ml aqueous solution of peanut peptides was added to each flask. The solution of peanut peptides was agitated (200 rpm) at 37°C in a water bath incubator overnight. Then the peanut peptides were eluted by using different concentrations of ethanol from macroporous resin. The eluted peanut peptides were collected, and then they were concentrated at 40°C on a rotary evaporator and freeze-dried. The peanut peptides, which possessed the strongest scavenging activity on DPPH radical, were chosen as the target of the next purification. The desalination percentage was calculated according to the following equation:
Gel Filtration and High Performance Liquid Chromatography
Peanut peptides showing obvious antioxidant activity were dissolved in distilled water and filtrated through 0.22-μm membrane. Then the filtrate was loaded onto a Sephadex G-15 gel filtration column (2.5 × 100 cm), which was previously equilibrated with distilled water. The column was then eluted with distilled water at a flow rate of 0.5 ml/min, while fractions showing antioxidant activity were pooled and lyophilized. The antioxidative fraction was further separated by reverse-phase high performance liquid chromatography (RP-HPLC) on a Primesphere C18 column (10 μm, 10 mm × 250 mm) using a linear gradient of acetonitrile (0–50%, v/v, 50 min) containing 0.1% trifluoroacetic acid (TFA) at a flow rate of 1.0 ml/min.
Identification of Purified Antioxidant Peptides
To identify molecular mass and amino acid sequence of the purified peptide, the pooled peak possessing the highest DPPH radical-scavenging activity was subjected to ultra-performance liquid chromatography (UPLC) for peptide separation in an Acquity UPLC BEH C18 column (2.1 × 120 mm) (Waters Corporation, Milford, MA, USA). Identification of the sequence of peanut peptides present in the fraction showing remarkable DPPH radical-scavenging activity was performed by matrix-assisted laser desorption/ionization time-of-flight/time-of-flight mass spectrometry (MALDI-TOF-TOF MS). Both MS and MS/MS data were acquired with a Waters Synapt Mass Quadrupole Time-of-Flight Mass Spectrometer (Waters Corporation, Milford, MA, USA). MALDI-TOF analysis was done covering a mass charge (m/z) range of 100–1500. Automatically, main ions were selected for the subsequent TOF/TOF analysis. The collision energies were set at 5 and 20 eV for MS and MS/MS, respectively. The spectra were interpreted using the peptide sequencing module of the MassLynx 4.1 software.
Statistical Analysis
All the tests were performed in triplicate. Data was expressed as mean, and standard deviation of three samples was subjected to analysis of variance (ANOVA) followed by Duncan's new multiple-range test. P values < 0.05 were regarded as not significant.
RESULTS AND DISCUSSION
DPPH Radical Scavenging Capacity
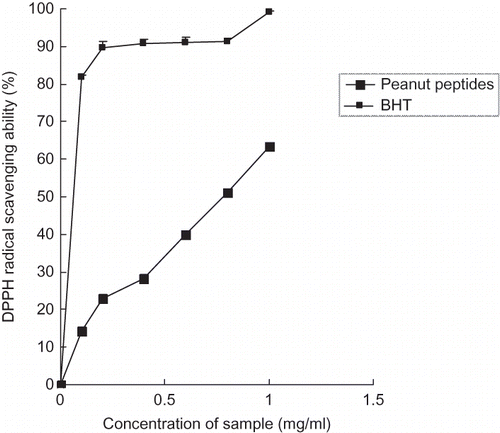
In order to evaluate the antioxidant potential of peanut peptides through free radical scavenging, the decoloration of DPPH radical dissolved in ethanol was monitored at 517 nm. The DPPH radical scavenging percentages in the presence of peanut peptides at different concentrations are shown in . Both peanut peptides and BHT possessed obvious scavenging effects on DPPH radical and the scavenging effects were of a concentration-dependent manner. At the same concentration of peanut peptides and BHT, the scavenging effect on DPPH radical decreased in this order: BHT > peanut peptides. The maximum scavenging effect of peanut peptides was 63.28% at the concentration of 1.0 mg/ml; the BHT was 99.16%. At the same time, the antioxidant activities of unfermented peanut meal were assayed also, but the unfermented peanut meal did not dissolve in the water and it did not show any obvious antioxidant activities in our test, so the data can be ignored. The fermentation is characterized by extensive hydrolysis of peanut meal protein to amino acids, peptides, and ammonia, resulting in improvement of the functionality, including antioxidant activity. In addition, the characteristics of B. subtilis also play a positive role in the properties and functionality of the fermented product. For example, improvement of the antioxidant activity of fermented okara using B. subtilis B2 has been reported by Zhu et al.[Citation28] The results indicated that the peanut peptides were good electron or hydrogen donators, the free radicals can easily rob electrons from the peanut peptides and become stable and less harmful or harmless intermediate products. Although the scavenging effect of peanut peptides was not as high as BHT at the same concentration, the origin of peanut peptides was natural and there is no potential health risk or side effects; thus, they can be used at higher concentrations to reach better radical-scavenging effects. Besides, some other ingredients such as flavonoids, polyphenolics, and pigments in peanut meal and B. subtilis metabolites may partially make a contribution to the DPPH radical-scavenging effect.
ABTS·+ Scavenging Activity
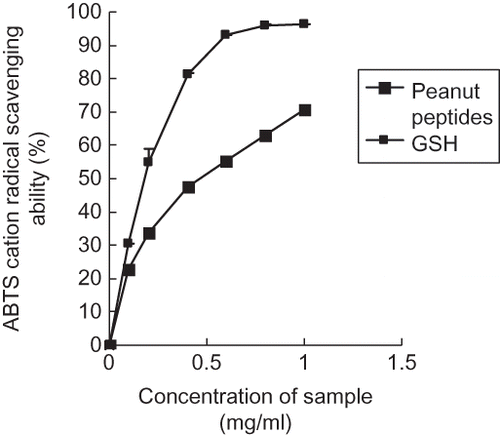
ABTS · + is another synthetic radical and more versatile than a DPPH radical, because the ABTS · + model can be used to assess the scavenging activity for the polar and non-polar samples, and the spectral interference is lessened as the absorption maximum often used is a wavelength not normally encountered by natural products.[Citation29] Therefore, it was necessary to further evaluate the antioxidant activity of peanut peptides against ABTS · +. In our ABTS radical-scavenging tests, the peanut peptides were also able to scavenge the ABTS · +. Further, the scavenging effects increased significantly with increasing concentrations (). The ABTS radical-scavenging activity of the peanut peptides is a little lower than that of GSH, but perhaps it is enough to extinguish a large proportion of the ABTS · + (the ABTS · + scavenging effect was 70.67% at the concentration of 1.0 mg/ml), which may be a good answer for its application in the food and pharmaceutical industries. Generally speaking, some specific protein fragments are inactive within the sequence of the parent protein, but after they are released by enzymatic hydrolysis, they may exert various physiological functions.[Citation8] In the course of B. subtilis fermentation, many proteases excreted by B. subtilis can cleave peptide bonds within peanut protein, and then some active sites and hydrophobic amino acid residuals were released, which may possess important antioxidant activity. All of this suggests that the peanut peptides were a good natural radical-scavenger, and they may become safe substitutes for synthetic antioxidants in the future, although their radical-scavenging was not as good as the synthetic ones.
Superoxide Anion Scavenging Activity
Superoxide anion is produced by a number of enzyme systems in autooxidation reactions and by nonenzymatic electron transfers that univalently reduce molecular oxygen. Although it is not highly reactive, it can produce hydrogen peroxide and hydroxyl radical through dismutation and other types of reaction and is the source of free radicals generated in vivo. Not only superoxide anion radical but also its derivatives are cell-damaging, which can cause damage to DNA and cell membranes. Therefore, it is of great importance to scavenge superoxide anion radical.[Citation30]
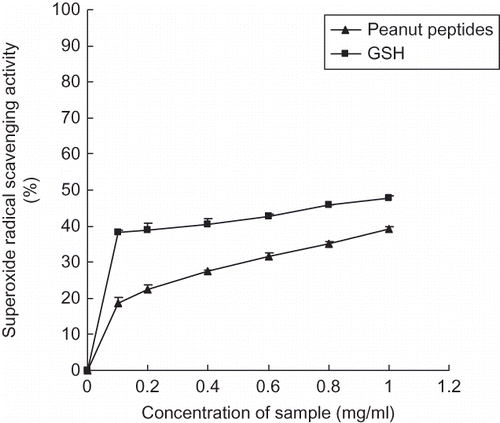
Superoxide anion scavenging effect of the peanut peptides is presented in . The peanut peptides tested in this experiment exhibited considerable scavenging activity on superoxide anion. Moreover, the scavenging effect increased significantly with the increasing concentrations. Addition of the peanut peptides at 1.0 mg/ml showed the highest superoxide anion scavenging activity (39.32%), which is a little lower than that of GSH at the same concentration. In vivo, superoxide anion radical is the origin of free radicals in other forms and hydrogen peroxide. Once superoxide anion radical is extinguished, the origin of other free radicals, which may be highly reactive, is eliminated also. Hence, the organelles and biomolecules can get protection from oxidative damage to some extent. The results show that the peanut peptides obtained from fermented peanut meal have good antioxidant and superoxide anion scavenging activities and they will be a potential source of natural antioxidant if they are used at much higher concentrations. Besides, Wang et al. have reported that fermented soybean product had shown antioxidant activities in vitro and in vivo studies.[Citation31]
Inhibition of Linoleic Acid Autooxidation
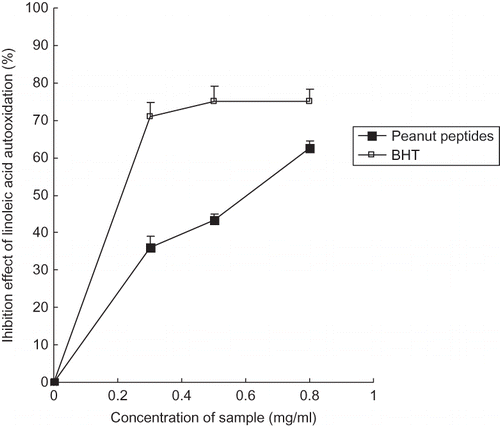
Lipid peroxidation leads to rapid development of rancid and stale flavors, and is considered as a primary mechanism of quality deterioration in lipid foods and oils.[Citation32] In the body, excessive production of free radicals affects lipid cell membranes to produce lipid peroxides. Lipid peroxides are likely involved in numerous pathological events, including inflammation, metabolic disorders, and cellular aging.[Citation33] The antioxidant effects of the peanut peptides on the peroxidation of linoleic acid were estimated at different concentrations. The results were shown in . The inhibition percentage of the peanut peptides on linoleic acid autoxidation increased sharply with increasing concentrations, and it came up to 62.69% at the concentration of 0.8 mg/ml. At the same concentration, the BHT showed inhibition of 75%, higher than peanut peptides. Usually, synthetic antioxidants possess stronger antioxidant activities than the natural ones. The results showed that the peanut peptides could remove free radicals, inhibit linoleic acid autooxidation, and protect linoleic acid from oxidation to a great extent. Membrane lipids in the body are rich in unsaturated fatty acids, which are most sensitive to oxidation. Especially, linoleic and arachidonic acids are targets of lipid peroxidation.[Citation34] In light of the inhibition effect of peanut peptides on linoleic acid autooxidation, it is postulated that the peanut peptides could effectively prevent unsaturated fatty acids from oxidation present in membrane lipids to protect the cell membrane from oxidative damage in the human body, if the peanut peptides are used in the food industry or people's daily diet.
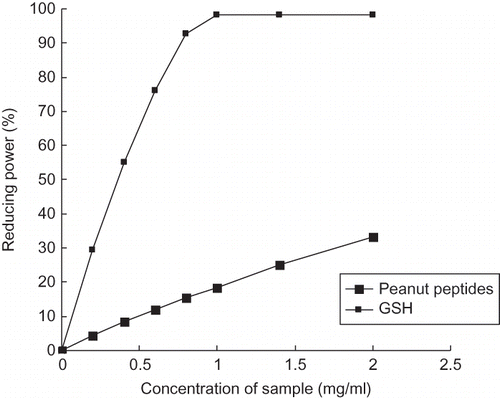
Reducing Power
The reducing power of a given compound often serves as a significant indicator of its potential antioxidant activity. Many reports have discovered that there is a direct correlation between antioxidant activities and reducing power of certain bioactive compounds.[Citation35 − Citation37] An electron-donating reducing agent is able to donate an electron to a free radical. As a result, the radical is neutralized and the reduced species subsequently acquires a proton from solution.[Citation38] The reducing power of peanut peptides is shown in compared with GSH as reference. In this assay, the ability of peanut peptides to reduce Fe3+ to Fe2+ was determined. The electron donation capacity of peanut peptides results in reduction of the Fe3+/ferric cyanide complex to the ferrous form. Therefore, measuring the formation of Perl's Prussian blue at 700 nm can monitor the Fe2+ complex concentration.[Citation39] As shown in , the reducing power of the peanut peptides increases significantly with increasing their concentrations, and they showed 32.8% of reducing power at the concentration of 2 mg/ml, lower than that of GSH at the same concentration. Presumably, B. subtilis hydrolyzed peanut protein and broke the compact structure of protein, so some low-molecular-weight bioactive peptides, which may contain antioxidant amino acids, were released and showed reducing power in our test. The results indicated that the peanut peptides possessed some degree of electron donation capacity, which may answer for their potential application as natural reducers in the food, pharmaceutical, and cosmetic industries, although their reducing power is not as high as that of GSH.
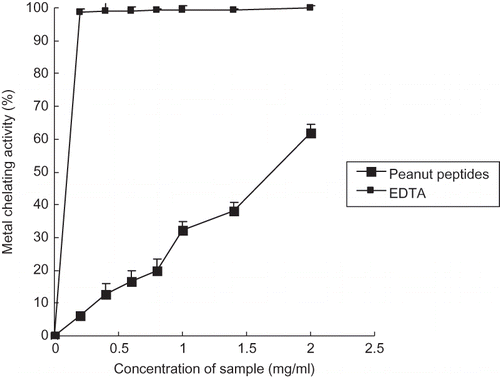
Metal Chelating Capacity
Transition metals may act as catalysts that promote the generation of the first few radicals, which initiate the oxidative chain reactions. Among the transition metals, iron is known as the most important lipid oxidation pro-oxidant due to its high reactivity. Fe2+ chelation may render important antioxidative effects by retarding metal-catalyzed oxidation.[Citation40] Fe2+ chelating activities of peanut peptides and EDTA are shown in . The Fe2+ chelating activity of peanut peptides was concentration dependent: the value increased with increasing concentration and reached 60.44% at the concentration of 2 mg/ml. In this assay, ferrozine can quantitatively form red complexes with Fe2+. In the presence of chelating agents the complex formation is disrupted, resulting in a decrease of absorbance at 562 nm. Therefore, measuring the color reduction can estimate the metal chelating activity of the coexisting chelator. Lower absorbance indicates higher metal chelating activity. The effective Fe2+ chelators may afford protection against oxidative damage by removing iron, which may otherwise promote the generation of some free radicals. Minimizing Fe2+ may afford protection against oxidative damage by inhibiting production of reactive oxygen species (ROS) and lipid peroxidation. It is believed that metal chelating capacity of peptides is related to some special amino acid residuals, such as acidic amino acid residuals, His residual, and so on. Carboxyl groups within acidic amino acid residuals and imidazole group within His residual can easily bind Fe2+ and form ring compounds.[Citation17,Citation41] The results of this test, shown in , revealed that the peanut peptides may lessen the availability of transition metals and inhibit the radical-mediated oxidative chain reaction in biological or food systems to a great extent by capturing Fe2+, and consequently improve human health and food quality, stability, and safety.
Purification of Antioxidant Peptides
Desalination of peanut peptides
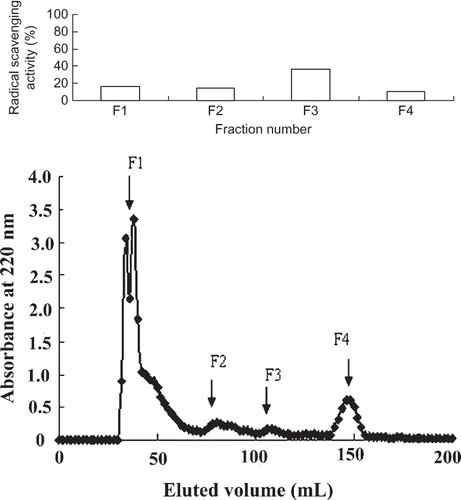
In order to preliminarily purify the peanut peptides, the peanut peptides were subjected to desalination with macroporous resin. The data in showed that the desalination percentages of the four eluted fractions were all more than 80%. This indicated that macroporous resin DA201-C could remove most salts in peanut peptides very well. The fraction eluted with 75% ethanol showed significantly (p > 0.05) higher scavenging effect on DPPH radical than others, so this fraction was chosen as the target of further purification.
Table 1 Desalination percentage of four fractions and their DPPH radical scavenging activities
Gel filtration and high performance liquid chromatography
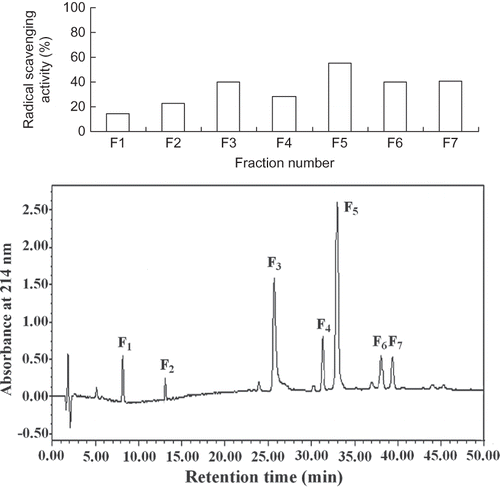
Considering scavenging effects on DPPH radical, the fraction eluted with 75% ethanol was employed for the purification and identification of antioxidant peptide. This fraction was dissolved in distilled water at 10 mg/ml, and loaded on a Sephadex G-15 gel column. It was separated according to molecular size. As shown in , four peptide fractions were isolated, among which fraction F3 was identified to possess significantly (p > 0.05) antioxidant activity than other fractions. In light of the activity analysis, the most potent fraction was purified with RP-HPLC on a Primesphere C18 column (10 μm, 10 mm × 250 mm), and the results were shown in . Seven clear peptide peaks were isolated and compared for their antioxidant activity on DPPH radical. Peak F5 showed significantly (p > 0.05) antioxidant activity than others. The amino acid sequences of the peptide, which possessed the highest antioxidant activity, were determined by MALDI-TOF-TOF MS.
Characterization of purified antioxidant peptides
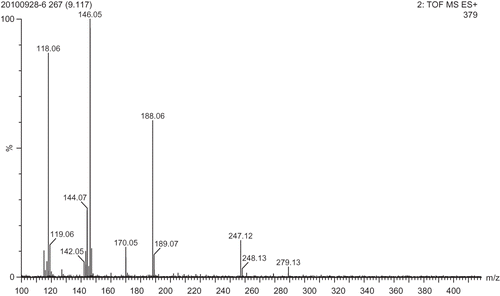
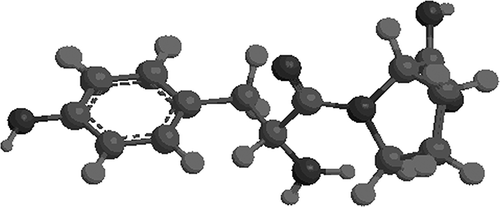
Among the seven peptide peaks, peak F5 was identified to possess the highest antioxidant activity on DPPH radical scavenging activity. The molecular mass of the antioxidant peptide was determined to be 278.13 Da. The precursor ion scan mass spectrum of the peptide and the MS/MS spectrum of a single charged ion with m/z at 278.13 Da was shown in . The amino acid sequence of the peptide was identified to be Tyr-Pro by the Biolynx peptide sequencer software from the MS/MS spectrum, and the molecular spatial structure of the Tyr-Pro is shown in . It is generally considered that aromatic amino acids (Phe, Tyr, Trp) are good donators of hydrogen and can scavenge free radicals very well because of their conjugated double-bond structure of a benzene ring. The Tyr can donate hydrogen atoms to free radicals, and then the amino acid itself becomes a free radical intermediate, phenoxyl free radical. The phenoxyl free radical can come up to stability via resonance. Besides, Tyr present in peptides sequences could improve antioxidant activities of antioxidant peptides. Pro contains a pyrrole ring in its molecular structure, and some researchers found that this amino acid showed some antioxidant activities.[Citation42] In the molecular structure of Tyr-Pro, there is an aromatic amino acid, Tyr in its N-terminus, Pro present in its C-terminus. Presumably, the special spatial structure of this peptide may contribute much to its antioxidant activities.
CONCLUSIONS
Annually, a lot of peanut meal is generated from the peanut oil industry, and some of the by-products are utilized, but the main part is often used as animal fodder or directly discarded. The peanut meal is a natural premium protein source that could be useful for applications in the food industry, health benefit products, or pharmaceuticals. Results from this study suggested that it is feasible to produce a natural antioxidant from peanut meal protein using B. subtilis fermentation.
ACKNOWLEDGMENTS
The authors would like to acknowledge all of their lab mates and friends who helped and encouraged them during the work. A special thank you goes to Professor Hui Zhang, Advisor.
REFERENCES
- Halliwell , B. and Gutteridge , J.M.C. 1999 . Free Radicals in Biology and Medicine , Oxford : Oxford University Press .
- Ozyurt , D. , Demirata , B. and Apak , R. 2007 . Determination of total antioxidant capacity by a new spectrphotometric method based on Ce(IV) reducing capacity measurement . Talanta , 71 ( 3 ) : 1155 – 1165 .
- Yang , J.-W. , Mau , J.-L. , Ko , P.-T. and Huang , L.-C. 2000 . Antioxidant properties of fermented soybean broth . Food Chemistry , 71 ( 2 ) : 249 – 254 .
- Prakash , D. , Upadhyay , G. , Singh , B.N. and Singh , H.B. 2007 . Antioxidant and free radical-scavenging activities of seeds and agri-wastes of some varieties of soybean (Glycine max) . Food Chemistry , 104 ( 2 ) : 783 – 790 .
- Pan , Y.M. , Zhang , X.P. , Wang , H.S. , Liang , Y. , Zhu , J.C. , Li , H.Y. , Zhang , Z. and Wu , Q.M. 2007 . Antioxidant potential of ethanolic extract of Polygonum cuspidatum and application in peanut oil . Food Chemistry , 105 ( 4 ) : 1518 – 1524 .
- Je , J.Y. , Qian , Z.J. , Byun , H.G. and Kim , S.K. 2007 . Purification and characterization of an antioxidant peptide obtained from tuna backbone protein by enzymatic hydrolysis . Process Biochemistry , 42 ( 5 ) : 840 – 846 .
- Arcan , I. and Yemenicioglu , A. 2007 . Antioxidant activity of protein extracts from heat-treated or thermally processed chickpeas and white beans . Food Chemistry , 103 ( 2 ) : 301 – 312 .
- Sarmadi , B.H. and Ismail , A. 2010 . Antioxidative peptides from food proteins: A review . Peptides , 31 ( 10 ) : 1949 – 1956 .
- Amarowicz , R. and Shahidi , F. 1997 . Antioxidant activity of peptide fractions of capelin protein hydrolysates . Food Chemistry , 58 ( 4 ) : 355 – 359 .
- Sakanaka , S. , Tachibana , Y. , Ishihara , N. and Juneja , L.R. 2004 . Antioxidant activity of egg-yolk protein hydrolysate in a linoleic acid oxidation system . Food Chemistry , 86 ( 1 ) : 99 – 103 .
- Je , J.Y. , Park , P.J. and Kim , S.K. 2005 . Antioxidant activity of a peptide isolated from Alaska Pollack (Theragra chalcogramma) frame protein hydrolysate . Food Research International , 38 ( 1 ) : 45 – 50 .
- Kim , S.Y. , Je , J.Y. and Kim , S.K. 2007 . Purification and characterization of antioxidant peptide from hoki (Johnius belengerii) frame protein by gastrointestinal digestion . The Journal of Nutritional Biochemistry , 18 ( 1 ) : 31 – 38 .
- Hwang , J.Y. , Shyu , Y.S. , Wang , Y.T. and Hwu , C.K. 2010 . Antioxidant properties of protein hydrolysate from defatted peanut kernels treated with esperase . LWT–Food Science and Technology , 43 ( 2 ) : 285 – 290 .
- Jiang , L.H. , Hua , D. , Wang , Z. and Xu , S.Y. 2009 . Aqueous enzymatic extraction of peanut oil and protein hydrolysate . Food and Bioproducts Processing , 88 ( 2–3 ) : 233 – 238 .
- Jamdar , S.N. , Rajalakshmi , V. , Pednekar , M.D. , Juan , F. , Yardi , V. and Sharma , A. 2010 . Influence of degree of hydrolysis on functional properties, antioxidant activity and ACE inhibitory activity of peanut protein hydrolysate . Food Chemistry , 121 ( 1 ) : 178 – 184 .
- Steinkraus , K.H. 1997 . Classification of fermented foods: Worldwide review of household fermentation techniques . Food Control , 8 ( 5–6 ) : 311 – 317 .
- Chen , H.M. , Muramoto , K. , Yamauchi , F. , Fujimoto , K. and Nokihara , K. 1998 . Antioxidative properties of histidine-containing peptides designed from peptide fragments found in the digests of a soybean protein . Journal of Agricultural Food Chemistry , 46 ( 1 ) : 49 – 53 .
- Rajapakse , N. , Mendis , E. , Jung , W.K. , Je , J.Y. and Kim , S.K. 2005 . Purification of a radical scavenging peptide from fermented mussel sauce and its antoxidant properties . Food Research International , 38 ( 2 ) : 175 – 182 .
- Zhang , J.H. , Zhang , H. , Wang , L. , Guo , X.N. , Wang , X.G. and Yao , H.Y. 2010 . Isolation and identification of antioxidant peptides from rice endosperm protein enzymatic hydrolysate by consecutive chromatography and MALDI-TOF/TOF MS/MS . Food Chemistry , 119 ( 1 ) : 226 – 234 .
- Turkoglu , A. , Duru , M.E. , Mercan , N. , Kivrak , I. and Gezer , K. 2007 . Antioxidant and antimicrobial activities of Laetiporus sulphureus (Bull.) Murrill . Food Chemistry , 101 ( 1 ) : 267 – 273 .
- Thana , P. , Machmudah , S. , Goto , M. , Sasaki , M. , Pavasant , P. and Shotipruk , A. 2008 . Response surface methodology to supercritical carbon dioxide extraction of astaxanthin from Haematococcus pluvialis . Bioresource Technology , 99 ( 8 ) : 3110 – 3115 .
- Xiao , H.S. , He , W.J. , Fu , W.Q. , Cao , H.Y. and Fan , Z.N. 1999 . A spectrophotometer method testing oxygen radicals . Progress in Biochemistry and Biophysics , 26 ( 2 ) : 180 – 182 .
- Osawa , T. and Namiki , M. 1985 . Natural antioxidant isolated from eucalyptus leaf waxes . Journal of Agricultural and Food Chemistry , 33 ( 5 ) : 777 – 780 .
- Chen , H.M. , Muramoto , K. and Yamauchi , F. 1995 . Structural analysis of antioxidative peptides from soybean β-conglycinin . Journal of Agricultural and Food Chemistry , 43 ( 3 ) : 574 – 578 .
- Yildirim , A. , Mavi , A. and Kara , A.A. 2001 . Determination of antioxidant and antimicrobial activities of Rumex crispus L . extracts. Journal of Agricultural and Food Chemistry , 49 ( 8 ) : 4083 – 4089 .
- Giménez , B. , Alemnán , A. , Montero , P. and Gómez-Guillén , M.C. 2009 . Antioxidant and functional properties of gelatin hydrolysates obtained from skin of sole and squid . Food Chemistry , 114 ( 3 ) : 976 – 983 .
- Zhang , F.X. , Wang , Z. and Xu , S.Y. 2009 . Macroporous resin purification of grass carp fish (Ctenpharyngodon idella) scale peptides with in vitro angiotensin-I converting enzyme (ACE) inhibitory ability . Food Chemistry , 117 ( 3 ) : 387 – 392 .
- Zhu , Y.P. , Fan , J.F. , Cheng , Y.Q. and Li , L.T. 2008 . Improvement of the antioxidant activity of Chinese traditional fermented okara (Meitauza) using Bacillus subtilis B2 . Food Control , 19 ( 7 ) : 654 – 661 .
- Re , R. , Pellegrini , N. , Proteggente , A. , Pannala , A. , Yang , M. and Rice-Evans , C. 1999 . Antioxidant activity applying an improved ABTS radical cation decolorization assay . Free Radical Biology and Medicine , 26 ( 9–10 ) : 1231 – 1237 .
- Macdonald , J. , Galley , H.F. and Webster , N.R. 2003 . Oxidative stress and gene expression in sepsis . British Journal of Anaesthesia , 90 ( 2 ) : 221 – 232 .
- Wang , D. , Wang , L.-J. , Zhu , F.-X. , Zhu , J.-Y. , Chen , X.D. , Zou , L. , Saito , M. and Li , L.-T. 2008 . In vitro and in vivo studies on the antioxidant activities of the aqueous extracts of Douchi (a traditional Chinese salt-fermented soybean food) . Food Chemistry , 107 ( 4 ) : 1421 – 1428 .
- Güntensperger , B. , Hammerli-Meier , D.E. and Escher , F.E. 1998 . Rosemary extract and precooking effects on lipid oxidation in heat sterilized meat . Journal of Food Science , 63 ( 6 ) : 955 – 957 .
- Wiseman , H. and Halliwell , B. 1996 . Damage to DNA by reactive oxygen and nitrogen species: Role of inflammatory disease and progression to cancer . Biochemical Journal , 313 ( 1 ) : 17 – 29 .
- Yu , L.L. 2001 . Free radical scavenging properties of conjugated linoleic acids . Journal of Agricultural and Food Chemistry , 49 ( 7 ) : 3452 – 3456 .
- Duh , P.-D. 1998 . Antioxidant activity of burdock (Arctium lappa Linne): Its scavenging effect on free radical and active oxygen . Journal of the American Oil Chemist's Society , 75 ( 4 ) : 455 – 465 .
- Duh , P.-D. , Tu , Y.-Y. and Yen , G.-C. 1999 . Antioxidant activity of water extracted of Harng Jyur (Chrysanthemum morifolium Ramat) . LWT–Food Science and Technology , 32 ( 5 ) : 269 – 277 .
- Moktan , B. , Saha , J. and Sarkar , P.K. 2008 . Antioxidant activities of soybean as affected by Bacillus-fermentation to kinema . Food Research International , 41 ( 6 ) : 586 – 593 .
- Wang , H. , Gao , X.D. , Zhou , G.C. , Cai , L. and Yao , W.B. 2008 . In vitro and in vivo antioxidant activity of aqueous extract from Choerospondias axillaris fruit . Food Chemistry , 106 ( 3 ) : 888 – 895 .
- Ferreira , I.C.F.R. , Baptista , P. , Vilas-Boas , M. and Barros , L. 2007 . Free-radical scavenging capacity and reducing power of wild edible mushrooms from northeast Portugal: Individual cap and stipe activity . Food Chemistry , 100 ( 4 ) : 1511 – 1516 .
- Kehrer , J.P. 2000 . The Haber-Weiss reaction and mechanisms of toxicity . Toxicology , 149 ( 1 ) : 43 – 50 .
- Je , J.-Y. , Qian , Z.-J. and Kim , S.-K. 2007 . Antioxidant peptide isolated from muscle protein of bullfrog, Rana catesbeiana Shaw . Journal of Medicinal Food , 10 ( 3 ) : 401 – 407 .
- Chen , H.M. , Muramoto , K. , Yamauchi , F. and Nokihara , K. 1996 . Antioxidant activity of designed peptides based on the antioxidative peptide isolated from digests of a soybean protein . Journal of Agricultural and Food Chemistry , 44 ( 9 ) : 2619 – 2623 .