Abstract
The effect of heat treatment on apple polyphenols was investigated at six different temperatures over three different lengths of time. Apple polyphenols, after heat treatment, maintained similar concentrations in solution compared to the control (at room temperature). The effect of pH on apple polyphenols was investigated at 11 different pH levels and at 2 different temperatures. The pH-optimum for apple polyphenols proved to be 5.0. Two apple polyphenol concentrations, three vitamin C concentrations, two sugar concentrations, and two different temperatures were chosen to investigate the effect of apple polyphenols on vitamin C. Apple polyphenols showed good stability and can be used in many types of food products.
INTRODUCTION
Diet plays an important role in human health. Some studies have estimated that one-third of all cancer cases and one-half of all cardiovascular diseases and cases of hypertension can be attributed to diet.[Citation1–Citation3] The possible health benefits of diets that include fruits and vegetables have been attributed to their phytochemical component.[Citation4–Citation6] Fruit and vegetables contain many different phytonutrients, which play an important role in preventing some diseases caused by oxidative stress.[Citation7] Polyphenols are one of the phytochemical groups whose “protective” properties include antioxidant, antimicrobial, anticancer, and cardiovascular protective activities.[Citation8–Citation13]
Apples can play a significant part in these diets and they represent an important source of bio-available phenolic compounds, such as flavonols (with quercetin glycosides as the main component), monomeric and oligomeric flavanols, dihydrochalcones (e.g., phloridzin), anthocyanidins, p-hydroxycinnamic, and p-hydroxybenzoic acids.[Citation14,Citation15] The content of phenolic compounds varies greatly among apple varieties as well as its peel and flesh, with apple peel containing a higher concentration of phenolic compounds than the flesh.[Citation16]
Apple pomace, a by-product from apple juice processing, is a rich source of polyphenols, minerals, and dietary fiber.[Citation17–Citation20] The apple pomace used in this study was supplied by a fruit juice factory in Yantai, China. In the last 3 years, apple polyphenols have been extracted by ultrasonic extraction and their antimicrobial function has been examined.[Citation21] Apple polyphenols have been shown to strongly inhibit the growth of six tested bacterial strains, including Staphyloccocus aureus. The minimum inhibition concentration of the polyphenols was 0.6 g l−1 for Flavobacterium SHL45, 0.7 g l−1 for Escherichia coli, 0.6 g l−1 for Pseudomonas fluorescens SHL5, 0.6 g l−1 for Pseudomonas fluorescens SHL7, 0.8 g l−1 for Bacillus subtilis, and 0.5 g l−1 for Staphyloccocus aureus, respectively.[Citation22] These results showed that apple polyphenols could be of potential use as a natural antimicrobial agent in improving food safety. If they possess good stability under a wide range of temperatures, pH values, and vitamin C concentrations, then apple polyphenols could be used widely in food products. The objective of this study was to examine the total polyphenol content (TPC) of apple juice using spectrophotometrical determination and to investigate the stability of apple polyphenols when subjected to different levels of heat treatment, pH, and vitamin C, treatments which model the main effects of food producing technologies.
MATERIALS AND METHODS
Materials and Reagents
Apple pomace extract, with high polyphenol content, was extracted from apple pomace using the ultrasonic method. The process was as follows: Approximately 10 g of apple pomace was weighed in a flask, then a small amount of vitamin C was added. Next, a certain percentage of ethanol solutions were added. The flask was sealed and polyphenols were extracted by ultrasonic method. The optimum process parameters are: 70% ethanol, solid-liquid ratio of 1:6 (g ml−1), and extracted at 70°C for 30 min. Under these conditions, the concentration of polyphenols extracted from apple pomace was 162.3 ± 0.5 μg ml−1. After extraction, vacuum filtration was used; the filtrate solution was rotary evaporated and then freeze-dried to achieve apple pomace extracts. Folin-Ciocalteu reagent and 2,6-dichlorophenol indophenol were purchased from Sigma-Aldrich Ltd. (Hungary). Gallic acid, sodium carbonate, citric acid, and sodium hydrogen carbonate were purchased from Reanal Ltd. (Hungary). All of the chemicals were analytical grade.
Determination of Apple Polyphenols Concentration
Total polyphenol content in apple pomace extracts was determined using Folin-Ciocalteu reagent.[Citation23,Citation24] The reaction mixture was prepared by mixing 0.1 ml of aqueous solution (concentration 1 g l−1) of the extract, 7.9 ml of distilled water, 0.5 ml of Folin-Ciocalteu reagent, and 1.5 ml of 20% sodium carbonate. After 2 h, the absorbance at 750 nm was measured against a blank that had been prepared in a similar manner but replacing the extract with distilled water. The total phenolic concentration was determined using a gallic acid calibration curve as a standard.
Effect of Heat Treatment on Apple Polyphenols
In order to model the pasteurization process in food production, six temperature levels (70, 75, 80, 85, 90, and 95°C) and three time durations (10, 20, and 30 min) were chosen to investigate the effect of heat treatment on apple polyphenols. The control was subjected to room temperature (20°C).
Effect of pH on Apple Polyphenols
The pH of food products varies widely. For example, the approximate pH of apple juice is 3.35–4.00, whereas for canned beets it is 4.90–5.80. In this experiment, some common pH levels in foods were selected to evaluate the stability of apple polyphenols under a range of pH. One g l−1 of polyphenol solution dissolved in distilled water was adjusted to pH 2.0, 2.5, 3.0, 3.5, 4.0, 4.5, 5.0, 5.5, 6.0, 6.5, and 7.0, respectively, using citric acid and sodium hydrogen carbonate. The control was 1 g l−1 of polyphenol solution with a pH of 3.55. The effect of pH on apple polyphenols was first investigated at room temperature (20°C). Then three pH levels (at which the highest concentration of apple polyphenols was observed) were chosen to investigate the effect of pH on apple polyphenols at 80 and 90°C.
Effect of Apple Polyphenols on Vitamin C Content
Vitamin C, also known as L-ascorbic acid, is a water-soluble vitamin and is present in many common foodstuffs. In order to investigate the effect of apple polyphenols on vitamin C content, two apple polyphenols concentrations (0 g 100 ml−1 and 0.1 g 100 ml−1); three levels of vitamin C content (10 mg 100 ml−1, 50 mg 100 ml−1, and 100 mg 100 ml−1); two levels of sugar content (0 g 100 ml−1 and 10 g 100 ml−1); and two temperature levels (20 and 90°C) were chosen to simulate food production process conditions.
Determination of Vitamin C Content
Vitamin C was analyzed according to the dichlorophenol indophenol titrimetric method as described in AOAC.[Citation25] Two grams of apple pomace was mixed with 50 milliliters of 2% oxalic acid solution, ground into a homogenate, and then filtered to obtain a filtrate. A 10-ml filtrate from this was titrated with dichlorophenol indophenols until color change. Results were expressed as mg/100 g of sample.
Statistical Analysis
p-Value of the t-test was performed to evaluate the effect of heat treatment, pH, and vitamin C on apple polyphenols. All data in tables and figures represent mean values ± standard deviation (n = 3). Differences are indicated as being significant if p < 0.05. All of the calculations were carried out using Statistica 8.0 software (Statsoft, Inc., Tulsa, OK, USA).
RESULTS AND DISCUSSION
Effect of Heat Treatment on Apple Polyphenols
Apple polyphenols have been reported to have good heat stability.[Citation26] The effect of heat treatment on apple polyphenols was investigated and the results are as follows (). As the temperature rose from 70 to 95°C, the concentration of apple polyphenols decreased slightly (from 750 to 700 ppm). However, there was only a small difference compared to the control (720 ppm).
Figure 1 3D surface plot of apple polyphenol concentration (ppm) against temperature (°C) and time (min).
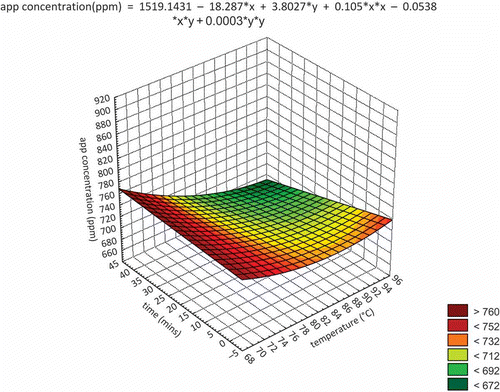
Interestingly, apple polyphenol content was higher after heat treatment at 70°C than it was in the control samples. There may be several possibilities for this, including polymerization, heat-degradation, or release of polyphenol molecules from some components (e.g., the cell wall, saccharides, and proteins).[Citation27–Citation29] As the heat treatment duration increased, the concentration of apple polyphenols changed slightly. However, there was no marked difference compared to the control. Samples heated for 40 min had a lower polyphenol concentration than the control. Samples heated for 20 min showed higher values compared to the control.
Effect of pH on Apple Polyphenols
Some studies have demonstrated that caffeic, chlorogenic, and gallic acids are not stable at high pH values. However, chlorogenic acid becomes stable to acid pH and heat, and can be stored when added to apple juice. (−)-Catechin, (−)-epigallocatechin, ferulic acid, rutin, and trans-cinnamic acid all resisted major pH-induced degradation.[Citation30,Citation31]
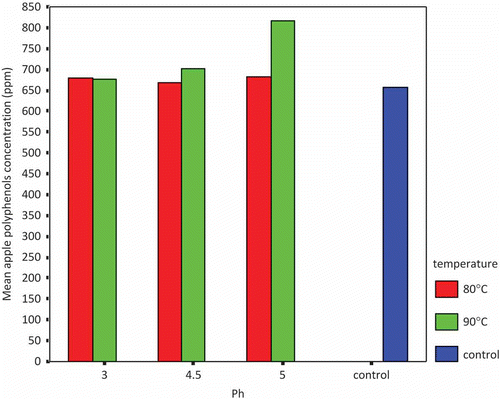
The stability of apple polyphenols was evaluated at the pH levels that occur in food products. The pH value of 1 g l−1 water solution of apple polyphenol was 3.55 (control). shows the effect of pH on apple polyphenols at two different temperatures (80 and 90°C). It indicates that at 90°C, the apple polyphenol concentration was higher than at 80°C and was also higher than the control.
There are several possible reasons for the results above: first, when apple polyphenols are subjected to heat treatment, some chemical reactions (e.g., polymerization, heat-degradation or release of polyphenol molecules from some compounds) will happen; second, apple polyphenols are acid compounds and easily dissolved in acid solutions. However, if the pH value is too low, polyphenol molecules will be degraded into other molecules.[Citation32]
Effect of Apple Polyphenols and Sugar Content on Vitamin
Vitamin C is a very important antioxidant agent in the human body. It is an antioxidant vitamin needed for the formation of collagen, which holds cells together, and for healthy teeth, gums, and blood vessels. It also improves iron absorption and resistance to infection. However, vitamin C is very sensitive to heat treatment, light levels, pH, and oxygen. Apple polyphenols are reported as having anti-oxidation and co-operative effects with vitamin C in food products.[Citation33–Citation35] In order to model the vitamin C-polyphenol interactions in fruit-based food products, solutions with sugar contents of 10 and 20 g 100 ml−1, and vitamin C contents of 10, 50, and 100 mg were prepared. The effect of adding 1 g l−1 of apple polyphenol was tested at room temperature and after pasteurization (90°C, 10 min).
Effect of Apple Polyphenols on Vitamin C
shows the effect of apple polyphenols on vitamin C. It indicates that apple polyphenol content had a significant effect on vitamin C. The samples containing apple polyphenols showed higher vitamin C content than the samples without apple polyphenols. This can be explained by the anti-oxidation and cooperation effect between vitamin C and apple polyphenols.
Table 1 Effect of apple polyphenols on vitamin C in samples with differing sugar contents at room temperature and 90°C
CONCLUSIONS
After being heat-treated, concentrations of apple polyphenols samples were approximately equal with the control at room temperature. The optimum pH value of apple polyphenols was 5.0. When samples were treated at pH value of 3.0–5.0 and at a temperature of 90°C, the apple polyphenol concentrations of the treated samples were similar. Heat treatment, concentration of sugar, and content of apple polyphenol were significantly affected by the vitamin C content; when these three factors acted on the vitamin C, samples of vitamin C content were significantly affected except for the samples with original vitamin C content of 10 mg/100 mL. Apple polyphenols possessed good heat and pH stability and could prevent vitamin C from oxidation. Thus, apple polyphenols extracted from apple pomace could be used in several food products as a functional food additive, preservative, etc.
ACKNOWLEDGMENTS
The authors gratefully acknowledge the technical support of Jakab David (Corvinus University of Budapest).
REFERENCES
- Lee, C.Y.; Smith, N.L. Apples: An important source of antioxidants in the American diet. New York Fruit Quarterly 2000, 8, 8–10.
- Willet, W.C. Diet and health: What should we eat. Science 1994, 254, 532–537.
- Wolfe, K.; Wu, X.; Liu, R.H. Antioxidant activity of apple peels. Journal of Agricultural and Food Chemistry 2003, 51, 609–614.
- Block, G.; Patterson, B.; Subar, A. Fruit, vegetables, and cancer prevention: A review of the epidemiological evidence. Nutrition and Cancer 1992, 18, 1–29.
- Lampe, J.W. Health effects of vegetables and fruit: Assessing mechanisms of action in human experimental studies. American Journal of Clinical Nutrition 1999, 70, 475S–490S.
- Liu, R.H. Health benefits of fruit and vegetables are from additive and synergistic combinations of phytochemicals. American Journal of Clinical Nutrition 2003, 78, 517–520.
- Kaur, C.; Kapoor, H.C. Antioxidants in fruits and vegetables—Millennium’s health. International Journal of Food Science and Technology 2001, 36, 703–725.
- Bendini, A.; Cerretani, L.; Pizzolante, L.; Toschi, T.G; Guzzo, F.; Ceoldo, S.; Marconi, A.M.; Andreetta, F.; Levi, M. Phenol content related to antioxidant and antimicrobial activities of Passiflora spp. extracts. European Food Research and Technology 2006, 223, 102–109.
- Chu, Y.H.; Chang, C.L.; Hsu, H.F. Flavonoid content of several vegetables and their antioxidant activity. Journal of the Science of Food and Agriculture 2000, 80, 561–566.
- Hertog, M.G.L.; Kromhout, D.; Aravanis, C.; Blackburn, H.; Buzina, R.; Fidanza, F.; Giampaoli, S.; Jansen, A.; Menotti, A. Flavonoid intake and the long-term risk of coronary heart disease and cancer in the Seven Countries Study. Archives of Internal Medicine 1995, 155, 381–386.
- Liu, R.H. Health benefits of dietary flavonoids: Flavonols and flavones. New York Fruit Quarterly 2002, 10, 21–23.
- Süzgeç, S.; Meriçli, A.H.; Houghton, P.J.; Çubukçua, B. Flavonoids of Helichrysum compactum and their antioxidant and antibacterial activity. Fitoterapia 2005, 76, 269–272.
- Yadav, D.N.; Patki, P.E.; Srihari, S.P.; Sharmaa, G.K.; Bawaa, A.S. Studies on polyphenol oxidase activity of heat stabilized whole wheat flour and its chapatti making quality. International Journal of Food Properties 2010, 13 (1), 142–154.
- Ekrami-Rad, N.; Khazaei, J.; Khoshtaghaza, M.H. Selected mechanical properties of pomegranate peel and fruit. International Journal of Food Properties 2011, 14 (3), 570–582.
- Escarpa, A.; Gonzalez, M. High-performance liquid chromatography with diode-array detection for the performance of phenolic compounds in peel and pulp from different apple varieties. Journal of Chromatography A 1998, 823, 331–337.
- Vrhosek, U.; Rigo, A.; Tonan, D.; Mattivi, F. Quantitation of polyphenols in different apple varieties. Journal of Agricultural and Food Chemistry 2004, 52, 6532–6538.
- Boyer, J.; Liu, R.H. Apple phytochemicals and their health benefits. Nutrition Journal 2004, 3, 5–19.
- Figuerola, F.; Hurtado, M.L.; Estevez, A.M.; Chiffelleb, I.; Asenjoa, F. Fibre concentrates from apple pomace and citrus peel as potential source for food enrichment. Food Chemistry 2005, 91, 395–401.
- Schieber, A.; Keller, P.; Carle, R. Determination of phenolic acids and flavonoids of apple and pear by high-performance liquid chromatography. Journal of Chromatography A 2001, 910, 265–273.
- Sudha, M.L.; Baskaran, V.; Leelavathi, K. Apple pomace as a source of dietary fibre and polyphenols and its effect of the rheological characteristics and cake making. Food Chemistry 2007, 104, 686–692.
- Suárez, B.; Álvarez, Á.L.; Garcíaa, Y.D.; Barriob, G.D.; Loboa, A.P.; Parrac, F. Phenolic profiles, antioxidant activity and in vitro antiviral properties of apple pomace. Food Chemistry 2010, 120 (1), 339–342.
- Sun, H.; Sun, A.; et al. Antimicrobial effect of apple polyphenols. Journal of Beijing Forestry University 2010, 32 (4), 280–283.
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventos, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin–Ciocalteu reagent. In: Methods in Enzymology, Vol. 299; Packer, L.; Ed.; Academic Press: San Diego, CA, 1999; 152–178.
- Djordjevic, T.M.; Siler-Marinkovic, S.S.; Dimitrijevic-Brankovic, S.I. Antioxidant activity and total phenolic content in some cereals and legumes. International Journal of Food Properties 2011, 14 (1), 175–184.
- AOAC. AOAC official method 985.33: Vitamin C in ready-to-feed milk-based infant formula, 2,6-dichloroindophenol titrimetric method. In: Official Methods of Analysis. Association of Official Analytical Chemists: Washington, DC, 1995.
- Vámos-Vigyázó, L.; Haard, N.F. Polyphenol oxidases and peroxidases in fruits and vegetables. Critical Reviews in Food Science and Nutrition 1981, 15, 49–127.
- Kagalkar, A.N.; Jagtap, U.B.; Jadhav, J.P.; Bapat, V.A.; Govindwar, S.P. Biotechnological strategies for phytoremediation of the sulfonated azo dye Direct Red 5B using Blumea malcolmii Hook. Bioresource Technology 2009, 100, 4104–4110.
- Murakami, M. Change in the radical-scavenging activity of quercetin and epigallocatechin gallate during heat treatment. Niigata University Academic Repository 2004, 55, 213–217.
- Siebert, K.J. Effects of protein-polyphenol interactions on beverage haze, stabilization, and analysis. Agricultural and Food Chemistry 1999, 47 (2), 353–362.
- Friedman, M.; Jürgens, H.S. Effect of pH on the stability of plant phenolic compounds. Agricultural and Food Chemistry 2000, 48 (6), 2101–2110.
- Idris, Y.M.A.; Mariod, A.A.; Hamad, S.I. Physicochemical properties, phenolic contents and antioxidant activity of Sudanese honey. International Journal of Food Properties 2011, 14 (2), 450–458.
- Sun, J.; Sun, A.; Bai, W. Study on the function and application of apple polyphenols. Nutrition and Health Care 2004, 10, 38–41.
- Pennington, J.; Bowes, G. Food Values of Portions Commonly Used, Lippincott Williams and Wilkins: USA, 1998.
- Smolin, L.; Grosvenor, J. Nutrition Science & Applications, Saunders College Publishing: UK, 2000.
- Basset, G.; Raymond, P.; Malek, L.; Brouquisse, R.; Changes in the expression and the enzymic properties of the 20S proteasome in sugar-starved maize roots. Evidence for an in vivo oxidation of the proteasome. Plant Physiology 2002, 128, 1149–1149.