Abstract
Gas chromatography, gas chromatography/mass spectrometry, headspace/gas chromatography/mass spectrometry, and Dean's switch technology were used to identify the structure and investigate the transformation of aroma components of Chinese Fenjiu under different ages (from 0 to 30 years). Results showed that the variation in physicochemical properties of Fenjiu was due to the synergistic effects of volatilization of low boiling point matters, oxidation of dissolved oxygen, balance between esterification and hydrolysis, weak interaction among molecules, and involvement of metal elements extracted from storage vessels. By simulating the natural storage environment and utilizing high-gravity rotating packed bed technique, a multistage-spraying rotating packed bed was developed locally and successfully applied to liquor aging. The liquor treated by this method had qualities equivalent to the liquors aged naturally for more than two years. The present study reveals the traditional aging process of Chinese liquor and offers a perspective into the utilitization of high-gravity rotating packed bed technique as an alternative tool for liquor aging.
INTRODUCTION
Chinese liquor, also known as samshu or firewater, is one of the famous distillates in the world. Along with brandy, whisky, rum, vodka, gin, and other distillates, they are called the seven distilled spirits in the world.[Citation1] Based on aroma characteristics, Chinese liquors can be classified into light aroma, strong aroma, sweet honey, soy sauce aroma, and miscellaneous styles.[Citation2] Fenjiu, principally obtained from sorghum, is one of the most popular light aroma style beverages and has a long history of over 1500 years.[Citation3] The fresh liquor is distilled using stream after fermentation and subsequently aged in sealed ceramic jars to develop balanced taste and aroma.
The freshly brewed liquor has undesirable characteristics, such as “peppery,” “harsh,” “green,” and “raw” flavor. These attributes are due to the hydrogen sulfides, mercaptans, and sulfides derived from sulfur-containing amino acid metabolism, as well as small amounts of acetaldehyde, acrolein, crotonaldehyde, methanol, free ammonia, and other low boiling point substances.[Citation4] However, the “mature” liquor has a pleasant and sweet mouthfeel resulting from a series of reactions during the aging process. The liquor properties are modified during the aging process whose effect is to strengthen some good characteristics while eliminating the unwanted ones. This progress is beneficial for the liquor quality and usually takes from six months to a few years. As a result, freezing of invested capital and financial burden for wineries will inevitably be incurred.
The quality of liquors has been known to be principally determined by its aroma compounds. Thus, exploring the composition and variations of aroma compounds in liquors is crucial in developing a new method of liquor aging to reduce aging time. A few studies have reported on the changes during the aging process.[Citation5 Citation Citation−7] Fan and Qian[Citation8] analyzed young and aged Chinese “Yanghe Daqu” liquor. However, none of them comprehensively explored the traditional aging process of liquors. In the past few years, scientists put more emphasis into shortening the aging time using different techniques, including physical methods[Citation9] (microwave,[Citation10] ultrasonic,[Citation11] infrared wave,[Citation12] laser,[Citation13] X-ray,[Citation14] magnetic,[Citation15] adsorption,[Citation16] and barrel-aging[Citation17,Citation18]); chemical methods (electrochemical[Citation19] and oxygenation[Citation20,Citation21]); biological methods;[Citation22,Citation23] and others. However, most of these methods present many limitations to Chinese liquor, such as reverting to original crudity or processing difficulty. Moreover, none of them can be applied in the industry. Thus, further studies are required to develop a new technology of accelerating liquor aging that can be used in wineries.
The purpose of the present work is to reveal the changes in liquor during the traditional aging process by comprehensively analyzing the transformation of aroma components, electric conductivity, and mineral contents in 65% Fenjiu, naturally stored for 0 to 30 years. Based on this objective, the current work explores the potential of the high-gravity rotating packed bed (RPB; HGRPB) technology employed in liquor aging to come up with a product comparable in quality with the conventionally aged wine.
MATERIALS AND METHODS
Chemicals and Liquors
The materials used in the present study were the following: tert-amyl alcohol (99.5%, Beijing chemical reagents), amyl acetate ester (99.5%, Beijing chemical reagents), and 2-ethylbutyric acid (99%, Acros Organics). All other commercially obtained reagents were of analytical grade without purification. Fenjiu (65% v/v), stored for 0, 0.5, 2, 5, 10, 16, 20, 25, and 30 years, were analyzed. They were all provided by Shanxi Xinghuacun Fenjiu Group.
Gas Chromatography/Mass Spectrometry (GC/MS) Analysis
An Agilent 7890 gas chromatograph equipped with a flame ionization detector (FID), 5975 mass spectrum detector (MSD), and a Dean's switch assembly was used. Ions were generated by the effect of electrons with energy of 70 eV in the 29–400 aμ mass range scanning mode. The transfer line temperature was 280°C. The MS QUAD temperature was 150°C, and the ion-source temperature was set to 230°C. An HP-FFAP MS capillary column (30 m × 0.25 mm inside diameter (ID); 0.25 μm film thickness; J&W Scientific) was used as the main column and directly connected to the MSD. An Agilent HP-5(30 m × 0.25 mm ID; 0.25 μm film thickness; J&W Scientific) was used as the secondary column. Helium was used as the carrier gas. The injection volume was 1 μL with a split ratio of 30:1. The injection port and FID temperatures were 250°C. The temperature program of the oven began at 45°C, held for four min, raised to 230°C at a rate of 5.5°C/min, and held for 20 min. The Dean's switch cut time was from 7.0 min to 9.6 min for ethanol, which interfered with other volatile compounds and switched to the secondary column. The mass spectra of all peaks were analyzed and identified by comparison with the mass spectra obtained with those present in NIST 05 Spectral Library and in Reference.[Citation24]
Headspace/GC/MS (HS/GC/MS) Analysis of Volatile Matters
HS/GC/MS was carried out using an Agilent 7694 plus GC/MS 5975C and was used to investigate low boiling point matters in a new liquor, two-year-old wine, and 10-year-old wine. The headspace was generated using 10 mL of diluted wine, 2.0 g NaCl, and 0.05 g EDTA in a 20 mL glass vial. The vials were then tightly capped with silicone septa and shaken. Equilibrium was reached in 2 h at 60°C.[Citation25] A one mL volume of headspace was injected after one min. The oven and injector temperatures, effect of electron energy, and other MS conditions were identical to those of the GC/MS analysis described previously, except that the HP-FFAP MS capillary column was the only column involved.
Gas Chromatographic Analysis
The following equipment and column were used for the GC analysis of the major aroma compounds without any previous treatment: a Shimadzu 2010 gas chromatograph fitted with a FID. Each sample was analyzed on a BP-21 FFAP capillary column (25 m × 0.32 mm ID; 0.25 μm film thickness; SGE, Victoria, Australia). Nitrogen was used as the carrier gas with a flow rate of one mL/min. The injector temperature was 250°C, and the detector temperature was 250°C. The temperature program was identical to that of the GC/MS analysis described previously. Samples of 1 μL at split mode of 30:1 were injected. Tert-amyl alcohol, amyl acetate ester, and 2-ethylbutyric acid served as the internal standard, and the quantitative analysis of the components followed the method introduced by Cai.[Citation24]
Chemical Analysis
The electrical conductivities of the liquors were measured using a DDSJ-308A electrical conductivity instrument (Leici, Shanghai) at a constant temperature of 25°C. The concentrations of Al, Ca, Cu, Fe, Mg, and Zn in the liquors were measured using the IRIS Intrepid II XSP ICP atomic emission spectrometer (MA, USA).
HGRPB Treatment
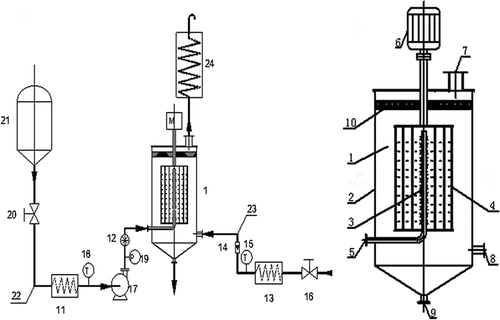
shows the sketches of the experimental system RPB units.[Citation26] The equipment is locally developed for liquor aging. During normal operations, after adjustment of the motor rotational speed the and the flow velocity of the liquor and oxygen, the freshly brewed liquor was pumped into the packed bed from a liquid inlet and then flowed into the spraying area through the slotted liquid distributor. The liquor was vaporized into thin films and tiny droplets using a rigorous centrifugal acceleration in the first stage of the concentric cylinders. Subsequently, it was sprayed along the transverse direction by centrifugal force onto the stuffing in the second and third stages. Therefore, the liquor contacted sufficiently with the ceramic wafer stuffing at the three stages of the concentric cylinders. At the same time, oxygen stream was introduced into the packed bed from the gas inlet, flowed lengthwise, contacted with the liquor and ceramic wafers completely, and finally left through the gas outlet pipe and entered into a condensation system to reduce liquor loss. The liquor was expelled from the bottom liquid outlet. Afterward, the composition of the treated liquor was analyzed using GC. The metal ions, electrical conductivity, and sensory evaluation were also studied. The experiment was replicated three times, and the results were obtained from the average of the three data.
Figure 1 Schematic diagram of multistage-spraying rotating packed bed (1: rotating packed bed; 2: shell; 3: liquid distributor; 4: spraying area; 5: liquid inlet; 6: motor; 7: gas outlet; 8: gas inlets; 9: liquid outlet; 10: trap; 11: liquid heater; 12: liquid flow controller; 13: gas heater; 14: gas flow controller; 15: gas thermometer; 16: gas valve; 17: pump; 18: liquid thermometer; 19: pressure gauge; 20: liquid valve; 21: liquid storage tank; 22: liquid pipeline; 23: gas pipeline; 24: condensation system).
Statistical Analysis
ANOVA was used to analyze the data obtained from the three replications, using the SPSS 16.0 software.
Sensory Evaluation
The liquor samples were evaluated by five state-level liquor sensory tasters following the method established by Shen.[Citation1] Number the treated liquor newly brewed, 0.5-year-aged, 2-year-aged, and 5-year-aged liquors, and equal liquor contents were poured into cups of the same size. The tasters appraised these liquors by observing the liquor color, smelling the liquor aroma, savoring the liquor taste, and judging the liquor quality. They then correspondingly graded and commented on each liquor sample. The final grades were obtained from the average of 10 results, and the comments were drawn from discussions among the tasters.
RESULTS AND DISCUSSION
GC-MS Analysis
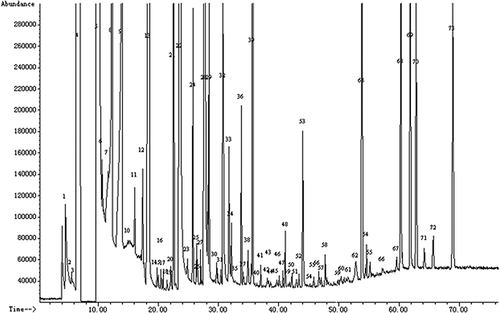
The flavor of a distilled beverage is affected by aroma compounds.[Citation27,Citation28] Every molecule may be responsible for a given sensory attribute. To explain reasonably the quality of liquors, the characteristic of aroma compounds has been seriously studied. GC/MS is systematically used for identification of volatile compounds present in the liquors. The aroma compounds in 65 Fenjiu were analyzed without pretreatment. The total ion chromatogram is shown in , where a total of 73 compounds are detected and 69 of them identified. In addition to the most abundant esters, a large number of alcohols, acids, ketones, aldehydes, lactones, furan derivatives, and phenolic compounds were found.
Figure 2 Total ion chromatogram of Fenjiu (1: acetaldehyde; 2: isobutylaldehyde; 3: ethyl formate; 4: ethyl acetate+acetal; 5: ethanol; 6: 2-butanol; 7: n-propanol; 8: 1,1-diethoxy isopentane; 9: isobutanol; 10: isoamyl acetate; 11: ethyl valerate; 12: butanol; 13: isoamyl alcohol; 14: ethyl caproate; 15: n-pentanol; 16: 3-methyl-3-buten-1-ol; 17: unknown; 18: 1,1,3-triethoxy-propane; 19: ethyl heptanoate; 20: 3-hydroxy butanone; 21: anisole; 22: ethyl lactate; 23: hexanol; 24: ethyl caprylate; 25: 2-hydroxy-3-methyl-butayric acid ethyl ester; 26: 1,2-propanediol; 27: heptanol; 28: acetic acid; 29: furfural; 30: benzaldehyde; 31: propionic acid; 32: 2,3-butanediol(levo); 33: isobutyric acid; 34: 2,3-butanediol(meso); 35: ethyl caprate; 36: butyric acid; 37: γ-butyrolactone; 38: isovaleric acid; 39: diethyl succinate; 40: 2,2-diethoxy-phenylethane; 41: unknown; 42: naphthalene; 43: n-pentanoic acid; 44: ethyl phenylacetate; 45: 1-nitro-4-vinylbenzene; 46: phenyl ethyl acetate; 47: ethyl laurate; 48: hexanoic acid; 49: unknown; 50: 1-methylnaphthalene; 51: benzoic acid; 52: diisobutyl succinate; 53: β-phenylethyl alcohol; 54: 1,5-dimethyl naphthalene; 55: enanthic acid; 56: 1,2- dimethyl naphthalene; 57: ethyl myristate; 58: 4-pentyl-γ- butyrolactone; 59: 2-methyl-diphenyl; 60: diisobutyl adipate; 61: ethyl pentadecanoate; 62: 4,4'-dimethoxy-diphenylmethane; 63: ethyl palmitate; 64: ethyl 11-hexadecenoate; 65: ethyl 9-hexadecenoate; 66: propanetriol; 67: ethyl stearate; 68: ethyl oleate; 69: ethyl linoleate; 70: diisobutyl phthalate; 71: octadeca-9,12,15-trienoate; 72: unknown; 73: dibutyl phthalate).
HS/GC/MS Analysis
Fresh distillates often have some undesirable characteristics, such as harsh, green, and raw savor. To distinguish the main components of the offensive savor in the fresh distillates, low boiling point matters in fresh and aged wines were analyzed by HS/GC/MS. Tentative identification was achieved by comparing the mass spectra, and the results are shown in . lists the compounds of Fenjiu at different wine ages, where 28 kinds of aroma components were detected in the fresh brewed wine. Six of these, namely, 2-methyl piperazine, DL-3-amino-n-butyric acid, 5-methyl thiazole, dihexyl sulfite, 2-methyl azetidine, and methoxy-benzaldoxime, were not found in the two-year and 10-year-aged wine. All these components were nitrogen- or sulfur-containing compounds and have undesirable smell.
Table 1 Aroma components of Fenjiu under different ages as detected by HS/GC/MS
Table 2 Aroma compound contents of Fenjiu under different ages and the liquor treated by HGRPB (mg/L)
Variations in Physicochemical Properties of Fenjiu During Storage
Liquors stored for 0 to 30 years were analyzed using GC, and 31 aroma components whose contents were over one mg/L were selected to investigate the relationship between transformation of aroma components and storage time. The results are shown in . lists the aroma compounds at different ages. The contents of most esters, such as ethyl formate, ethyl acetate + acetal, isoamyl acetate, ethyl caproate, ethyl lactate, ethyl caprylate, and ethyl palmitate, decreased markedly with aging, except for ethyl caprate and diethyl succinate. In contrast to esters, all acid contents, such as acetic acid, butyric acid, and hexanoic acid, were higher in aged liquors compared with young liquors. Further, most aldehydes contents, including acetaldehyde, isobutyl aldehyde, and isovaleric aldehyde, displayed a decreasing trend with the increase in wine age, which are ascribed to the following factors:
Volatilization of Low Boiling Point Matters
The acetaldehyde, isobutyl aldehyde, methanol, and isovaleric aldehyde contents decreased with storage time. also shows that some nitrogen- and sulfur-containing compounds gradually disappear. During storage, these compounds were apt to volatilize because of their low boiling point. Thereby, the offensively harsh, green, and raw flavor was eliminated and “mature” smell developed.
In addition, as the main flavor component, esters contributed to the floral, fruity, sweet, and apple-like aroma and exerted an important influence on the sensory characteristics of the liquor.[Citation29,Citation30] shows that the ethyl acetate and ethyl lactate contents decreased with liquor age. However, the decreasing amount of ethyl acetate was greater than that of the ethyl lactate because ethyl acetate has a relatively lower boiling point and volatilizes easily compared with ethyl lactate. Moreover, the effects of β-phenylethyl alcohol and 3-hydroxy butanone must be taken into account. β-phenylethyl alcohol possesses honey-flavored rose aroma, which makes Fenjiu not only smell sweet, but also features a lasting fragrance. 3-hydroxy butanone is also one of the most important aroma substances. Their concentration increases lately with storage time, probably because some smaller, lower boiling point aroma compounds escape from the ceramic jar, whereas the larger ones with higher boiling point cannot migrate and are concentrated during storage.[Citation8] Therefore, volatilization of low boiling point matters is an effective way of eliminating the offensive savor and enhances mature smell.
Activities of Dissolved Oxygen and Balance Between Esterification and Hydrolysis
shows that the low alcohol and most aldehyde compound contents were reduced due to dissolved oxygen activities. Chinese liquor is stored in ceramic jars, which induces certain effects on the liquor aging. During ceramic sintering, organics and other gases contained in clay are ruled out when the sintering temperature is at 750°C. As a result, numerous millipores of ceramic jars are formed.[Citation31] Oxygen dissolves in liquor through these millipores, which enhances oxidation reactions of alcohols and aldehydes, transforming them to acids. Oxygen is also assumed to play a major role in many chemical reactions that occur during the aging process.[Citation32 Citation Citation−34] shows that most esters showed decreasing trend, whereas acids show the opposite. The decrease of esters can be attributed to their hydrolysis into acids. The balance between esterification and hydrolysis[Citation35] is also an important transformation factor during storage.
Effect of the Storage Vessels' Material
Storage position and environment affect the evolution of wine and liquors.[Citation36] Inorganic elements existing in ceramic jar gradually migrate into the liquor during storage and promote liquor aging.[Citation31] Thus, the electrical conductivities and metal elements of Fenjiu at different ages were analyzed. The results are shown in . The relationship between electrical conductivity (Y) and the liquor age (X) showed a remarkable correlation according to the following equation, with R 2 equal to 0.931:
Furthermore, the six metal elements and the electrical conductivities of liquors stored for one year in vessels made of stainless steel jar, black pottery, and red pottery were measured. The results are shown in , which shows that metal cations are gradually extracted from the vessels during storage and take part in some of the physicochemical reactions.[Citation31] Obviously, the electrical conductivity of the liquor increased with storage time for two reasons. On the one hand, the concentrations of H+ increased with the increase in the liquors acid contents. On the other hand, metal cations were extracted from the ceramic jars and dissolved in liquors. The more metal elements are present, the better is the liquor taste. These results agree with the findings of other studies[Citation37,Citation38] and indicate that the influence of the storage vessels' material on liquor aging cannot be discounted.
To sum up, the variations in the physicochemical properties of Fenjiu during the aging process are the integrated results of the volatilization of low boiling point matters, oxidation of dissolved oxygen, balance between esterification, and hydrolysis, weak interaction between molecules, and involvement of the metal elements extracted from the storage vessels. The process usually takes a very long time. However, if a method is available that can make the gas (dissolved oxygen), liquid (liquor), and solid phase (vessel) contact adequately, then the physical and chemical reactions speeds during storage would be enhanced; therefore, the aroma will be better, and the liquor taste will be sweeter, softer, and more refined.
Effect of HGRPB on Newly Brewed Liquor
In the present study, the freshly brewed liquors were treated using the home-developed multistage-spraying RPB. The basic principle of HGRPB is to create a high-gravity environment using centrifugal forces, where the liquor, oxygen, and ceramic wafers interacted rigorously with one other. Hence, the rate of molecular diffusion and mass transfer can be accelerated tremendously to 2–3 orders of magnitude faster than those in conventional gravity environment.[Citation39,Citation40] The treated liquors were subsequently analyzed by GC (). The metal element contents and electrical conductivities are shown in .
and shows that most esters exist at decreased levels, and the corresponding acids exist at increased levels in the treated liquor compared with those of the freshly brewed one. Owing to low boiling point and easy oxidization, lower alcohol and aldehydes contents reduced. Similarly, higher alcohol contents, such as isobuanol, isoamyl alcohol, and others, increased because of their high boiling point. Moreover, typical aroma components, such as β-phenylethyl alcohol and 3-hydroxy butanone, existed at increased levels. The metal elements and electrical conductivities in the liquor treated by the multistage-spraying RPB displayed an increasing trend compared with the newly brewed liquor. The phenomena were identical to the trend of naturally aged liquor.
Organoleptic sensory evaluation plays an important role in determining liquor quality. The colors, smell, taste, and style properties are the key deciding factors.[Citation41] lists the mean scores of each liquor sample based on the evaluation of the five state-level wine reviewers, which shows that the liquor treated by the multistage-spraying RPB obtained a high value in sensory score. Thus, HGRPB treatment effectively improves liquor quality, accelerates liquor aging, and shows good potential as an alternative application method for liquor aging.
Table 3 Metal ion contents (mg/L) and electrical conductivities (μs/cm) of Fenjiu under different ages (year)
Table 4 Metal ion contents (mg/L) and electrical conductivities (μs/cm) of liquors stored for one year in vessels made of different materials
Table 5 Organoleptic investigation results of the liquors
CONCLUSION
In the present study, conclusion is made that the variations in the physicochemical properties of Fenjiu during aging process are the integrated results of volatilization of low boiling point matters, oxidation of dissolved oxygen, balance between esterification and hydrolysis, weak interaction between molecules, and participation of metal elements extracted from storage vessels. Meanwhile, high-gravity technology is first introduced in the liquor aging process, and a multistage-spraying RPB was locally developed to simulate natural storage environment. The indexes of the liquor treated by HGRPB validated the potential of multistage-spraying RPB as a good application for liquor aging. Application for Chinese patent has been made for this invention, and its use has been authorized.
ACKNOWLEDGMENT
This work was supported in part by the Key Project (No. 20080311082) and in part by Graduate Innovative Project (No. 20093029) of Shanxi Province. The authors would like to thank the state-level wine reviewers in Shanxi Xinghuacun Fenjiu Group for the organoleptic investigation.
REFERENCES
- Shen , Y.F. 1998 . Manual of Chinese Liquor Manufactures Technology , Beijing , China : Chinese Light Industry Press .
- Fan , W.L. , Xu , Y. and Zhang , Y.H. 2007 . Characterization of pyrazines in some Chinese liquor and their approximate concentrations . Journal of Agricultural and Food Chemistry , 55 ( 24 ) : 9956 – 9962 .
- Li , Z.F. , Wang , N. , Raghavan , G.S.V. and Vigneault , C. 2011 . Volatiles evaluation and dielectric properties measurements of Chinese spirits for quality assessment . Food Bioprocess Technology , 4 ( 2 ) : 247 – 253 .
- Yin , Y.G. , He , G.D. and Shi , J. 2005 . Experiment of liquor aging by high voltage pulse electric field . Liquor-Making Science Technology , 12 : 47 – 50 .
- Wang , J.G. 2008 . Physical and chemical analysis of Jiaxing rice wine and its flavor change in storage . China Brewing , 23 : 89 – 91 .
- Jiang , Y.L. and Cheng , W. 2003 . Study on maotai-flavor liquor aging in storage period . Liquor Making , 30 ( 1 ) : 20 – 22 .
- Wang , D.Y. , He , H.Z. , Xiao , X.L. and Zhang , L.H. 1990 . Mechanism of traditional aging process for Chinese liquor . China Brewing , 6 : 25 – 28 .
- Fan , W.L. and Qian , M.C. 2005 . Headspace solid phase microextraction and gas chromatography-olfactometry dilution analysis of young and aged Chinese “Yanghe Daqu” liquors . Journal of Agricultural and Food Chemistry , 53 ( 20 ) : 7931 – 7938 .
- Liang , C.H. , Jiang , Y.T. and Xing , H.X. 2007 . Accelerated aging of alcoholic drink with physical methods . China Brewing , 7 : 5 – 7 .
- Zhang , L.H. and Wan , H.Z. 2008 . Application of microwave technique in the production of health wine . Liquor-Making Science Technology , 1 : 82 – 83 .
- Chang , A.C. and Chen , F.C. 2002 . The application of 20 kHz ultrasonic waves to accelerate the aging of different wines . Food Chemistry , 79 ( 4 ) : 501 – 506 .
- Jiang , Y.T. and Zhang , Y. 2001 . Discussion on the application of infrared technology in accelerating the ripening of wine and vinegar . Infrared , 4 : 14 – 17 .
- Huang , H.C. and Ma , Z.Q. 1994 . A technique of the Shaoxing Jia Fan rice wine mellowed by N2 laser . Applied Laser , 14 : 221 – 223 .
- Liao , Z.L. A new method of liquor aging by x-ray . Chinese Patent No. 86103652 . 1986 .
- Xiao , L.M. , Zeng , X.A. , Chen , Y. and Yang , H.F. 2004 . FTIR analysis of fresh dry red wine treated by electromagnetic field . Food Science , 25 ( 1 ) : 152 – 155 .
- Jiang , L.L. , Ma , R.S. and Wen , Y.Y. 2006 . Adsorb ability on fusel oil in white spirit's by macroporous adsorbent resin . Liquor Making , 33 ( 1 ) : 33 – 34 .
- Apetrei , I.M. , Rodríguez-Méndez , M.L. , Apetrei , C. , Nevares , I. , del. Alamo , M. and de Saja , J.A. . 2012 . Monitoring of evolution during red wine aging in oak barrrels and alternative method by means of an electronic panel test . Food Research International , 45 ( 1 ) : 244 – 249 .
- Hernández-Orte , P. , Lapeña , A.C. , Peña-Gallego , A. , Astrain , J. , Baron , C. , Pardo , I. , Polo , L. , Ferrer , S. , Cacho , J. and Ferreira , V. 2008 . Biogenic amine determination in wine fermented in oak barrels: Factors affecting formation . Food Research International , 41 ( 7 ) : 697 – 706 .
- Zeng , X.A. , Yu , S.J. , Zhang , L. and Chen , X.D. 2008 . The effects of AC electric field on wine maturation . Innovative Food Science and Emerging Technologies , 9 ( 4 ) : 464 – 468 .
- Li , H.T. , Wang , B. and Li , C.L. 2004 . Study on the effect of aging and turbidity removal by ozone urging to distillate spirits . Liquor Making , 31 ( 2 ) : 75 – 77 .
- Ortega , A.F. , Lopez-Toledano , A. , Mayen , M. , Merida , J. and Medina , M. 2003 . Changes in color and phenolic compounds during oxidative aging of sherry white wine . Journal of Food Science , 68 ( 8 ) : 2461 – 2468 .
- Muñoz , D. , Peinado , R.A. , Medina , M. and Moreno , J. 2005 . Biological aging of sherry wine using pure culture of two flor yeast strains under controlled microaeration . Journal of Agricultural and Food Chemistry , 53 ( 13 ) : 5258 – 5264 .
- Moreno , J.A. , Zea , L. , Moyano , L. and Medina , M. 2005 . Aroma compounds as markers of the changes in sherry wines subjected to biological aging . Food Control , 16 ( 4 ) : 333 – 338 .
- Cai , X.Y. , Yin , J.J. and Hu , G.D. 1994 . Study on determination aroma components in Chinese liquor by direct injection using FFAP column . Liquor Making Science Technology , 61 : 18 – 22 .
- Mestres , M. , Busto , O. and Guasch , J. 1997 . Chromatographic analysis of volatile sulphur compounds in wines using the static headspace technique with flame photometric detection . Journal of Chromatography A , 773 ( 1–2 ) : 261 – 269 .
- Zhang , S.W. , Qiao , H. , Wang , W. , Zhang , F.Z. and Liu , Z.Y. A new method of accelerate liquor aging and its appliance . Chinese Patent No. ZL 200810054772.8 . 2008 .
- Lobo , A.P. , Madrera , R. and R; Alonso , J.J.M. 2005 . A study of cider distillates using sensory and chromatographic data and chemometric analysis . Journal of Food Science , 70 ( 3 ) : 204 – 207 .
- Peña , Y. , Lillo , M. , Agosin , E. , Belangcic , A. and Latrille , E. 2005 . Chemical markers for tracking the sensory contribution of production stages in muscat wine distillates . Journal of Food Science , 70 ( 7 ) : 432 – 441 .
- Madrera , R.R. , Gomis , D.B. and Mangas Alonso , J.J. 2003 . Characterization of cider brandy on the basis of aging time . Journal of Food Science , 68 ( 6 ) : 1958 – 1961 .
- Zhao , Y. , Xu , Y. , Li , J. , Fan , W. and Jiang , W. 2009 . Profile of volatile compounds in 11 Brandies by headspace solid-phase microextraction followed by gas chromatography-mass spectrometry . Journal of Food Science , 74 ( 2 ) : 90 – 99 .
- Wang , J.G. and Feng , D.M. 2009 . Discussions on aging mechanism of storing wine in pottery . China Brewing , 9 : 127 – 130 .
- Zea , L. , Moyano , L. and Medina , M. 2010 . Changes in aroma profile of sherry wines during the oxidative ageing . International Journal of Food Science and Technology , 45 ( 11 ) : 2425 – 2432 .
- Nevares , I. , del Alamo , M. and Gonzalez-Muñoz , C. 2010 . Dissolved oxygen distribution during micro-oxygenation. Determination of representative measurement points in hydroalcoholic solution and wines . Analytica Chimica Acta , 660 ( 1–2 ) : 232 – 239 .
- Oliveira , C.M. , Ferreira , A.C.S. , de Freitas , V. and Silva , A.M.S. 2011 . Oxidation mechanisms occurring in wines . Food Research International , 44 ( 5 ) : 1115 – 1126 .
- Vianna , E. and Ebeler , S.E. 2001 . Monitoring ester formation in grape juice fermentation using solid phase microextraction coupled with gas chromatography-mass spectrometry . Journal of Agricultural and Food Chemistry , 49 ( 2 ) : 589 – 595 .
- Mas , A. , Puig , J. , Lladoa , N. and Zamora , F. 2002 . Sealing and storage position effects on wine evolution . Journal of Food Science , 67 ( 4 ) : 1374 – 1378 .
- Luo , H.B. , Zhang , S.Y. , Zhao , J.S. , Lu , Z.M. and Wu , S.Y. 2007 . Colloid properties of distilling liquor and its application (I) . Liquor-Making Science Technology , 1 : 17 – 19 .
- Luo , H.B. , Zhao , J.S. , Wu , S.Y. and Zhang , S.Y. 2007 . Colloid properties of distilling liquor and its application (II) . Liquor-Making Science Technology , 2 : 20 – 21 .
- Lin , C.C. and Su , Y.R. 2008 . Performance of rotating packed beds in removing ozene from gaseous streams . Separation and Purification Technology , 61 ( 3 ) : 311 – 316 .
- Wang , G.Q. , Xu , Z.C. , Yu , Y.L. and Ji , J.B. 2008 . Performance of a rotating zigzag bed-A new HIGEE . Chemical Engineering and Processing , 47 ( 12 ) : 2131 – 2139 .
- Hsieh , CW. , Huang , YH. , Lai , CH. , Ho , WJ and Ko , W.C. 2010 . Develop a novel method for removing fusel alcohols from rice spirits using nanofiltration . Journal of Food Science , 75 ( 2 ) : 25 – 29 .
