Abstract
Operational parameters of Rancimat, including sample weight (3, 6, 9 g), airflow rate (10, 15, 20 L/h) and temperature (110, 120, 130°C) were evaluated to determine their effects on oxidative stability index, temperature coefficient, Q10 number, and shelf life prediction of ghee (anhydrous milk fat). These parameters showed statistically significant effects on the oxidative stability index. It was observed that when the sample weight and airflow rate at a given temperature were at saturated air condition, the oxidative stability indexes showed no significant differences (p < 0). As the temperature increased, oxidative stability index decreased and average coefficient of variation was minimal at 120°C. The conditions under which the ghee sample was saturated with air and had a relatively lower oxidative stability index, was with sample weight of 6 g, temperature at 120°C and an airflow rate of 15 L/h. Temperature coefficient and Q10 number were independent of sample weight and air flow rate, however, they had a significant effect on shelf life prediction of ghee.
INTRODUCTION
Oxidative stability is an important parameter for the quality assessment of milk fat and vegetable fats and oils. Lipids undergo oxidative degradation during processing and storage, resulting in an alteration of major quality parameters such as color, flavor, aroma, and nutritive value, affecting their suitability for consumption. The majority of these changes are initiated by reactive oxygen species, which lead to the formation of various primary and secondary products like carbonyls, peroxides, and hydroperoxides.[Citation1,Citation2] Therefore, assessing the extent of oxidative degradation of fats, oils and foods containing lipids is essential to the food industry.[Citation3]
Rapid methods are required to predict the stability and shelf life of edible oils and fats. The determination of oxidative stability with the active oxygen method (AOM; AOCS Method Cd 12–57) and Schaal oven method, involves the use of elevated temperatures to increase the reaction rate of lipid oxidation. However, both are costly and labor intensive.[Citation4] Rancimat method developed by Hadorn and Zurcher[Citation5] is used to assess oxidative stability index (OSI) and it agrees well with AOM method. It has gained acceptance, owing to its ease of use, reproducibility, continuous measurement requiring no periodic analytical determinations and uses no organic solvents for titrations.[Citation6,Citation7] This method is based on the measurement of conductance, produced by volatile organic acids collected in deionised water. In this test, the fat sample is placed in a vessel, subjected to an above-ambient temperature while oxygen is bubbled through it, to accelerate the oxidative process until short-chain volatile acids are produced. In a separate vessel, the acids formed are immediately dissolved in distilled water, and the conductivity of this solution is monitored at ambient temperature.[Citation7] The end point of Rancimat test is determined by induction period (time before rapid deterioration of fat occurs), which is used to establish relative stability of fats. The oxidative stability index (OSI) obtained from the Rancimat correlated well with stability of fats under various conditions of lipid oxidation and also with data obtained from independent sensory and/or analytical methods.[Citation8Citation10]
This method may also provide some other useful information regarding oxidative stability of edible fats. Plotting the logarithms of OSIs versus elevated temperatures and extrapolating it to room temperature, the shelf-life of the fat sample at ambient conditions can be predicted. The slope of the curves represents the temperature coefficients for fat samples.[Citation6] Temperature acceleration factor, known as Q10 number, which predicts the increase in oxidation rate with a 10°C increase in temperature, can also be calculated.[Citation11]
Sample weight, air flow rate, and temperature are the operational parameters that can be adjusted easily in the Rancimat method and may affect the determination of the OSI, temperature coefficient, and Q10 number (oxidative stability measures) as well as the shelf-life prediction of ghee. A number of studies have investigated the effect of these parameters independently and/or in combination for vegetable oils and fish oils. However, no studies have been reported to date regarding the effect of these three operating parameters of Rancimat on oxidative stability of ghee (Anhydrous milk fat). Therefore, the aim of the present work was to investigate the effect of operational parameters on the determination of the oxidative stability and to predict the shelf-life of ghee (AMF) using Rancimat.
MATERIALS AND METHODS
Materials
Freshly prepared ghee was procured from experimental dairy plant, National Dairy Research Institute, Karnal and stored at 4°C. The peroxide value of ghee was determined according to IS: 3508.[Citation12]
Apparatus
A Metrohm Rancimat model 743 (Herisau, Switzerland) capable of operating over a temperature range of 50 to 220°C was used. The glassware were rigorously cleaned between each run to avoid any contamination that would catalyze peroxidation. The tubes were soaked in hot water detergent overnight followed by cleaning with hot water, distilled water, and then thoroughly dried in an oven. Measuring vessels, electrodes, and connecting tubes were cleaned with detergent, washed off by running warm water and were rinsed several times with alcohol and distilled water before use.
OSI Measurement
A stream of air was bubbled into ghee (AMF) samples (3, 6, and 9 g) in a reaction vessel placed in an electric heating block. Effluent air containing volatile organic acids from the ghee (AMF) sample were collected in a measuring vessel containing double distilled water (60 mL). The conductivity of the water was measured automatically as oxidation proceeded. Filtered, cleaned, and dried air was allowed to bubble through the hot ghee at the rates of 10, 15, and 20 L/h. The OSIs of the ghee samples were recorded at 110, 120, and 130°C. In each run, eight samples were accommodated in the equipment and analyzed simultaneously.
Shelf-Life Prediction, Temperature Coefficient and Q10 Number Calculation
The logarithms of OSIs versus elevated temperatures (110, 120, and 130°C) were plotted and lines were fitted to the data. Equations for each line were also determined. Slopes of the lines yielded temperature coefficients. The shelf-lives as OSIs at 37°C (OSI37) were calculated from the corresponding equations. Q10 numbers were calculated as the OSIs at T/OSI time at T + 10°C.[Citation6,Citation7,Citation11]
Statistical Analysis
All determinations were carried out in triplicate and data was subjected to analysis of variance. Analysis of variance was performed using the ANOVA, according to the SYSTAT software. P values less than 0.05 were considered statistically significant.
RESULTS AND DISCUSSION
The OSIs and coefficient of variation (CV) of treatment combinations (ghee sample weight × air flow rate) at different temperatures are presented in . All ghee samples had zero peroxide values prior to the start of the Rancimat test.
Table 1 The oxidative stability index (OSI) and coefficient of variation (CV) of the treatment combinations at different temperatures
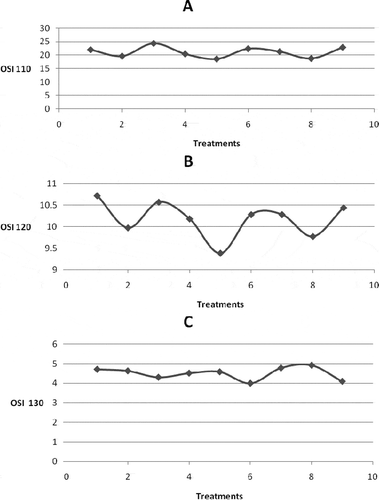
At 110°C with sample weight of 3, 6, and 9 g, the air flow rates of 10, 15, and 20 L/h gave statistically different OSIs of ghee. It is evident from that at 110°C, lowest OSI was observed at 6 g sample weight and air flow rate of 15 L/h, whereas highest OSI was observed at 3 g sample weight and air flow rate of 20 L/h at same temperature. Hill and Perkins[Citation13] reported significantly different OSIs for an oil sample size of 2.5 g at air flow rates of 12–20 L/h. It was interesting to find that the OSI of the ghee sample at an air flow rate of 20 L/h was significantly higher than that of low air flow rates. Jebe et al.[Citation14] reported that temperature stabilization at a sample weight of 2.5 g was difficult when using the Rancimat method and thus recommended a 5 g sample weight. However, it seems that air-saturated conditions cannot be maintained for low ghee sample weights exposed to high air flow rates. The rigorously turbulent status of the ghee sample resulted in more air escaping from the ghee sample than used for lipid oxidation.[Citation15] At 110 and 120°C, for all air flow rates and sample weight of 6 g ghee the OSI was minimal. These relatively low OSIs for 6 g sample size might be because of the creation of sufficient air saturated conditions which was necessary for lipid oxidation as against 3 and 9 g sample weights, whereas the above trend was not observed for 130°C temperature as the OSIs for 9 g sample size were higher from that of 6 g sample size. This suggests that the concentration of air in the ghee sample was not sufficient for the creation of air-saturated conditions essential for oxidation. It can, therefore, be interpreted that treatment combinations 1, 3, 4, 6, 7, and 9 in cannot create the air-saturated conditions whereas, a relatively large CV for treatment combinations two and eight suggests that the conditions under which oxidation occurred varied more than the others. Hence, the preferred conditions at 110°C are 6 g sample weight with an air flow rate of 15L/h.
As expected, OSIs significantly decreased with temperature (). As shown in , at 110 and 120°C, a nearly similar trend for the OSIs was observed, but not at 130°C. For all treatment combinations, the OSIs at 110°C were higher than corresponding OSIs at 120 and 130°C. The average CV of all the treatment combinations varied considerably among all the three temperatures and was minimal at 120°C (0.023). These observations indicated that the conditions under which the sample oxidizes varies with temperature. As seen in , it can be interpreted that the optimum condition for determining OSI, where the sample was saturated with air (OSI is minimum) with a relatively lower CV, was sample weight of 6 g, air flow rate of 15 L/h and the temperature of 120°C.
Figure 1 The variation trend of the oxidative stability index (OSI) for different treatment combinations (ghee sample size × air flow rate) at (a) 110°C, (b) 120°C, and (c) 130°C.
represents the data calculated from the linear relationship between the natural logarithm of OSI and the temperature for the treatment combinations in . There were non-significant differences among the treatment combinations in terms of the temperature coefficients and Q10 numbers. Thus, these two parameters can be determined for ghee independent of the sample weight and air flow rate. The results obtained revealed that the temperature coefficient for ghee had a mean value of –3.24 × 10−2°C−1. The temperature coefficient for soybean oil was found to be –3.12 × 10−2°C−1.[Citation15] A mean value of 2.07 for Q10 number indicated that an increase in temperature by 10°C approximately halves the OSI of ghee.
Table 2 The results calculated from the linear relationship between the natural logarithm of the OSI assessed by the rancimat test and the temperature for the treatment combinations in
Statistically significant differences between the calculated OSIs for the treatment combinations at 37°C (OSI37) showed that sample weight of ghee and air flow rate have a definite effect on the shelf-life prediction for ghee. Frankel[Citation11] stated that the extrapolation of the stability results obtained by the Rancimat test at ambient storage leads to either over prediction or under prediction of the actual shelf life depending on the type of oil. Mendez et al.[Citation7] attributed this to the different mechanism of peroxidation and peroxide decomposition kinetics under accelerated conditions of the Rancimat test from the corresponding mechanisms at ambient storage conditions. Nevertheless, the Rancimat method correlated highly (r = 0.966; P = 0.000) with rapeseed oil stability measured by peroxide development.[Citation16] Variations between shelf life predictions from long-term storage studies and the OSI test can be minimized by choosing the correct levels of these operational parameters in the Rancimat method depending on the type of the product. Thus, by using a Rancimat, we can rapidly assess the quality of fats, oils, and fat containing dairy foods which can save a lot of time and is very precious in quality control as well as research and development laboratories.
CONCLUSION
Temperature, sample weight, and air flow rate showed a significant effect on shelf-life prediction of ghee. Therefore, choosing the right levels of these operational parameters in the Rancimat method may produce the least possible difference between predictions from long-term storage studies and the OSI test.
ACKNOWLEDGEMENTS
This work was supported by the National Agricultural Innovation Project (component 4: Basic and strategic research C30029), Indian Council of Agricultural Research. New Delhi, India.
REFERENCES
- Andersson, K.; Lingnert, H. Kinetic studies of oxygen dependence during initial lipid oxidation in rapeseed oil. Journal of Food Science 1999, 64(2), 262–266.
- Wagner, K.H.; Elmadfa, I. Effects of tocopherols and their mixtures on the oxidative stability of olive oil and linseed oil under heating. European Journal of Lipid Science and Technology 2001, 103, 624–629.
- Addis, P. Occurrence of lipid oxidation products in foods. Food and Chemical Toxicology 1986, 24, 1021–1030.
- Laubli, M.; Bruttel, P. Determination of the oxidative stability of fats and oils: Comparison between the active oxygen method (AOCS Cd 12-57) and the Rancimat Method. Journal of American Oil Chemists Society 1986, 63(6), 792–795.
- Hadorn, H.; Zurcher, K. ZurBestimmung der Oxydations-stabilitat von Olen und Fetten. DtschLebensmRundsch 1974, 70, 57–65.
- Hasenhuettl, G.; Wan, P. Temperature effects on the determination of oxidative stability with the Metrohm Rancimat. Journal of American Oil Chemists Society 1992, 69, 525–527.
- Mendez, E.; Sanhueza, J.; Speisky, H.; Valenzuela, A. Validation of the Rancimat test for the assessment of the relative stability of fish oils. Journal of American Oil Chemists Society 1996, 73, 1033–1037.
- Anwar, F.; Bhanger, M.; Kazi, T. Relationship between Rancimat and active oxygen method values at varying temperatures for several oils and fats. Journal of American Oil Chemists Society 2003, 80, 151–155.
- Kowalski, B.; Ratusz, K.; Kowalska, D.; Bekas, W. Determination of the oxidative stability of vegetable oils by differential scanning calorimetry and Rancimat measurements. European Journal of Lipid Science and Technology 2004, 106, 165–169.
- Velasco, J.; Andersen, M.; Skibsted, L. Evaluation of oxidative stability of vegetable oils by monitoring the tendency to radical formation. A comparison of electron spins resonance spectroscopy with the Rancimat method and differential scanning calorimetry. Food Chemistry 2004, 85, 623–632.
- Frankel, E. Lipid oxidation. The Only Press: Dundee, 1998. 99–114.
- IS: 3508. Indian standards, Methods for sampling and testing for ghee (butter fat) 1966, Bureau of Indian Standards, New Delhi.
- Hill, S.E.; Perkins, E.G. Determination of oxidation stability of soybean oil with the oxidative stability instrument: Operation parameter effects. Journal of American Oil Chemists Society 1995, 72, 741–743.
- Jebe, T.A.; Matlock, M.G.; Sleeter, R.T. Collaborative study of the oil stability index analysis. Journal of American Oil Chemists Society 1993, 70, 1055–1061.
- Farhoosh, R. Effect of operational parameters of the Rancimat method on determination of the oxidative stability measures and shelf-life prediction of soybean oil. Journal of American Oil Chemists Society 2007, 84, 205–209.
- Gordon, M.; Mursi, E. A comparison of oil stability based on the metrohm Rancimat with storage at 200°C. Journal of American Oil Chemists Society 1994, 71(6), 649–651.
