Abstract
Acid hydrolysis and shaking extraction methods were used to extract nine genotypes of Thai rice grains. The extracts were determined total phenolic including anthocyanin contents and antioxidant capacities using 2,2-diphenyl-1-picrylhydrazyl and the ferric thiocyanate assays from white, red, and black rice grains. The results demonstrated that the extraction methods affected phenolic antioxidant of rice grains. The values of insoluble phenolic compounds were significantly higher than those of soluble phenolic compounds, which was about 1.3 times. The soluble anthocyanin content of grains was low comparing to the insoluble anthocyanin content. In addition, among studied rice grains, black rice possessed the highest phenolic and anthocyanin contents as well as antioxidant capacities. These findings suggested that black rice can be further explored as a functional food.
INTRODUCTION
The major rice exporters in the world market have been the nations of mainland Southeast Asia such as Thailand, Burma, Cambodia, and South (or southern) Vietnam.[Citation1] Since consumers are concerned about health, nutritional quality of rice has received more attention. Hence, the data of bioactive compounds in Thai rice should be reported to inform rice nutrition value and promote Thai rice grains. Rice is a rich source of many bioactive compounds including phenolic antioxidants that have the potential to reduce the risk of disease, such as reducing the risk of coronary heart disease and cancer,[Citation2] and preventing oxidative damage of lipid and low-density lipoproteins.[Citation3] Data for rice are limited but suggest that the predominant phenolic compounds are phenolic acids.[Citation4] Phenolic acids can be classified as free phenolic acids/soluble forms and bound phenolic acids/insoluble forms. The level of soluble phenolic acids provided an index of grain resistance.[Citation5] Insoluble phenolic compounds are typically involved in cell wall structure mostly bound to polysaccharides of the cell wall. Insoluble phenolic compounds account for the major part of polyphenols in whole grains and suggest that their contents may be underestimated in many studies. In consequence their contribution to biological activity is largely unknown.
Monotonous consumption of rice may lead to deficiencies of essential minerals, vitamins, and other nutritional compositions.[Citation6] This is not caused by nutritional deficiency in rice grain itself, but due to it being traditionally eaten in the form of the milled white kernel. Milling of the brown rice to obtain milled rice removes bran layers that are rich in protein, dietary fiber, minerals, carotenoids, and other nutrients,[Citation7] leading to loss of most of the nutritional components of the rice grain.
This study was conducted to evaluate the effect of extraction methods (shaking and acid hydrolysis) on total phenolic content (TPC) of white, red, and black rice (Oryza sativa L.) grains genotypes from indica subspecies. Moreover, the antioxidant activities and the anthocyanin content (ATC) of the rice extracts were evaluated.
MATERIALS AND METHODS
Materials
Three batches of nine rice grains were obtained by Community Enterprise Network, a group of farmers of organic food, Ubon Ratchathani province in Thailand. The moisture content of rice grains on a dry weight basis according to AOAC[Citation8] ranged from 11.52–13.17 g/100 g. Three black-grained rice genotypes (Hawm nil, Hawm kanya, and Kum), two red-grained rice genotypes (Sang yod and Red Jasmine), and four white-grained rice genotypes (Hawm Ubon, Lao tek, Jasmine rice 105, and Sin lek) were harvested from March to June 2011. The rice materials were kept in a chiller at 4°C for no longer than two months. The different grains are shown in , and their characteristics are reported in .
Figure 1 Type of rice. (a) Hawm nil; (b) Hawm kanya; (c) Kum; (d) Jasmine rice 105; (e) Sang yod; (f) Red Jasmine; (g) Lao tek; (h) Hawm Ubon; (i) Sin lek. (Color figure available online.)
Table 1 Characteristics of Thai rice grainsFootnote ¥
Folin Ciocalteu reagent, 2,2-diphenyl-1-picrylhydrazyl (DPPH), and sodium carbonate, were purchased from Sigma Chemical Co., Ltd (St. Louis, USA). Ferulic acid was purchased from Acros Organics (New Jersey, USA). The other chemicals and solvents used in this experiment were analytical grade purchased from Sigma-Aldrich Co., Ltd (Steinheim, Germany).
Color of Rice Grain
The color of rice grain samples was measured with a Spectrophotometer (Minolta CM-3500d, Tokyo, Japan). Color measurements were expressed as tristimulus parameters, L*, a*, and b*. L* indicates lightness (100 = white and 0 = black). a* indicates redness–greenness and b* indicates yellowness–blueness.[Citation9]
Sample Preparation
Rice grains were ground into fine flour by using a Cyclone Sample Mill (Model 3010-018, Udy Corporation, Fort Collins, CO, USA) through a 0.5 mm sieve screen and stored at 4°C before analysis.
Extraction of Rice Phenolics
Rice flour (20 g) was extracted using two methods. First, the rice grains were extracted twice with 95 g/100 g ethanol (shaking method or Method 1) at a ratio of 1:3 (w/v). The mixture was kept on a mechanical shaker (Innova 4080, New Brunswick Scientific, USA) for 1 h at 25°C. After centrifuging (Model Super T 21, Sorvall, USA) at 4000 rpm (1430 g) for 15 min, the supernatant obtained was concentrated to dryness by using a rotary evaporator (Model NEI, EYELA, Japan) at 45°C. The sample was dried in a freeze dryer and stored in aluminum foil after flushing with nitrogen at –20°C until analysis. For acid hydrolysis method (Method 2), rice flour (20 g) was extracted with 1.5N HCl in 95 g/100 g ethanol at a ratio of 1:3 (w/v). After that the extraction process was similar to Method 1. The dried extracts from Method 1 and 2 including reference samples (α-tocopherol, ferulic acid, and p-coumaric acid) were used to estimate the antioxidant properties by the DPPH and ferric thiocyanate (FTC) methods. The extracts were also used to evaluate TPC and ATC. All analyses were performed in triplicate.
Determination of Antioxidant Properties
The total free radical-scavenging capacity of extracts or reference samples was determined by using the DPPH and the FTC methods in a linoleic acid emulsion system.
The free radical scavenging activity of the extracts or reference samples was evaluated using the stable radical DPPH according to the method of singleton and Rossi.[Citation10] The DPPH radical scavenging activity of samples was calculated from [Ao – (A 1 – As )]/Ao × 100. Ao is the absorbance of the control solution (containing only DPPH); A 1 is the absorbance of the DPPH solution containing plant extract; and As is the absorbance of the sample extract solution without DPPH. All determinations were performed in triplicate.
The antioxidant activity in a linoleic acid emulsion system of the extracts or reference samples was determined using the FTC method,[Citation11] with some modifications. Each sample in absolute ethanol (0.5 ml) was mixed with 0.5 ml of 5.21 g/100 g linoleic acid, 1 ml of 0.05 M phosphate buffer (pH 7), and 0.5 ml of distilled water and placed in a screw capped tube. The reaction mixture was incubated in the dark at 40°C in an oven. Aliquots of 0.1 ml were removed every 24 h during incubation and the degree of oxidation was measured by sequentially adding ethanol (9.7 ml, 75 g/100 g), ammonium thiocyanate (0.1 ml, 30 g/100 g), and ferrous chloride (0.1 ml, 0.02 M in 3.5 g/100 g HCl). After the mixture was rested for 3 min, the peroxide value was determined by monitoring absorbance at 500 nm until the absorbance of the control reached the maximum. The degree of linoleic acid peroxidation was calculated using the following formula: Antioxidant activity = [(Acontrol − Asample )/Acontrol ] × 100. The antioxidant activity was plotted against sample concentration in order to determine the concentration required to achieve a 50% inhibition of linoleic acid oxidation [AA50]. The antioxidant efficiency was calculated in term of 1/AA50. All tests and analyses were carried out in triplicate and averaged.
Determination of TPC
The TPC of extracts was determined using the Folin-Ciocalteu's phenol reagent (modified from Kähkonen et al.[Citation12]). The concentration of total phenolic compounds in all plant extracts was expressed as mg of ferulic acid equivalent (FAE) per g dry weight of rice grain using a linear equation. All determinations were performed in triplicate.
Determination of ATC
The extracts were used to determine ATC. It was determined by the pH differential method.[Citation13] To measure the absorbance at pH 1.0 and 4.5, the crude extract was diluted 20 times with pH 1.0 potassium chloride buffer and pH 4.5 sodium acetate buffers, respectively. The ATC of crude extract was calculated in terms of cyanidin-3-glycoside. The concentration of anthocyanin pigment was calculated by the following equation:
The concentration of anthocyanin compounds in all plant extracts was expressed as mg/g dry weight of rice grain.
Statistical Analysis
Each experiment, from sample preparation to analysis, was repeated in triplicate, and the data were analyzed by SPSS software program (SPSS Inc., Chicago, IL, USA). The general linear model procedure was applied and Duncan's multiple range test was used to compare the mean values at p < 0.05. Mean values and pooled standard error of the mean (SEM) were then estimated.
RESULTS AND DISCUSSION
Most of studied rice grains are slender grain (L/M ratio more than 3) except genotype gum (L/M ratio = 2.71) and Hawm kanya (L/M ratio = 3.00). Extra long grains were found in Hawm Ubon, Laotek, Jasmine105, Sinlek, and Red jasmine genotypes (more than 7.0 mm long). The longest rice grain was found in genotype Sinlek (7.64 mm long). Typically, long grain kernels are roughly 7 mm long. Hawmnil genotype is long grain. Hawm kanya, Kum, and Sangyod genotypes are medium rice grains (6.27–6.47 mm long) ( and ). Typically, the red rice had L* and b* values of color parameters smaller than the white rice, but larger than the black rice (). Black and red rice contains color pigment. L* and b* mean lightness and yellowness of samples, hence black rice showed the lowest values of L* and b*.
To assay the antioxidant capacity and phenolic constituents of rice that are covalently linked to cellular components for the extraction, acid hydrolysis was performed. In whole rice grains, phenolic compounds are present in free/soluble and bound/insoluble forms.[Citation14] In this report, the values of TPCs, ATCs, and antioxidant capacities were compared with different extraction methods (shaking and acid hydrolysis). The values obtained from shaking (Method 1) were soluble forms and those from acid hydrolysis (Method 2) were insoluble forms.
Properties of Shaking Extraction of Rice Grains
TPC, ATC, and antioxidant activity of nine genotypes of Thai rice grains were investigated. The TPC differed among the different types of rice genotypes. Red jasmine genotypes showed the highest value of TPC () more than white and black rice groups. This was consistent to Muntana and Prasong.[Citation15] Typically, pigmented rice, such as red and black rice, composed of high content of phenolic compounds.[Citation15] Interestingly, the black rice group showed similar soluble phenolic content as some white rice, such as jasmine 105 and Hawn Ubon, whereas, ATC of the black rice genotypes showed higher than that of red and white rice groups (). The results suggested that rice composed of other phenolic class, such as phenolic acid, showed high value of phenolic content in white rice but low value of anthocyanin pigment. Moreover, phenolic acid was extracted easily comparing to anthocyanin. Since most grain phenolic compounds such as phenolic acids occur in the outer layers of grains.[Citation16] Antioxidant activities of the black rice group exhibited higher than that of the white and red genotypes (). The results cause by the high antioxidant capacity of anthocyanin more than that of phenolic acids.[Citation17]
Figure 2 Total phenolic content of rice extracted by shaking (Method 1) and acid hydrolysis procedures (Method 2).

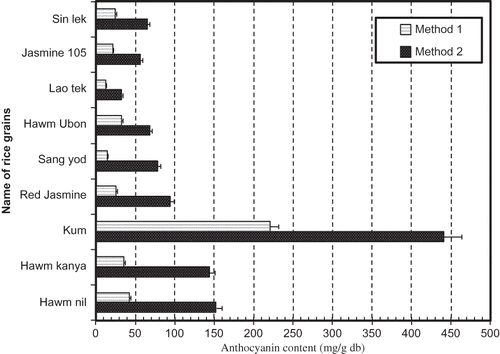
Figure 3 Total anthocyanin content of rice extracted by shaking (Method 1) and acid hydrolysis procedures (Method 2).
Significant differences in antioxidant activities among some Thai rice samples can be attributed to several complex factors including cultivar, growing environment, and harvesting conditions. The antioxidant activity assessed by the FTC method of Thai rice samples showed the same order as DPPH activity (). Ferric is used as stimulator in FTC method. Iron is a well-described initiator of free radical oxidations, stimulating lipid peroxidation, whereas free radical scavenger is major mechanism of DPPH method.[Citation18] Hence there is a relation between these two methods. The scavenging ability and antioxidant capacity of all rice grains were comparable to that of α-tocopherol.
Properties of Acid Hydrolysis Extraction of Rice Grains
The effect of acid hydrolysis of nine genotypes of rice grains on total phenolic and ATC including antioxidant activities using DPPH and FTC methods was shown in and and . The order of TPC and ATC were different from DPPH radical scavenging activity of rice grains. Black rice exhibited higher TPC than the red and white rice groups (). Some phenolic compounds in rice grains occur as insoluble phenolic and esterified phenolic compounds.[Citation19] Acid hydrolysis enhances the extraction of total insoluble phenolic compounds, thus breaking more easily the linkages between phenolic compounds and other constituents such as sugar and protein.[Citation20] The results clearly showed that most grain phenolic compounds were in the insoluble form (). Therefore, many reports showed underestimated phenolic compounds of grains because there were not included the insoluble phenolic compounds. Insoluble phenolic compounds in rice grains are associated with cell wall materials that may survive upper gastrointestinal digestion conditions and may finally reach the colon. On the basis of our results, most of the grain phenolic compounds may be released in the colon to exert their health benefits locally and beyond after absorption.
Table 2 Characterization of rice extract obtained from shaking method (Method 1) and acid hydrolysis method (Method 2)Footnote ¥
The order of ATC of rice grain groups obtained by acid hydrolysis was similar to that of phenolic content (). The black rice group exhibited the highest value of ATC. The soluble ATC in grains were low comparing to the insoluble ATC. They were all significantly different (p < 0.05) from each other (). The soluble ATC followed a slightly similar pattern as insoluble ATC in all rice grains, because of the large contribution from insoluble anthocyanins. The soluble ATC of Hawn kanya and Hawm Ubon genotypes were similar, although their insoluble ATCs were different. This could be attributed to the different groups of anthocyanin of rice. The anthocyanin founding in each genotype of rice were different. The principal anthocyanin in rice is cyanidin-3-glucoside, followed, in minor proportion by peonidin-3-glucoside. Small quantities of other derivatives of cyanidin have also been found: cyanidin-3-gentiobioside, cyanidin-3-rhamnoside, cyanidin-3,5-diglucoside, and cyanidin-3-rhamnoglucoside.[Citation21] Typically, extraction of anthocyanins is commonly carried out under conditions with methanol or ethanol containing a small amount of acid with the objective of obtaining the flavylium cation form, which is red and stable in a highly acid medium.[Citation22] That is the reason why acid hydrolysis extraction showed higher value of anthocyanin than that of shaking method.
The order of scavenging activity of rice grain genotypes was: Kum > Hawn nil > Hawm kanya = Red jasmine > Sang yod = Lao tek > Hawm ubon > Jasmine 105 = sin lek. Moreover, Kum genotype showed the highest DPPH activity, antioxidant efficiency, total phenolic cotent, and anthocyanin pigment. The extraction method affected to antioxidant capacities of rice grain. This is attributable to higher phenolic content in insoluble extracts compared to soluble extract (). It can be seen that rice extracts from acid hydrolysis method was almost 1.3 times more effective (in DPPH in scavenging) than that from shaking method ().
Relationship of Rice Properties Between Extraction Methods
To assess the contribution of TPC to antioxidant activity of Thai rice grains, the relationship among TPC, ATC, DPPH free radical scavenging activity, and FTC method was investigated (). A high correlation (r > 0.9, p < 0.0001) was found between DPPH and FTC method. Interestingly, the close correlation between anthocyanin pigment and antioxidant activity obtained from FTC method was more than that from DPPH method. This is consistent to Satué-Gracia et al.[Citation23] They reported that anthocyanin exhibited several antioxidant mechanisms including hydrogen donation, metal chelation, and protein binding. A strong linear relation between TPC obtained from acid hydrolysis extraction, and anthocyanin pigment as well as antioxidant capacities from both methods were found (), indicating that the insoluble phenolic compounds might be the major contributors to the antioxidant activities of these extracts.
Table 3 Correlation coefficients between phenolics, anthocyanin, DPPH activity, antioxidant capacity obtained from FTC method, and yield among all rice genotypesFootnote ¥
CONCLUSIONS
The results have shown that phytochemical contents of rice grains have been underestimated in the literature without including the insoluble phytochemicals. Among the rice grain genotypes that were tested, Kum genotype had the highest content of phenolic compounds followed by Hawm kanya and Hawn nil, while white rice, Jasmine 105, had the lowest phenolic content. The results also show the major portions of phytochemicals in the grains are present in the insoluble form.
ACKNOWLEDGEMENTS
This study was supported by a grant from University of the Thai Chamber of Commerce (UTCC). The authors wish to express gratitude to Professor Michael H. Gordon for any suggestions.
REFERENCES
- Dawe , D. 2002 . The changing structure of the world rice market, 1950–2000 . Food Policy , 27 ( 4 ) : 355 – 370 .
- Fitó , M. , Cladellas , M. , De la Torre , R. , Martí , J. , Alcántara , M. , Pujadas-Bastardes , M. , Marrugat , J. , Bruguera , J. , López- Sabater , M.C. , Vila , J. and Covas , M.I. 2005 . Antioxidant effect of virgin olive oil in patients with stable coronary heart disease: A randomized, crossover, controlled, clinical trial . Atherosclerosis , 181 : 149 – 158 .
- Tatiana , L.C and Stanley , T.O. 2006 . Additive or synergetic effects of phenolic compounds on human low density lipoprotein oxidation . Food and Chemical Toxicology , 44 ( 4 ) : 510 – 516 .
- Qiu , Y. , Liu , Q. and Beta , T. 2009 . Antioxidant activity of commercial wild rice and identification of flavonoid compounds in active fractions . Journal of Agricultural and Food Chemistry , 57 ( 16 ) : 7543 – 7551 .
- Ramputh , A. , Teshome , A. , Bergvinson , D.J. , Nozzolillo , C. and Arnason , J.T. 1999 . Soluble phenolic content as an indicator of sorghum grain resistance to Sitophilus oryzae (Coleoptera: Curculionidae) . Journal of Stored Products Research , 35 : 57 – 64 .
- Abdul-Hamid , A. , Raja Sulaiman , R.R. , Osman , A. and Saari , N. 2007 . Preliminary study of the chemical composition of rice milling fractions stabilized by microwave heating . Journal of Food Composition and Analysis , 20 ( 7 ) : 627 – 637 .
- Bouis , H.E. , Chassy , B.M. and Ochanda , J.O. 2003 . Genetically modified food crops and their contribution to human nutrition and food quality . Trends in Food Science & Technology , 14 : 191 – 209 .
- AOAC . 1990 . Official Methods of Analysis , 684 Washington , DC : Association of Official Analytical Chemists . 16th Ed
- Bao , J.S. , Cai , Y. , Sun , M. , Wang , G.Y. and Anthocyanins , Corke, H. 2005 . flavonols and free radical scavenging activity of Chinese bayberry (Myrica rubra) extracts and their color properties and stability . Journal of Agricultural and Food Chemistry , 53 : 2327 – 2332 .
- Singleton , V.L. and Rossi , J.R. 1965 . Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents . American Journal of Enology and Viticulture , 16 : 144 – 153 .
- Hu , C. , Zawistowski , J. , Ling , W. and Kitts , D.D. 2003 . Black rice (Oryza sativa L. indica) pigmented fraction suppresses both reactive oxygen species and nitric oxide in chemical and biological model systems . Journal of Agricultural and Food Chemistry , 51 ( 18 ) : 5271 – 5277 .
- Kähkonen , M.P. , Hopia , A.I. , Vuorela , H.J. , Rauha , J.P. , Pihlaja , K. and Kujala , T.S. 1999 . Antioxidant activity of plant extracts containing phenolic compounds . Journal of Agricultural and Food Chemistry , 47 ( 10 ) : 3954 – 3962 .
- Giusti , M. and Wrolstad , R.E. 2005 . “ Characterization and measurement of anthocyanins by UV-visible spectroscopy ” . In Handbook of Food Analytical Chemistry , Edited by: Wrolstad , R.E. and Schwartz , S.J . 19 – 31 . Wiley : New York .
- Sosulski , F.W. and Sosulski , K. 1985 . Processing and composition of wild oat groats (Avena fatua L.) . Journal of Food Engineering , 4 ( 3 ) : 189 – 203 .
- Muntana , N. and Prasong , S. 2011 . Study on total phenolic contents and their antioxidant activities of Thai white, red, and black rice bran extracts . Pakistan Journal of Biological Sciences , 13 ( 4 ) : 170 – 174 .
- Oki , T. , Masuda , M. , Kobayashi , M. , Nishiba , Y. , Furuta , S. , Suda , I. and Sato , T. 2002 . Polymeric procyanidins as radical-scavenging components in red-hulled rice . Journal of Agricultural and Food Chemistry , 50 ( 26 ) : 7524 – 7529 .
- Adom , K.K. and Liu , R.H. 2002 . Antioxidant activity of grains . Journal of Agricultural and Food Chemistry , 50 : 6182 – 6187 .
- Negi , P.S. , Jayaprakasha , G.K. and Jena , B.S. 2010 . Evaluation of antioxidant and antimutagenic activities of the extracts from the fruit rinds of Garcinia cowa . International Journal of Food Properties , 13 ( 6 ) : 1256 – 1265 .
- Cai , Y. , Luo , Q. , Sun , M. and Corke , H. 2004 . Antioxidant activity and phenolic compounds of 112 traditional Chinese medicinal plants associated with anticancer . Life Sciences , 74 : 2157 – 2184 .
- Anesini , C. , Ferraro , G.E. and Filip , R. 2008 . Total polyphenol content and antioxidant capacity of commercially available tea (Camellia sinensis) in Argentina . Journal of Agricultural and Food Chemistry , 56 : 9225 – 9229 .
- Nuutila , A.M. , Kammiovirta , K. and Oksman-Caldentey , K.M. 2002 . Comparison of methods for the hydrolysis of flavonoids and phenolic acids from onion and spinach for HPLC analysis . Food Chemistry , 76 ( 4 ) : 519 – 525 .
- Escribano-Bailón , M.T. , Santos-Buelga , C. and Rivas-Gonzalo , J.C. 2004 . Anthocyanins in cereals . Journal of Chromatography , 1054 : 129 – 141 .
- Satué-Gracia , M.T. , Heinonen , M. and Frankel , E.N. 1997 . Anthocyanins as antioxidants on human low-density lipoprotein and lecithin−liposome systems . Journal of Agricultural and Food Chemistry , 45 ( 9 ) : 3362 – 3367 .
