Abstract
Fresh blueberries were selected as trial materials. Two blueberry juice samples were sterilized using pulsed electric field and thermal treatments respectively. Their qualities were analyzed and compared by evaluating the microorganisms in the samples. The changes in the quality of fresh blueberry juice samples during storage were also assessed. The results showed that the inactivation of Escherichia coli increased as electric field strength and treatment time increased. Pulsed electric field treatments at 35 kV/cm and 82 μs reduced E. coli by 5.12 logarithms. Pulsed electric field had less effect on juice quality, but after heat treatment, the vitamin C of blueberry juice sample was reduced by 14.78%, whereas its anthocyanin content was reduced by 3.64%. After sterilization, no significant change was observed in the relative reducing sugar, total acid, phenol contents, and Brix value. The vitamin C and anthocyanins contents of the fresh blueberry juice samples treated with pulsed electric field were higher than those treated with heat sterilization.
INTRODUCTION
Blueberries, also known as bilberries, are classified under the Ericaceae family and Vaccinium genus (Vaccinium spp.) and are perennial deciduous or evergreen shrubs. The blueberry fruit is dark blue, nearly circular, and rich in functional components, such as vitamins, pectin, and mineral elements. Blueberries also have the highest anthocyanin content among a variety of fruits. In general, 100 g fresh blueberries contain 100 to 200 mg anthocyanins. Thus, blueberries are known as the “king of berries.”[Citation1,Citation2] The anthocyanins, polyphenols, and other active substances in blueberries provide antioxidant, anti-aging, anti-tumor, vision-improving, and immunity-enhancing functions, among others.[Citation3–Citation6] Therefore, blueberries have been identified as one of the five healthy foods by the United Nations Food and Agriculture Organization.
Fresh fruit juice is always preferred by consumers because of its high nutritional content, aromatic ingredients, and wide variety of flavors. Blueberry juice is heat-sensitive. Thus, traditional pasteurization will decrease not only its unique flavor but also its nutrients. At present, non-thermal sterilization has been gradually applied to food production processes. Pulsed electric field (PEF) treatment is a widely studied non-heat sterilization technology[Citation7,Citation8] that utilizes the PEF of a high-voltage pulse generator to sterilize food. The basic process deals with cryogenically cooled food placed between the poles using instantaneous high pressure. However, the PEF microbial mechanism is unclear. The bactericidal mechanism has been recognized by most professionals as the direct contact of PEF with the microbial cell membrane through an external electric field. This contact destroys the structure of the cell membrane and leads to “electroporation” and, finally, microbial inactivation in a certain period of time.[Citation9–Citation11]
At present, negligible data have been reported on the reduction of the nutritional value of blueberry juice due to PEF during the sterilization process.[Citation12,Citation13] Escherichia coli was used as the target organism to study the bactericidal effect of PEF in blueberry juice samples, analyze the impact of common physical and chemical properties by the PEF treatment, and provide a theoretical basis for PEF in the actual process.
MATERIALS AND METHODS
Materials
The blueberries were provided by Organic Food Co., Ltd., Dandong City, Liaoning Province, China. The fruits were ripened and refrigerated at −80°C. The reagents, namely, sodium hydroxide, ethanol, copper sulphate, sodium tartrate, acetic acid, disodium EDTA, Fast Blue salt B, ascorbic acid, Folin-phenol reagent, and sodium carbonate, among others, were of analytical grade and provided by Beijing Blue Eagle Reagent Co., Ltd., China.
Juice Preparation
The blueberries were placed into distilled water at a ratio of 1:6 after blanching, pressed in an organization stamp mill, and the slurry was centrifuged for 20 min at 4°C and 4000 r/min. The supernatant was filtered, and the conductivity of the filtrate was adjusted with 1 mol/L of NaCl to 1.4 and 1.8 ms/cm. The mixture was sterilized at 121°C for 20 min, and the pH was adjusted to 3.3 to obtain the blueberry juice samples.
Processing and Method of Bacterial Count
E. coli was inoculated into the nutrient broth at 37°C. The culture was shaken for 14 h and left to grow until the cell concentration reached 109 CFU/mL to 1010 CFU/mL. Twenty mL of the bacteria culture was placed in 2 L sterilized blueberry juice, and each sample had a cell concentration of 107 CFU/mL to 108 CFU/mL. The blueberry juice samples before and after the PEF treatment were placed on plates, and the cells were counted at 37°C after 48 h. The death rate log s suggests the bactericidal effect of PEF treatment:[Citation14]
PEF Sterilization Parameters
E. coli was inoculated into the blueberry juice samples with electric field strengths at 20, 25, 30, and 35 kV/cm. The processing times were 27, 54, and 82 μs, whereas conductivity was at 1.4 and 1.8 ms/cm. The waveform was a unipolar square wave. Electric field strength (E) and total processing time (t) can be calculated as follows:[Citation15]
Blueberry Juice Samples
The blueberry juice control samples were obtained directly from freshly squeezed blueberries.
The heated blueberry juice samples were subjected to heat treatment for 15 s to simulate the pasteurization temperature of 90°C.
The PEF-treated blueberry juice samples were obtained with the following PEF processing parameters: 30 kV/cm field strength, 54 μs processing time, and 1.4 ms/cm conductivity.
Identification of the Total Number of Colonies
The GB4789.2-1994 tablet count method was used to determine the total number of colonies in the PEF-treated, heated, and control samples.[Citation16,Citation17]
Impact of PEF Sterilization on the Physical and Chemical Properties of Blueberry Juice Samples
The changes in the blueberry juice samples were studied through physical and chemical indicators, such as vitamin C content, anthocyanins, total phenols, titratable acids, and reducing sugars, as well as Brix and color after PEF treatment.
The vitamin C contents of the samples were determined using the GB/T 5009.159-2003 method (“Determination of ascorbic acid in food”).[Citation18]
The anthocyanin contents were determined using the pH differential method.[Citation19]
The titratable acids of the samples were obtained using the potentiometric titration method.[Citation18]
The percentages of soluble solids (Brix) were obtained using the refractometer method, where the temperature of the sample solution was measured. Non-standard temperatures (not equal to 20°C) were adjusted using the temperature correction table.[Citation20]
The phenolic contents of the samples were determined using the Folin–Ciocalteu method.[Citation21]
The percentages of soluble reducing sugars were determined via direct titration.[Citation22]
The tristimulus colors CIE L* a* b* of the samples were determined using a colorimeter. CIE L* indicates the brightness, a* indicates the red value, and b* indicates the yellow value.
The L* value ranges from 0 to 100. The higher the L* values, the whiter the sample surface. The following formulas can be used to calculate the hue angle (hab), the saturation (C*), and total color difference (ΔE), respectively:[Citation23]
Statistical Analyses
The experiments in this study were performed in triplicate, and the results were expressed as mean ± standard deviation. All statistical analyses were performed using the SPSS 16.0 statistical software.
RESULTS AND DISCUSSION
Influence of PEF Field Strength and Treatment Time on the Bactericidal Effect of Blueberry Juice Samples
As shown in , at constant conductivity, the increase in electric field strength or processing time enhanced the inactivation effect of PEF on E. coli in the blueberry juice samples. This trend was similar to the findings of Ayman and Shesha,[Citation8] Sanchez-Moreno et al.,[Citation11] and Francisco et al.[Citation24]
Figure 1 Bactericidal effect of PEF on the blueberry juice samples. (a) PEF intensity and processing time; (b) PEF intensity and conductivity.
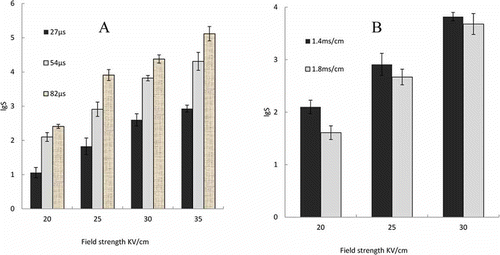
In this experiment, when the electric field strength was 20 kV/cm and the processing time was 27 μs, the total number of E. coli colonies decreased by 1.06 logarithms. When the electric field strength was 35 kV/cm and the processing time was 82 μs, the total number of E. coli colonies decreased by 5.12 logarithms. The linear fit result of the inactivation effect under different electric field strengths indicates that the critical electric field strength of E. coli is 12.9 kV/cm. Previous studies have shown that the critical electric field strength of E. coli was 11.21 kV/cm to 12.19 kV/cm.[Citation24,Citation25] This small difference may be attributed to the processing conditions and different E. coli strains.
Influence of PEF Field Strength and Conductivity on the Bactericidal Effect of Blueberry Juice Samples
In , when the electric field strength is increased, the inactivation effect of PEF on E. coli increases. However, at the same electric field strength, the inactivation effect of PEF on E. coli decreased when the conductivity increased. This result is consistent with those of Zhong et al.[Citation26] This decrease may be attributed to the following. First, the increased electrical conductivity caused a deformity in the square wave and increased its rising time. The pulse width could not be ignored. Thus, the active time was reduced. Second, conductivity determined the resistance of the treatment chamber, thus allowing the decrease in electric field strength and fluctuation of material temperature. Third, greater conductivity requires more density input energy, which leads to better PEF inactivation effect. Therefore, conductivity is an important factor of the PEF sterilization effect.
Table 1 Effect of PEF on the physical and chemical properties of the blueberry juice samples
Influence of PEF on the Physical and Chemical Properties of Blueberry Juice Samples
To explore the impact of PEF on the physical and chemical indicators of the blueberry juice samples, the blueberry juice control and heat-treated samples at high-voltage PEF were compared. The vitamin C, anthocyanins, total phenols, titratable acidity, reducing sugars, Brix, and color, as well as other physical and chemical indicators, were used to test the effect of PEF under the following treatment conditions: 30 kV/cm field strength, 54 μs processing time, 1.4 ms/cm conductivity. The heated samples were treated at 90°C for 15 s, whereas the control group received no treatment. details the indicators of the blueberry juice samples. The PEF-treated group was compared with the control group, which appeared almost unchanged. After heat treatment, the vitamin C of the PEF-treated blueberry juice sample was reduced by 14.78%, whereas its anthocyanin content was reduced by 3.64%.
exhibits the color change of the blueberry juice samples after different treatments. The total color ΔE values of the blueberry juice samples were 2.44 and 6.08 after PEF and heat treatment, respectively. After heat treatment, hab increased from 5.66 to 15.45, and the b* value increased from 3.17 to 8.72. PEF treatment maintained the purple color, fragrant aroma, and nutritional content of the blueberry juice samples. In contrast, heat sterilization resulted in darker blueberry juice color and reduced flavor, vitamin C, and anthocyanin content.
Table 2 Effect of PEF on the color of the blueberry juice samples
Changes in the Microbiological and Physicochemical Properties of the Blueberry Juice Samples after PEF and Heat Treatments During the Storage Period
The trends of the total acid and soluble solids contents in the blueberry juice samples were relatively stable under the different treatment and sterilization methods and during storage at 4°C for 30 days. This result indicates that PEF treatment had a small impact on the total acid and soluble solids contents of the blueberry juice samples ( and ).
Figure 2 Small changes in the properties of stored blueberry juice samples induced by different sterilization processes. (a) Acid content; (b) soluble solid content; (c) phenol content.
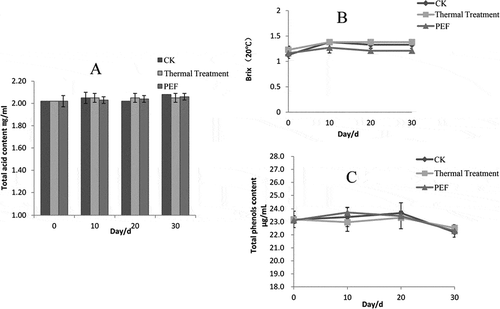
As shown in , compared with that of the control sample, the phenol contents of the PEF-treated and heated blueberry juice samples showed no significant difference after 30 days at 4°C. During the early storage period, the phenol content of the blueberry juice samples slightly increased because of the degradation of anthocyanins and other polyphenols. Then, the total phenolic content in the blueberry juice samples slightly decreased after 20 days because the hydroxyl on the phenolic substances became susceptible to oxidation, generating a black material with metallic reaction and inducing enzymatic and non-enzymatic browning while the total phenol content decreased.
The total number of colonies reached 4.57 log when the blueberry juice samples were stored for 30 days without any treatment. Then, the juice began to spoil. Meanwhile, the total number of colonies in the PEF-treated sample was 1.3 log, which meets the commercial sterility requirements (GB19297-2003). The total number of colonies in the heat-treated samples was detected after 10 days of storage. The number increased to 1.1 log 30 days later. These results indicate that the bactericidal effect of the PEF and heat treatments met the health standards for fruit and vegetable juices ().
Figure 3 Significant changes in the properties of stored blueberry juice samples induced by different sterilization processes. (a) reducing sugar content; (b) bacterial microorganisms; (c) anthocyanin content; (d) vitamin C content.
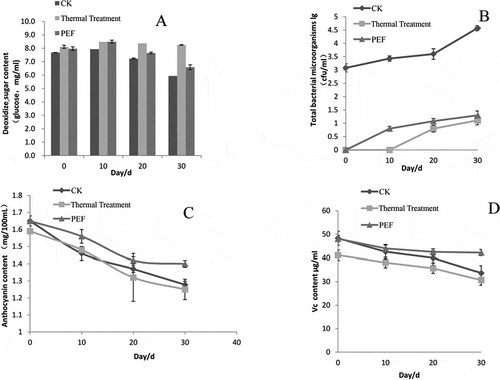
The sugar contents of the PEF-treated and control blueberry juice samples later decreased during storage by about 22%. On the other hand, the heated blueberry juice samples were more stable during the storage period ().
When the storage time was increased, the anthocyanin content of the different treated blueberry juice samples dropped significantly, suggesting that the anthocyanin storability was poor and unstable. After 30 days of storage, the anthocyanin content of the control, PEF-treated, and heated blueberry juice samples decreased by 22.55, 15.15, and 21.38%, respectively ().
The vitamin C content of the control sample decreased to 30.21%, whereas that of the heated sample decreased by 25.39% after 30 days. The PEF-treated sample had a moderate declining slope against storage time, and its degradation rate was only 13.96%. This result indicates that PEF treatment maintained the vitamin C of the blueberry juice samples better than the heat treatment ().
CONCLUSION
The results showed that PEF had an inactivation effect on E. coli in the blueberry juice samples when the electric field strength and processing time were increased. The total number of E. coli colonies was reduced to 5.12 log after PEF treatment at 35 kV/cm and 82 μs. The reducing sugar, total acid, phenols, and soluble solids contents of the blueberry juice samples changed slightly after the PEF and heat treatments. These values are only slightly different from those of the control sample. In general, the vitamin C of the blueberry juice samples were easily oxidized during 30 days of storage at 4°C. The retention rate of the vitamin C in the blueberry juice samples after PEF treatment was 87.87%, which is 13.27% higher than that of the heated sample. The anthocyanins in the blueberry juice samples have anti-oxidant and other physiological functions, but they are unstable and easily decompose. After 30 days of storage, the anthocyanin retention of the blueberry juice samples after PEF treatment was 84.84%, which is 6.23% higher than that of the heated sample. In conclusion, compared with heated blueberry juice, PEF-treated blueberry juice can be stored without losing its original color and nutrients.
ACKNOWLEDGMENT
This work was supported by “The Fundamental Research Funds for the Central Universities of China” (No. BLYJ201203)
REFERENCES
- Astadi, I.R.; Astut, I.M.; Santos, O.U. In vitro antioxidant activity of anthocyanins of black soybean seed coat in human low-density lipoprotein (LDL). Food Chemistry 2009, 112, 659–663.
- Du, Q.; Zheng, J.; Xu, Y. Composition of anthocyanins in mulberry and their antioxidant activity. Food Composition and Analysis 2008, 21, 390–395.
- Faria, A.; Calhau, C.; Defreitas, V. Anthocyanins as antioxidants and tumor cell growth modulators. Journal of Agricultural and Food Chemistry 2011, 54, 2392–2397.
- Mazza, G.; Kay, C.D.; Cottrell, T.; Holub, B.J. Absorption of anthocyanins from blueberries and serum antioxidant status in human subjects. Journal of Agricultural and Food Chemistry 2002, 50, 7731–7737.
- Tian, Q.; Giusti, M.M.; Stoner, G.D.; Schwartz, S.J. Screening for anthocyanins using high-performance liquid chromatography coupled to electrospray ionization tandem mass spectrometry with precursor-ion analysis, product-ion analysis, common-neutral-loss analysis, and selected reaction monitoring. Journal of Chromatography. A 2005, 1091, 72–82.
- Yi, W.; Akoh, C.C.; Fischer, J.; Krewer, G. Effects of phenolic compounds in blueberries and muscadine grapes on HepG2 cell viability and apoptosis. Food Research International 2006, 39, 628–638.
- Bendicho, S.; Barbosa-Canovas, G.V.; Martin, O. Reduction of protease activity in simulated milk ultra filtrate by continuous flow high-intensity pulsed electric field treatments. Journal of Food Science 2003, 68, 952–958.
- El-Hag, A.; Jayaram, S.H./(ICDL 2008), 30 June–3 July, IEEE 2008, p. 1–4. Effect of biological cell size and shape on killing efficiency of pulsed electric field. IEEE International Conference on Dielectric Liquids.
- Evrendilek, G.A.; Zhang, Q.H. Effects of pulse polarity and pulsed delaying time on pulsed electric fields- induced pasteurization of E. coli O157: H7. Journal of Food Engineering 2005, 68, 271–276.
- Jin, Z.L.; Zhang, H.Q.; Li, S.; Kim, M. Quality of apple sauces processed by pulsed electric fields and HTST pasteurization. International Journal of Food Science and Technology 2009, 44, 829–839.
- Sánchez-Moreno, C.; Ancos, D.B.; Plaza, L.; Elez-Martínez, P.; Cano, M.P. Nutritional approaches and health-related properties of plant foods processed by high pressure and pulsed electric fields. Critical Reviews in Food Science and Nutrition 2009, 49, 552–576.
- Chen, T.; Yang, R.J.; Zhang, S.; Zhao, W. Effects of pulsed electric fields on the sterilization and carotenoid content of carrot juice. Food and Fermentation Industries 2010, 36, 41–44 (in Chinese).
- Esteve, M.J.; Barba, F.J.; Palop, S. The effects of non-thermal processing on carotenoids in orange juice. Czech Journal of Food Sciences 2009, 27, 304–306.
- Li, J.; Xiao, J.F.; Chen, J.; Liao, X.J. Inactivation of Escherichia coli and Staphylococcus aureus in apple juice by pulsed electric fields. Food and Fermentation Industries 2010, 36, 41–45 (in Chinese).
- Walkling-Ribeiro, M.; Noci, F.; Cronin, D.A.; Riener, J.; Lyng, J.G.; Morgan, D.J. Reduction of staphylococcusaureus and quality changes in apple juice processed by ultraviolet irradiation, pre-heating, and pulsed electric fields. Journal of Food Engineering 2008, 89, 267–273.
- Salda, A.G.; Puértolas, E.; López, N. Comparing the PEF resistance and occurrence of sublethal injury on different strains of Escherichia coli, Salmonella Typhimurium, Listeria monocytogenes, and Staphylococcus aureus in media of pH 4 and 7. Innovative Food Science and Emerging Technologies 2009, 10, 160–165.
- Marx, M.; Stuparic, M.; Schieber, A. Effects of thermal processing on trans-cis-isomerization of β-carotene in carrot juices and carotene-containing preparations. Food Chemistry 2003, 83, 609–617.
- Jayathilakan, K.; Sultana, K.; Radhakrishna, K.; Sharma, G.K. Effect of irradiation on differential scanning calorimetric profile of fluidised bed dried mutton. International Journal of Food Properties 2012, 15, 202–210.
- Legua, P.; Melgarejo, P.; Martinez, J.J.; Martinez, R.; Hernandez, F. Evaluation of Spanish pomegranate juices: Organic acids, sugars, and anthocyanins. International Journal of Food Properties 2012, 15, 481–494.
- Siddiq, M.; Iezzoni, A.; Khan, A.; Breen, P.; Sebolt, A.M.; Dolan, K.D.; Ravi, R. Characterization of new tart cherry (Prunus cerasus L.): selections based on fruit quality, total anthocyanins, and antioxidant capacity. International Journal of Food Properties 2011, 14, 471–480.
- Pavagadhi, S.; Joseph, G.S.; Jena, B.S. Antioxidant principles in peltophorum ferrugineum flower extracts. International Journal of Food Properties 2012, 15, 549–557.
- Gungor, N.; Sengul, M. Antioxidant activity, total phenolic content, and selected physicochemical properties of white mulberry (Morus alba L.) fruits. International Journal of Food Properties 2008, 11, 44–52.
- Rolle, L.; Torchio, F.; Ferrandino, A.; Guidoni, S. Influence of wine-grape skin hardness on the kinetics of anthocyanin extraction. International Journal of Food Properties 2012, 15,249–261.
- Francisco, T.; Clara, C.; Maria, J.E.; Ana, F. Effect of high-intensity pulsed electric fields processing and conventional heat treatment on orange-carrot juice carotenoids. Journal of Agricultural and Food Chemistry 2005, 53, 9519–9525.
- Zhong, K.; Wu, J.H.; Wang, Z.F. Inactivation kinetics and secondary structural change of PEF-treated HRP and PPO. Food Chemistry 2006, 104, 483–491.
- Zhong, K.; Hu, X.S.; Zhao, G.H. Inactivation and conformational change of horseradish peroxidase induced by pulsed electric field. Food Chemistry 2005, 92, 473–479.
