Abstract
In this study, the mineral composition of germinated brown rice, brown rice, and white rice was evaluated. Brown rice grain was processed through a combination of chemical pretreatment and low oxygen treatment, after which germination was confirmed through imaging under a microscope. Using energy dispersive X-ray analysis, Mg, Al, and Cl were found to be slightly higher in germinated brown rice than in brown rice and white rice. These variations in the mineral content of germinated brown rice were attributed to the joint effect of the germination process and the prior soaking. The inability of energy dispersive X-ray to detect other minerals suggested that it was not sensitive and, hence, it was not suitable for studying elemental distribution in rice grains, or maybe the elements were not present in the rice grains studied.
INTRODUCTION
Morphologically, germination is the transformation of an embryo into a seedling.[Citation1] Physiologically, it is the resumption of suspended metabolism and growth and the switching on of the transcription of the genome.[Citation1] Biochemically, it is the sequential differentiation of oxidative and synthetic pathways.[Citation2] The first step in germination is imbibitions.[Citation2] In this process, water penetrates the grain coat and begins to soften the hard, dry tissues inside. The water uptake causes the grains to swell up, and the coat eventually splits, open allowing water to enter at a faster rate. The water begins to activate the biochemistry of the dormant embryo.[Citation2]
Various requirements must be satisfied before germination can occur. In most cases, there must be sufficient oxygen to allow aerobic respiration, suitable temperature to permit various metabolic processes to continue at an adequate rate, and enough moisture for growth and development.[Citation1] The biochemical and molecular factors affecting germination have been the object of study.[Citation3] Ritchie and Gilroy[Citation4] proposed a detailed model describing the biochemical and molecular factors preceding germination, based on the barley aleurone which shows how gibberellic acid (GA) ultimately causes the transcription of hydrolytic enzymes including α-amylase. Additionally, the germination of monocots, like rice, has been extensively studied.[Citation5−Citation8]
The germination of seeds can be enhanced by factors like chemical pretreatment[Citation9] and anoxia.[Citation10] The effects of hydrogen peroxide (H2O2),[Citation11] sodium hypochlorite (NaOCl),[Citation12] temperature,[Citation13] and anoxia[Citation10] have been reported by different researchers. In agriculture, H2O2 serves as a signalling molecule that enhances the tolerance of seeds to various stresses[Citation14] and enhance the germination of seedlings.[Citation9,Citation11] Anoxia and sodium hypochlorite pretreatment are other factors that have been proven to enhance the germination of seedlings and the activation of α-amylase.[Citation10] In fact, among all grains studied by Perata et al.,[Citation10] only rice had an exceptional ability to germinate under anoxia. Furthermore, energy dispersive X-ray (EDX) analysis is used to study the elemental and chemical compositions of a wide range of materials.[Citation15] Specifically, it can be used to study elemental composition of seeds and grains.[Citation16] Also, the elemental composition of rice has been reported based on the use of different analytical methods.[Citation17−Citation19]
EDX is appealing because it is a relatively simple analytical technique that does not require rigorous sample preparation.[Citation20] However, to the best of the authors knowledge, EDX analysis of brown rice (BR) and especially germinated brown rice (GBR) is sadly lacking. Therefore, the mineral composition of rice grains were studied using EDX to determine the suitability of this method for elemental determination in rice grains. The focus was on the levels of P, K, Mg, Ca, S, Si, and Zn within these deposits. It is believed this is the first study of mineral composition in GBR using EDX spectroscopy and the first comparison of its composition with that of BR and WR. The suitability of an analytical method clearly needs to be determined to enable researchers to choose an ideal method for their studies.
MATERIALS AND METHODS
Chemicals
Hydrogen peroxide (H2O2) was purchased from Bendosen Laboratory Chemicals (Selangor, Malaysia) and sodium hypochlorite was purchased from Takrif Bestari Sdn Bhd. (Selangor, Malaysia).
Rice Sample
One lot (100 kg) of MR220 and MR219 varieties of BR and WR were procured from a local milling factory in Tanjung Karang, Selangor, Malaysia and stored at 4oC. These are the commonly consumed rice varieties in Malaysia. The required quantity of rice was taken out only when needed for the experimental work. The moisture contents of BR and WR were 5.27 and 7.74%, respectively, as determined by a moisture analyzer (MLB 50-3, Kern & Sohn GmbH, Germany) at 110oC.
Germination Process
BR (100 g) was surface sterilized for 30 min with 0.1% sodium hypochlorite (1:5, w/v) in a beaker. The BR was rinsed with several changes of clean tap water and then soaked for 3 to 6 h in 0.5% H2O2. As a control, clean tap water without H2O2 was used to soak the BR. The pre-germination was carried out in a dark room at 35oC, after which the water was drained. The pre-germinated BR was transferred to a square plastic container and allowed to germinate in the darkness of an oven at 35°C for up to 24 h. The plastic container was closed with a lid to create a low oxygen tension. Room temperature was used to germinate BR as a control. The grains were then frozen at –80°C before analysis.
Imaging of Rice Kernels During Germination
Photographs of GBR kernels were taken with a Hirox KH-770 3D Digital Microscope (Tokyo, Japan) to see the developmental changes of their sprouts at different germination intervals. Rice was viewed under this microscope directly at different time intervals without special sample preparation.
Sample Preparation for EDX Analysis
The rice kernels of GBR were cut in half along the cross-sectional axis. Samples were attached to a SEM stub using a double-backed cellophane tape. The stub and samples were examined, photographed, and connected to an EDX for elemental analyses.
EDX
EDX analyses of WR, BR, and GBR were carried out as reported previously.[Citation20] Briefly, analyses were performed at 20 kVp and 80μA with a magnification of 100 X using the scanning mode of Leo 1455 VPSEM (UK) and INCA microanalysis systems (Oxford Instrument Analytical, Bucks, England). The elements were observed using mapping and point identification, with thresholding of α = 2. All spectra were collected for 60 s with aperture, spot size, tilt, and detector distance kept the same for all analyses. Only those spectra collected with greater than 1000 count s−1 and less than 5% dead time were used. Elements in the grains were matched against a standard spectra of elements originally calibrated onto the system, and their values (%) were automatically calculated by the system software. X-ray counts for all the spectra were collected by integrating the peaks at the selected window widths. Actual counts were obtained after subtracting background counts from the total number of counts in the element. Expression was based on a format that entails expressing peak values in the form of peak-to-background (P/B) ratio, defined as the number of counts above the background of peak divided by the number of background counts.[Citation21]
Analysis of Results
All analyses were done in triplicate. Analysis of variance (ANOVA) was done using SPSS 17.0 software (SPSS Inc., Chicago, IL, USA) to assess the significance of the differences between the means at p < 0.05.
RESULTS AND DISCUSSION
Germination Process
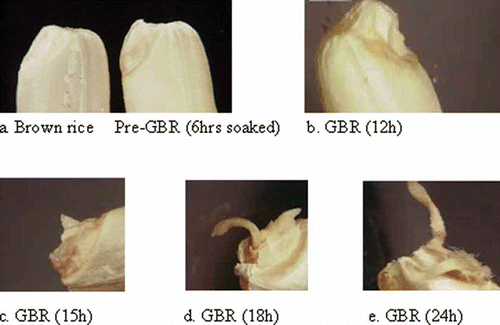
As shown in , soaking of BR in H2O2 solution for 6 h increased the germination percentage (91.5% at a higher temperature) when compared to the control (soaked in tap water) in which the germination was 81.5%. However, pretreatment with H2O2 solution for 3 h at 35oC was not different from soaking in water. shows that H2O2 pretreatment generally resulted in an improvement in germination percentages when matched with the corresponding controls, soaked in water, only for a few exceptions. This is also true for pretreatment at room temperature. Surprisingly, pre-germination times of 3 and 6 h did not show any difference except when H2O2 solution was used at 35oC.
Table 1 Percentage of germination of BR by using 0.5% H2O2 compared with water
As previously reported, H2O2 pretreatment is used to break the seed dormancy of rice to enhance its germination.[Citation22] This effect of H2O2 has also been reported for other grains,[Citation23,Citation24] establishing it as a germination enhancer. The molecular mechanisms underlying germination of rice seeds are still being unravelled. However, it is known that an important mechanism involved in the germination of cereal seeds is GA signalling, and only rice is known to respond to this vital molecule under anoxic conditions.[Citation10] The fact that α-amylase is synthesized under anaerobic conditions in rice seeds, but not in barley and wheat, suggests that the biochemical mechanisms for germination in rice seeds differ from those of other grains,[Citation10] hence the reason for germinating BR under low oxygen tension.
Sasaki et al.[Citation11] reported that H2O2 pretreatment of seeds affected the germination rate and the growth rate of germinated seedlings at different temperatures. This underscores the choice of different temperatures for the current study. shows that H2O2 pretreatment enhanced the elongation of sprouts. The elongation of sprouts more than 2 mm after final germination was significantly higher (p < 0.05) for the H2O2 solution than the control, especially at 35oC using a pre-germination time of 6 h. Although, after a 24 h germination at 35oC, sprout length was predominantly between 1 and 2 mm, and at 27oC it was mostly equal to or less than 1 mm. The results, therefore, suggest that higher temperatures germinated more BR under low oxygen conditions similar to the results of Roberts[Citation25] reported. There was no significant difference between soaking in H2O2 or water between 1 and 2 mm sprout length except for a 3 h pre-germination time. For the same sprout length, a temperature of 35oC gave better results (p < 0.05) than 27oC, while the difference between the 3 and 6 h pre-germination times was not significant (p > 0.05).
Table 2 Percentage of germination of BR with shoot length
Imaging of Rice Kernel During Germination
The appearance of GBR at various germination times was digitally captured, to determine the best germination conditions. When BR was soaked in H2O2 solution at 35oC and kept in darkness (), germination, i.e., radicle emergence, started at 15 h and was almost complete by 24 h after water uptake. The photographs show that radicle elongation increased as germination time progressed. shows that the kernel was already swollen after 6 h of soaking. This was because soaking increased the respiration rate, which is known to continue for about 12 h.[Citation26] Over the course of 24 h, germination had successfully taken place.
EDX Analysis
EDX analysis was done using one GBR kernel germinated at 35oC in H2O2 solution after a 6 h soaking. shows that GBR contained significantly higher levels (p < 0.05) of Cl compared to BR and WR. The levels of Si, P, K, and Ca were similar in BR and GBR but significantly different (p < 0.05) from those in WR. GBR contained significantly higher levels of Mg than BR, but had lower Mg levels than WR. The level of Al was similar in GBR and WR, which had significantly lower levels than in BR. Surprisingly, although germination is a catabolic process that uses up reserves present in the cotyledon for development and growth of the embryo, minerals were spared in this process as shown from the results in which only Mg, Al, and Cl differed significantly between GBR and BR. X-ray analyses of WR and GBR could not detect Fe, Zn, and Se even though they were reported present by Manjusha et al.[Citation18] and Chung et al.[Citation27] based on atomic absorption spectrometry (AAS). These elements are normally found in WR and BR; therefore, our findings suggest that EDX analysis does not have the necessary sensitivity for rice grain analysis.
Table 3 Average relative values (percentage weight) for elemental distribution of GBR compared to BR and WR
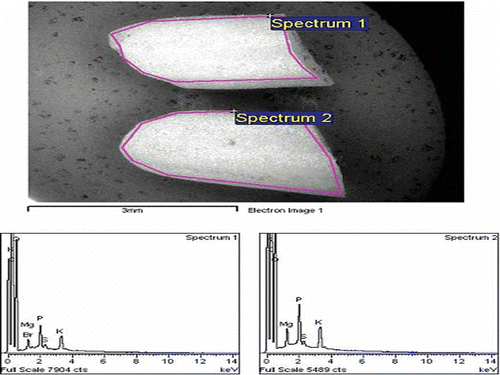
and show the distribution of elements in GBR and WR respectively, using a mapping method. The mapping of one whole GBR kernel showed that seven elements were detected in GBR compared to four elements in WR. Si, Cl, and Ca deposits were not detected in WR on EDX, while Br was only detected in WR. shows that the elements present in the upper and lower halves of the same GBR kernel are different. The upper half of GBR only had Mg, Cl, K, and Ca, while the lower half had three additional minerals (Si, P, and S). The mineral contents in the lower half of GBR were higher than in the upper part, and Ca was the lowest compared to the other minerals. shows that elements present in the upper and lower halves of the same WR kernel are similar; they both have Mg, P, K, and S, although the lower part had higher amounts. and further show the EDX spectra together with elements obtained from two halves of GBR and WR grains, respectively.
Figure 2 EDX mapping for one whole kernel of GBR which has been cut into two equal halves showing elements detected therein: (a) upper half of GBR; (b) lower half of GBR. (Color figure available online.)
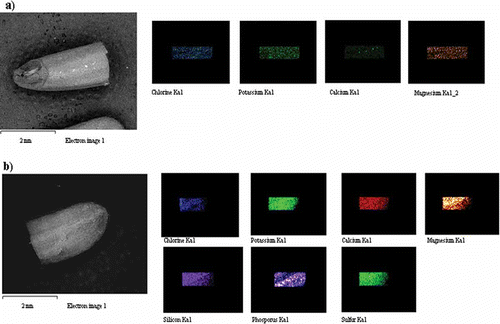
Figure 3 EDX mapping for one whole kernel of WR which has been cut into two equal halves showing elements detected therein: (a) upper half of WR; (b) lower half of WR. (Color figure available online.)

Figure 4 EDX spectra for the upper (spectrum 1) and lower (spectrum 2) halves of GBR grain, and the intensity of corresponding elements detected. (Color figure available online.)
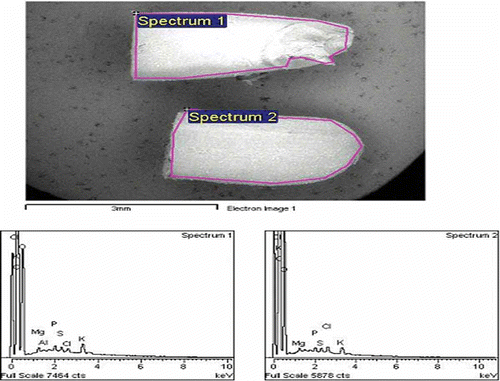
Figure 5 EDX spectra for the upper (spectrum 1) and lower (spectrum 2) halves of WR grain, and the intensity of corresponding elements detected. (Color figure available online.)
Several elements are known to be present in rice including but not limited to Cr, Cd, Pb, Fe, Mg, Zn, Se, Na, K, Br, As, Rb, Zn, Co, and Sc;[Citation18,Citation19,Citation27] however, their detection depends on which analytical technique is used. This current study shows how EDX can detect some but not all elements found in rice, suggesting that it may not be sensitive enough, and likely not a suitable method, to study elemental distribution in rice. However, considering the ease of conducting EDX and the lack of sample preparation,[Citation20] it may be useful in studying specific elements in rice grains, like those detected in the current study, without destroying the grains. Furthermore, the rice varieties did not contain other undetected elements since, generally speaking, the mineral composition of rice is determined by rice variety, degree of milling and growing environment.[Citation28] Interestingly, there are no studies reporting the mineral composition of the Malaysian rice varieties analyzed in the current study, making comparisons difficult.
Finally, it is common knowledge that EDX can be used as a screening tool to detect elements in grains, and it was also shown that EDX can be used to study the elemental composition of WR, BR, and GBR together with their distribution through mapping.
CONCLUSION
The current study confirmed the previously reported germination enhancing effects of chemical pretreatment, higher temperatures, and low oxygen tension on rice seeds. Additionally, it was also found that Mg, Al, and Cl are affected by germination. Further studies are needed to determine the full mineral composition of these rice varieties and how the minerals are affected by germination by using analytical techniques other than EDX. However, EDX may be useful in studying any of the specific minerals that the current study has shown to be detectable by EDX.
ACKNOWLEGMENTS
This work was funded by Padiberas Nasional Berhad (BERNAS), Selangor, Malaysia. The authors thank the staff of the Laboratory of Molecular Biomedicine and Electron Microscopy Unit, Institute of Bioscience, Universiti Putra Malaysia for their advice and technical assistance in this study.
REFERENCES
- Andrade, A.C.S.; Cunha, R.; Souza, A.F.; Reis, R.B.; Almeida, K.J. Physiological and morphological aspects of seed viability of a neotropical savannah tree, Eugenia dysenterica DC. Seed Science and Technology 2003, 31, 125–137.
- Nonogaki, H. Seed germination — The biochemical and molecular mechanisms. Breeding Science 2006, 56, 93–105.
- Limami, A.M.; Rouillon, C.; Glevarec, G.; Gallais, A.; Hirel, B. Genetic and physiological analysis of germination efficiency in maize in relation to nitrogen metabolism reveals the importance of cytosolic glutamine synthetase. Plant Physiology 2002, 130, 1860–1870.
- Ritchie, S.; Gilroy, S. Gibberellins: Regulating genes and germination. New Phytologist 1998, 140, 363–383.
- Cohn, M.A.; Hughes, J.A. Seed dormancy in red rice. I. Effect of temperature on dry-after ripening. Weed Science 1981, 29, 402–404.
- Cohn, M.A.; Butera, D.L. Seed dormancy in red rice. II. Response to cytokinins. Weed Science 1982, 30, 200–205.
- Cohn, M.A.; Butera, D.L.; Hughes, J.A. Seed dormancy in red rice. III. Response to nitrite, nitrate, and ammonium. Plant Physiology 1983, 73, 381–384.
- Cohn, M.A.; Castle, L. Dormancy in red rice. IV. Response of unimbibed and imbibing seeds to nitrogen dioxide. Plant Physiology 1984, 60, 552–556.
- Riffle, J.W.; Springfield, H.W. Hydrogen peroxide increases germination and reduces microflora on seed of several southwestern woody species. Forest Science 1968, 14, 96–101.
- Perata, P.; Geshi, N.; Yamaguchi, J.; Akazawa, T. Effect of anoxia on the induction of α-amylase in cereal seeds. Planta 1993, 191, 402–408.
- Sasaki, K.; Kishitani, S.; Abe, F.; Sato, T. Promotion of seedling growth of seeds of rice (Oryza sativa L. cv. Hitomebore) by treatment with H2O2 before sowing. Plant Production Science 2005, 8, 509–514.
- Miyoshi, K.; Mii, M. Enhancement of seed germination and Protocorm formation in Calanthe discolor (Orchidaceae) by NaOCl and polyphenol absorbent treatments. Plant Tissue Culture Letters 1995, 12, 267–272.
- Roberts, E.H. The viability of rice seed in relation to temperature, moisture content, and gaseous environment. Annals of Botany 1961, 25, 381–390.
- He, L.; Gao, Z.; Li, R. Pretreatment of seed with H2O2 enhances drought tolerance of wheat (Triticum aestivum L.) seedlings. African Journal of Biotechnology 2009, 8,6151–6157.
- Bamford, S.A.; Wegrzynek, D.; Chinea-Cano, E.; Markowicz, A. Application of X-ray fluorescence techniques for the determination of hazardous and essential trace elements in environmental and biological materials. Nukleonika 2004, 49, 87–95.
- Lott, J.N.A.; Greenwood, J.S.; Vollmer, C.M. Energy-dispersive x-Ray Analysis of Phosphorus, Potassium, Magnesium, and Calcium in globoid crystals in protein bodies from different regions of Cucurbita maxima embryos. Plant Physiology 1978, 61, 984–988.
- Maihara, V.A.; Vasconcellos, M.B.A. Determination of trace elements in Brazilian rice grains and in biological reference materials by neutron activation analysis. Journal of Radioanalytical and Nuclear Chemistry 1989, 132, 329–337.
- Manjusha, R.; Dash, K.; Karunasagar, D.; Arunachalam, J. Determination of trace elements in Indian rice by ETAAS and ICP-AES. Atomic Spectroscopy 2008, 29, 51–55.
- Park, C.J.; Suh, J.K. Determination of trace elements in rice flour by isotope dilution inductively coupled plasma mass spectrometry. Journal of Analytical and Atomic Spectrometry 1997, 12, 573–577.
- Turhan, S.; Zararsiz, A.; Karabacak, H. Determination of element levels in selected wild mushroom species in Turkey using non-destructive analytical techniques, International Journal of Food Properties 2010, 13, 723–731.
- Ockenden, I.; Lott, J.N.A. Beam sensitivity of globoid crystals within seed protein bodies and commercially prepared phytates during X-ray microanalysis. Scanning Microscopy 1991, 5, 767–768.
- Naredo, M.E.B.; Juriano, A.B.; Lu, B.R.; Guzman, F.D.; Jackson M.T. Response to seed dormancy breaking treatment in rice species (Oryza L.). Seed Science and Technology 1998, 26, 675–689.
- Cavusoglu, K.; Kabar, K. Effects of hydrogen peroxide on the germination and early seedling growth of barley under NaCl and high temperature stresses. EurAsian Journal of Bioscience 2010, 4, 70–79.
- Gondim, F.A.; Gomes-Filho, E.; Lacerda, C.F.; Prisco, J.T.; Neto, A.D.A.; Marques, E.C. Pretreatment with H2O2 in maize seeds: effects on germination and seedling acclimation to salt stress. Brazilian Journal of Plant Physiology 2010, 22, 103–112.
- Roberts, E.H. Dormancy in rice seed: III. The influence of temperature, moisture, and gaseous environment. Journal of Experimantal Botany 1962, 13, 75–94.
- Jann, R.C.; Amen, R.D. What is germination?. In: The Physiology and Biochemistry of Seed Dormancy and Germination; Khan, A.A.; Ed.; North Holland Publishing: Amsterdam, 1977; 7–28.
- Chung, Y.S.; Chung, Y.J.; Cho, K.H.; Lee, J.H. Determination of trace and toxic elements in Korean rice CRM by INAA, ICP, and AAS. Journal of Radioanalytical and Nuclear Chemistry 1996, 215, 129–134.
- McKevith, B. Nutritional aspects of cereals. Nutrition Bulletin 2004, 29, 111–142.