Abstract
Green tea (Camellia sinensis) is a prosperous source of polyphenols, especially catechins. In the current research, an effort was made to optimize the extraction conditions for maximum yield of catechins from the local green tea Qi-Men. For the purpose, three different solvents were used, i.e., aqueous ethanol (50%), aqueous methanol (50%), and water at different time intervals (20, 40, and 60 min). Green tea catechins were quantified through HPLC using a C18 column and UV detector. The antioxidant activity of green tea catechins was measured through in vitro tests including DPPH radical scavenging ability and antioxidant activity. Results showed that extraction through aqueous ethanol resulted in maximum yield of green tea catechins (17400 ± 0.19 mg/100 g green tea leaves. Moreover, epigallocatechin, epigallocatechin gallate, epicatechin gallate, and epicatechin ranged from 4.26 ± 0.09 to 6.4 ± 0.2, 12.1 ± 0.123 to 17.7 ± 0.3, 1.32 ± 0.03 to 1.81 ± 0.02, 5.48 ± 0.099 to 8.6 ± 0.2 g/100 g of dry-extract, respectively. Furthermore, highest antiradical (80.65 ± 3.69%) and antioxidant activity (67.12 ± 3.08%) were observed in catechins extracted through aqueous ethanol.
INTRODUCTION
Green tea is now gaining significant attention both in technical and consumer communities due to its health benefits against variety of ailments. The presence of polyphenols (catechins) is responsible for health benefits associated with green tea consumption. Catechins are flavanols that constitute the majority of soluble solids of green tea leaves. The major fractions are epigallocatechin gallate (EGCG), epigallocatechin (EGC), epicatechin gallate (ECG), and epicatechin (EC). Among these, EGCG is the predominant fraction contributing more than 50% of the polyphenols. Generally, catechins are lower molecular weight, colorless, water-soluble compounds with a bitter and astringent taste. Other components present in tea leaves include proteins, carbohydrates, lipids, sterols, vitamins, xanthic bases (caffeine and theophylline), volatiles compounds, minerals, and trace elements.[Citation1,Citation2] The chemical composition of green tea varies with the geographical area and growing conditions, e.g., climate, season, agricultural practices, variety, age, and position of the leaf.[Citation3]
Free radicals and reactive oxygen species (ROS) produced from oxidative metabolism inhibit the activities of some protective enzymes and induce destructive effects on cellular components.[Citation4] Green tea and its functional ingredients hold the ability to trap ROS, thus reducing the damage to lipid membranes, proteins, and nucleic acid. Among tea catechins, EGCG is the most effective in attenuating ROS.[Citation5] Extraction of active components from green tea requires special attention as it affects the overall yield of catechins.[Citation6,Citation7] During the extraction process catechins may combine with sugars and proteins. Furthermore, these are also prone to oxidation, high process temperature, and alkaline conditions which all result in their subsequent degradation.
The present project was designed to obtain maximum extraction of crude catechins from green tea leaves using different solvents (water, aqueous ethanol, and equeous methanol) at various time intervals (20, 40, and 60 min). Crude catechins obtained were subjected to HPLC analysis to quantify EC, EGC, EGCG, and ECG. Extracted crude catechins were also subjected to different in vitro tests to find out radical scavenging and antioxidant activity. This research work will help to determine solvents ratio and time combination that will result in maximum catechins extraction from locally grown green tea variety (Qi-Men). Measurement of antioxidant and DPPH scavenging activity will further help to elaborate the ability of extracted catechins to scavenge free radicals.
MATERIALS AND METHODS
Materials
Dried green tea leaves of the Qi-Men variety were obtained from the National Tea Research Institute (NTRI), Shinkiari, Mansehra, Pakistan. Solvents (ethanol, methanol, ethyl acetate, chloroform, acetonitrile, and acetic acid) and Folin–Ciocalteus reagent was purchased from Merck (Merck KGaA, Darmstadt, Germany). Standards (catechin, EGCG, EGC, and EC) and DPPH reagent was purchased from Sigma (Sigma-Aldrich, St. Louis, MO, USA). Special care was taken to maintain the temperature and time during optimization. Standards for catechins were kept under prescribed condition during storage before use.
Chemical Composition of Green Tea
Green tea leaves were ground (Renker, Model: GMO 1 grinder) and analyzed for moisture, ash, crude protein, crude fat, crude fiber, and nitrogen free extract (NFE) according to their respective standard methods described in AACC.[Citation8] Total alkaloids including caffeine, theophylline, and theobromine were also estimated.[Citation9] Green tea leaves were subjected to mineral analysis for sodium, potassium, calcium, zinc, and iron.[Citation10]
Polyphenols
Total polyphenols (TP) in green tea leaves were determined using the Folin-Ciocalteu method.[Citation11] For the purpose, 50 μL of sample was added to 250 μL of Folin-Ciocalteu’s reagent then 750 μL of 20% Na2CO3 solution was added and volume was made upto 5 mL with distilled water. After 2 h, absorbance was measured at 765 nm with a UV/visible light Spectrophotometer (CECIL CE7200) against a control.
Extraction of Catechins
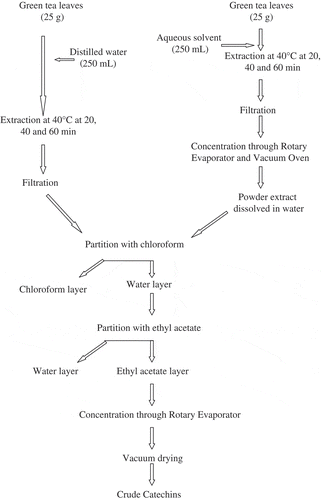
Crude catechins from green tea leaves (25 g) were extracted using three solvents (250 mL) namely water, aqueous ethanol (50%), and aqueous methanol (50%) for three time periods (20, 40, and 60 min) and constant temperature of 40°C. After extraction, solvents extracts (aqueous ethanol and methanol) were filtered and concentrated in a Rotary Evaporator (Eyela, Japan). These were further dried in a Vacuum Oven (DZF-6020). The resultant powdered extracts were dissolved in water and along with the water extract, subjected to separation partition by chloroform and ethyl acetate through separating funnel to obtain their respective catechins rich fractions followed by rotary evaporation and vacuum drying () following the method described by Row and Jin.[Citation12]
HPLC Analysis of Catechins
Crude catechins were analyzed for active ingredients (EC, EGCG, EGC, ECG) by HPLC (PerkinElmer, Series 200, USA) containing C18 column (250 mm × 4.6 mm, 5.0 μm particle size) and an autosampler. Injection amount was 10 μL (0.5 g crude catechins in 10 mL ethanol) and column temperature was 40°C. During catechins quantification, mobile phase including solvent A (acetonitrile/acetic acid/water 6:1:193) and solvent B (acetonitrile/acetic acid/water 60:1:139) was used with a flow rate of 1 mL/min. Gradient elution was performed consisting of 100% (v) solvent A to 100% (v) solvent B by linear gradient during the first 15 min and then 100% (v) solvent B until 60 min. Quantification of individual components was carried out by UV detector at 280 nm.[Citation13]
Antioxidant Activity (In Vitro Studies)
Free radical scavenging activity (DPPH assay)
DPPH radical scavenging activity of catechins was measured according to the method described by Brand-Williams et al.[Citation14] Accordingly, 1 mL of DPPH was added to each 4 mL sample (prepared by adding 1 g of crude catechins in 5 mL of ethanol) with incubation at room temperature for 30 min. Absorbance was noted at 520 nm using a spectrophotometer.
Antioxidant activity
Antioxidant activity of the catechins was determined following the protocol of Taga et al.[Citation15] Oxidation of β-carotene emulsion was measured spectrophotometrically by reading absorbance at 470 nm at 0, 10, 20, 30, and 40 min. The degradation rate of the catechins extracts was calculated using following equation.
Statistical Analysis
The experiment was run in triplicate and completely randomized design (CRD) was applied and resultant data was subjected to statistical analysis using Cohort version 6.1 as statistical package (Costat-2003). Analysis of variance technique (ANOVA) was used to determine the level of significance[Citation16] and means showing the effects of solvents, time and their interaction were further compared through using Duncan Multiple Range test.
RESULTS
Composition of Green Tea
Proximate analysis of green tea (dry weight basis) indicated that moisture, protein, fat, fiber, ash, and NFE were 4.88 ± 0.09, 18.06 ± 1.06, 2.49 ± 0.13, 15.35 ± 1.05, 5.60 ± 0.21, and 53.68 ± 1.75%, respectively. TP and alkaloids were found to be 30.31 ± 1.78 and 2.51 ± 0.09%, respectively. Minerals including sodium, potassium, calcium, manganese, iron, and zinc in green tea were 7.55 ± 0.14, 1703.13 ± 51.50, 374.75 ± 19.65, 65.22 ± 3.83, 20.53 ± 0.76, and 3.06 ± 0.12 mg/100 g, respectively ().
Table 1 Composition of green tea
Extraction and HPLC Assay of Catechins
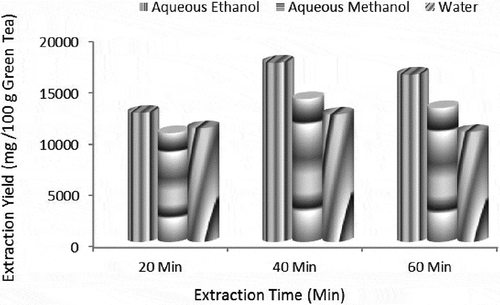
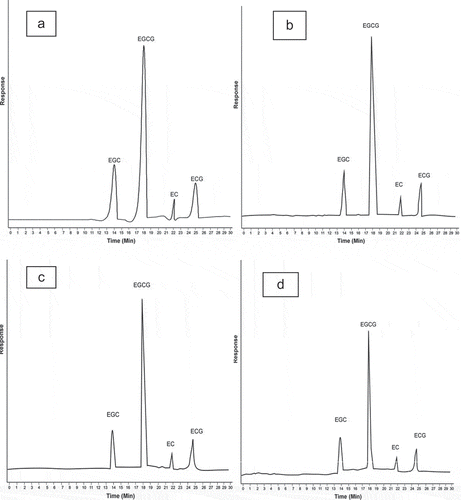
Solvent extraction is a basic technique for the extraction of tea catechins and is influenced by many factors like quantity of tea sample, solvent, time, ratio of solvent to material, and temperature. Among different solvents aqueous ethanol resulted in maximum catechins extraction (17400 ± 0.19 mg/100 g) after 40 min, whereas lowest yield was recorded with methanol after 20 min (). indicated amount of individual catechins in green tea quantified through HPLC, i.e., EGC, EGCG, ECG, and EC that were significantly affected by solvent and extraction time. Chromatograms are shown in . Among three solvents, aqueous ethanol resulted in maximum yield of EGC (6.362 ± 0.152 g/100 g extract), EGCG (17.657 ± 0.24 g/100 g extract), ECG (1.810 ± 0.018 g/100 g extract) and EC (8.624 ± 0.161 g/100 g). Regarding extraction time 40 min gave highest recovery of EGC, EGCG, ECG, and EC. Results also confirmed that among individual catechins, EGCG was present in highest quantity and EC was in lowest values. However, order of catechins was EGCG > EGC > ECG > EC.
Table 2 Extraction yield of EGC, EGCG, ECG, and EC (g/100 g of dry extract)
In Vitro Studies
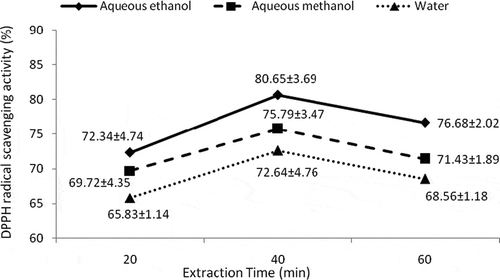
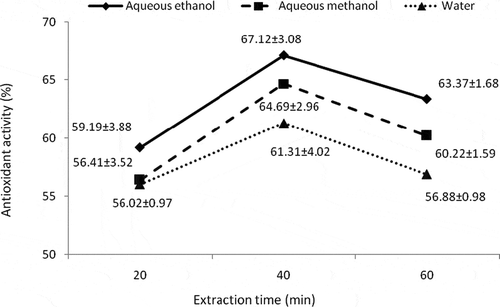
Means for DPPH radical scavenging activity indicated that catechins extracted by aqueous ethanol after 40 min showed the highest DPPH radical scavenging activity (80.65 ± 3.69%) followed by methanol extracted catechins (75.79 ± 3.47%), whereas lowest activity was shown by water extracted catechins i.e., 65.83 ± 1.14% (). Likewise highest antioxidant activity (67.12 ± 3.08%) was shown by catechins extracted with aqueous ethanol after 40 min () followed by methanol and water extracted catechins.
DISCUSSION
The catechins are responsible for the biological activities of green tea. They have shown promising results in curing various ailments that include metabolic disorders, cardiovascular disparities, and cancer insurgence. For the concern, efforts were directed to analyze and extract green tea catechins (GTC) from a variety cultivated in Pakistan. In the first step, chemical composition of green tea was assessed, presented in . The results regarding proximate composition are comparable with the previous findings, i.e., moisture, protein, lipid, sugars, fiber, and ash contents in different green tea samples were 2.2–5.0, 18.2–30.7, 3.5–5.3, 28.6–39.2, 10–19.5, and 5.4–7.4%, respectively.[Citation17] However, catechins are functional ingredients of green tea. In this regard, results were comparable with that of Asghar and Masood.[Citation18] They estimated TP contents in green tea as 31.3 ± 0.095%, however, Anesini et al.[Citation19] contradicted with others and highlighted that TPC fall in the range of 14.32 ± 0.45 to 21.02 ± 1.54%. Likewise, the same research group tested total alkaloids in green tea and they stated its range from 1.9–3.5%.
The results for mineral contents are corroborated with the earlier findings that Ca, Fe, Na, and K in different green tea samples were in the range of 390–740, 10.4–38, 3–11, and 1900–2800 mg/100 g, respectively.[Citation17] In another research experiment, minerals including Zn, Mn, Fe, Ca, Na, and K in 21 green tea samples varied from 2.10–3.49, 66.07–159.54, 14.45–35.22, 361.81–547.50, 3.77–11.70, and 1190.05–1699.41 mg/100 g, respectively.[Citation20] The compositional variations in green tea with special reference to proximate, TP, and minerals were due to differences in variety, season, geographic origin, agronomic practices (soil, water, minerals, and fertilizers), age, and position of leaf on harvested shoot.
In the second phase, extraction of GTC was carried out using different solvents. The results for the extraction yield of catechins are supported by previous findings that aqueous ethanol results in better extraction of tea flavonoids than water, aqueous methanol, and acetone[Citation21] and it is also more effective for prolonged extraction of 30 min than water.[Citation2,Citation22] Higher extraction yield of EGCG was obtained with ethanol (50%) than methanol (50%) and water that is in agreement with results published by Druzynska et al.[Citation23] who documented that aqueous ethanol could give better extraction yield of catechins than aqueous methanol. These findings showed higher GTC as compared to similar work conducted in other parts of the globe. Therefore, extracts were evaluated through HPLC to determine the presence of specific functional ingredients, e.g., EGCG, EGC, EC, and ECG. The efficacy of GTC was further evaluated through determining their antioxidant activity by some in vitro tests.
The EGC quantification results through HPLC are also in conformity with findings of Wang and Helliwell,[Citation21] as they obtained highest EGC 68.7 ± 0.4 mg/g using 40% ethanol at 30 min. The amount of EGCG in extracted samples of catechins is supported by findings of different scientists groups as they reported that in HPLC analyzed green tea samples EGCG was higher in quantity than other catechins. The results for ECG are similar to the findings of Friedman et al.;[Citation24] they stated that amount of ECG in water extracted samples (1.2 ± 0.16 to 27.1 ± 0.6 mg/g) was less than ethanol extracted samples (2.4 ± 1.8 to 40.5 ± 1.6 mg/g). Lowest value of EC is in line with work of Lin et al.[Citation3] they documented the range 1.26 ± 0.24 to 7.27 ± 0.47 and 6.60 to 10.3 mg/g, respectively for EC in HPLC analyzed green tea samples. In current exploration, higher concentrations of EGCG, EGC, ECG, and EC obtained through aqueous ethanol might be due to closer polarity of catechins with ethanol, whereas water assisted tea leaves in spreading matrix structure.
The antioxidant activity of GTC extracted through different solvents yielded some varied responses. In an earlier study, it was described that DPPH radical scavenging ability of green tea is due to galloyl moiety attached to the falvanol at 3-position along with ortho-trihydroxyl group in B ring.[Citation18] Moreover, it is already narrated in a few studies that EGCG is the most effective scavenger of superoxide anion, hydroxyl radical and 1, DPPH than other catechins compounds.[Citation19] Therefore, EGCG has the highest scavenging activity because of both trihydroxyl groups at carbons 3’, 4’, and 5’ on the B-ring and a gallate moiety esterified at carbon 3’ on the C-ring. In the present study, ethanolic extracted catechins possessed better antiradical and antioxidant activity because of high EGCG concentration. Moreover, higher DPPH activity in the present study might be due to presence of active ingredients. Therefore, DPPH activity is indirectly related to different solvents and extraction time.[Citation7,Citation25] The hypothesis can be validated as highest DPPH scavenging ability was observed in catechins obtained after time of 40 min. The existing results for DPPH assay and antioxidant activity are in-line with the findings of many researchers who also reported better antiradical activity from ethanolic extracts than water extracts.[Citation18]
CONCLUSION
Green tea is a good source of bioactive molecules (catechins) including EGC, ECG, EGCG, and EC. Among catechins, EGCG is present in the highest amount, whereas EC in minimum quantity. The green tea leaves slurred with aqueous ethanol for 40 min resulted in maximum extraction of catechins and EGCG. The aqueous ethanol performed better than methanol and water for catechins extraction. The quantification of individual components revealed that EGCG was present in higher proportions. The powdered green tea extracts showed significant antioxidant activities. However, EGCG extract showed maximum antioxidant activity than remaining fractions.
NOMENCLATURE
| ANOVA | = | Analysis of variance |
| Ca | = | Calcium |
| CRD | = | Completely randomized design |
| DNA | = | Deoxyribonucleic acid |
| DPPH | = | 1,1-diphenyl-2-picrylhydrazyl radical |
| EC | = | Epicatechin |
| ECD | = | Electrochemical detector |
| ECG | = | Epicatechin gallate |
| EGC | = | Epigallocatechin |
| EGCG | = | Epigallocatechin gallate |
| Fe | = | Iron |
| g | = | Gram |
| HPLC | = | High performance liquid chromatography |
| K | = | Potassium |
| mg | = | Milligram |
| min | = | Minute |
| Mn | = | Manganese |
| Na | = | Sodium |
| NFE | = | Nitrogen free extract |
| ref | = | Reference |
| TP | = | Total polyphenols |
| TPC | = | Total polyphenols contents |
| Zn | = | Zinc |
ACKNOWLEDGMENT
Rabia Shabir Ahmad is thankful to the Higher Education Commission of Pakistan for providing financial support to complete this project entitled ‘‘Extraction of green tea catechins for the preparation of functional drink: Correlation with lifestyle-related disorders.”
REFERENCES
- Cabrera, C.; Artacho, R.; Gimenez, R. Beneficial effects of green tea—A review. Journal of American College of Nutrition 2006, 25, 79–99.
- Liang, Y.R.; Xu, Y.R. Effect of extraction temperature on cream and extractability of black tea (Camellia sinensis (L.) O. Kuntze). International Journal of Food Science and Technology 2003, 38, 37–45.
- Lin, Y.S.; Tsai, Y.J.; Tsay, J.S.; Lin, J.K. Factors affecting the levels of tea polyphenols and caffeine in tea leaves. Journal of Agriculture and Food Chemistry 2003, 51, 1864–1873.
- Bauer, V.; Sotnikova, R.; Machova, J.; Matyas, S.; Pucovsky, V.; Stefek, M. Reactive oxygen species induced smooth muscle responses in the intestine vessels and airways and the effect of antioxidants. Life Sciences 1999, 65, 1909–1917.
- Khan, N.; Mukhtar, H. Tea polyphenols for health promotion. Life Sciences 2007, 81, 519–533.
- Danrong, Z.; Yuqiong, C.; Dejiang, N. Effect of water quality on the nutritional components and antioxidant activity of green tea extracts. Food Chemistry 2009, 113, 110–114.
- Liang, Y.R.; Yea, Q.J.; Liang, H.; Lu, J.L.; Du, Y.Y.; Dong, J.J. Chemical and instrumental assessment of green tea sensory preference. International Journal of Food Properties 2008, 11, 258–272.
- AACC Approved Methods of the American Association of Cereal Chemists, 10th Ed; The American Association of Cereal Chemists: St Paul, MN, USA, 2000.
- Meterc, D.; Petermann, M.; Weidner, E. Drying of aqueous green tea extracts using a supercritical fluid spray process. The Journal of Supercritical Fluids 2008, 45, 253–259.
- AOAC International. Official Methods of Analysis of Association of Official Analytical Chemists International. In: Horwitz, W.; Ed.; Association of Analytical Communities: Galthersburg, MD, USA, 17th Ed, Second revivion. 2003.
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventos, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent. Methods in Enzymology 1999, 299, 152–178.
- Row, K.H.; Jin, Y. Recovery of catechin compounds from Korean tea by solvent extraction. Bioresource Technology 2006, 97, 790–793.
- Liang, H.; Liang, Y.; Dong, J.; Lu, J. Tea extraction methods in relation to control of epimerization of tea catechins. Journal of Science of Food and Agriculture 2007, 87, 1748–1752.
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Useof a free radical method to evaluate antioxidant activity. Lebensmittel-Wissenschaft und-Technologie 1995, 28, 25–30.
- Taga, M.S.; Miller, E.E.; Pratt, D.E. China seeds as source of natural lipid antioxidation. Journal of the American Oil Chemists’ Society 1984, 61, 928–931.
- Steel, R.G.D.; Torrie, J.H.; Dickey, D. Principles and Procedures of Statistics: A Biometrical Approach, 3rd Ed; McGraw Hill Book Co Inc.: New York, 1997.
- Chee, D.C.; Juneja, L.R. Coeneral chemical composition of green tea and its infusion. In: Chemistry and Applications of Green Tea; Yamamoto, T.; Juneja, L.R.; Chee, D.C.; Kim, M.; Ed.; CRC Press: Japan, 1997.
- Asghar, Z.; Masood, Z. Evaluation of antioxidant properties of silymarin and its potential to inhibit peroxyl radicals in vitro. Pakistan Journal of Pharmaceutical Sciences 2008, 21 (3), 249–254.
- Anesini, C.; Ferraro, G.E.; Filip, R. Total polyphenol content and antioxidant capacity of commercially available tea (Camellia sinensis) in Argentina. Journal of Agriculture and Food Chemistry 2008, 56 (19), 9225–9229.
- Fernandez-Caceres, P.L.; Martın, M.J.; Pablos, F.; Gonzalez, A.G. Differentiation of tea (Camellia sinensis) varieties and their geographical origin according to their metal content. Journal of Agriculture and Food Chemistry 2001, 49, 4775–4779.
- Wang, H.; Helliwell, K. Determination of flavonols in green and black tea leaves and green tea infusions by high-performance liquid chromatography. Food Research International 2001, 34, 223–227.
- Rusak, G.; Komes, D.; Likic, S.; Horzic, D.; Kovac, M. Phenolic content and antioxidative capacity of green and white tea extracts depending on extraction conditions and solvent used. Food Chemistry 2008, 110, 852–858.
- Druzynska, B.; Stepniewska, A.; Wolosiak, R. The influence of time and type of solvent on efficiency of the extraction of polyphenols from green tea and antioxidant properties obtained extracts. ACTA Scientiarum Polonorum Technologia Alimentaria 2007, 6 (1), 27–36.
- Friedman, M.; Levin, C.E.; Choi, S.H.; Kozukue, E.; Kozukue, N. HPLC analysis of catechins, theaflavins, and alkaloids in commercial teas and green tea dietary supplements: Comparison of water and 80% ethanol/water extracts. Journal of Food Science 2006, 71 (6), 328–337.
- Nanjo, F.; Goto, K.; Seto, R.; Suzuki, M.; Sakai, M.; Hara, Y. Scavenging effects of tea catechins and their derivatives on l l-diphenyl-2-picrylhydrazyl radical. Free Radical Biology and Medicine 1996, 21 (6), 895–902.