Abstract
The antioxidant efficacy of basil extracts was estimated in stabilization of sunflower oil. The basil essential oil was analyzed by gas chromatography–mass spectrometry. Twenty-two compounds were identified representing 93.74% of the total essential oil. Basil methanolic extract was thermally evaluated by heating at 185°C. At the 100 min heating time, the extract exhibited antioxidant activity higher than that of butylated hydroxytoluene. Different concentrations of methanolic extract were added to sunflower oil. Selected parameters (i.e., weight gain, induction period to primary oil oxidation, peroxide value, conjugated dienes, and conjugated trienes) were considered for evaluating the effectiveness of basil in stabilization of sunflower oil. Basil methanolic extract showed good antioxidant activity according to synthetic antioxidants. Basil may be used as a natural antioxidants to prevent vegetable oils oxidation.
INTRODUCTION
Lipid peroxidation is responsible for the quality deterioration of vegetable oils, fats, and other food systems. Oxidation lowers the nutritive value of vegetable oils and produces off-flavor compounds in food. It also causes aging, heart diseases, stoke, emphysema, mutagenesis, and carcinogenesis.[Citation1] The oil industry has to pay special attention in this context, as oils, fats, and fatty foods suffer stability problems. Oil manufacturers aim at producing foods that maintain their shelf life and nutritional quality over a defined period. Thus, the use of antioxidants to minimize the oxidation of lipids in food materials is extensively practiced. For this purpose, synthetic antioxidants, such as butylated hydroxyanisole (BHA), butylated hydroxytoluene (BHT), ter-butyl hydroquinone (TBHQ) have been used as food additives for over 50 years.[Citation2] In present times, there is increasing consumer awareness and health-consciousness which results in pressure to avoid the use of synthetic additives that may be implicated in many health risks, including cancer and carcinogenesis.[Citation3] In these circumstances, research of safer, natural antioxidant is the goal of many laboratories. Plant extracts provide phenolic antioxidants that might exhibit strong activity. Subjects of investigation have been various spice, herb, and olive mill waste extracts, as well as fruits and vegetables.[Citation4–Citation6]
Among natural antioxidants, basil, Ocimum basilicum L. (Lamiaceae), has been widely accepted as one of the species with a strong antioxidant activity.[Citation7,Citation8] Basil is an annual and perennial herb which grows in several regions around the world.[Citation9] Its essential oil has been used traditionally in food as a flavoring agent, in perfumery, and medical industries in the treatment of headaches, coughs, diarrhea, constipation, warts, worms, and kidney malfunctions.[Citation10]
Ocimum basilicum L. is also reported as a source of aroma compounds, and it possesses a range of biological activities such as insect repellent, nematocidal, antibacterial, antifungal agents, and antioxidant activities.[Citation11,Citation12] It is well known that the high antioxidant capacity of basil extracts in the medicine and food industry is attributed to the presence of a substantial concentration of rosmarinic acid.[Citation13,Citation14] Other caffeic acid derivatives, such as chicoric acid, were also found in high concentrations.[Citation15]
The effectiveness of different natural sources in stabilizing vegetable oils has been previously studied. In this way, garlic and pomegranate peel extracts were employed for the stabilization of sunflower oil (SFO).[Citation16,Citation17] In a previous study, polyphenolic extract from olive mill waste water was used for the stabilization of refined oils.[Citation18] To the best of the authors knowledge, only Juntachote et al.[Citation19] tested the effectiveness of both Holy basil powder and its ethanolic extracts in preventing/minimizing lipid oxidation in cooked ground pork. No previous studies have been presented so far on basil describing its efficiency for the stabilization of food systems, especially SFO. The purpose of this work was to estimate the efficiency of basil extracts against oil oxidative deterioration. Refined, bleached and deodorized (RBD) SFO was chosen to evaluate the antioxidant efficiency of basil extracts, because of its wide use among Tunisian population and due to its higher content of polyunsaturated fatty acids. Furthermore, the stabilization effect is more pronounced in SFO.
MATERIALS AND METHODS
Materials
A sample of RBD SFO, without additives, was received from a local commercial refining plant (Agrimed, Sfax). Fresh basil (Ocimum basilicum L.) leaves were harvested from Sfax province in the center of Tunisia in October 2009. All the chemicals and reagents used were of analytical reagent grade and were purchased from Fluka, or Sigma Chemical Co (St. Louis, MO, USA).
Solvent Extraction
Dried basil leaves (500 g) were chopped using a grinder and extracted with organic solvents of increasing polarity: hexane, ethyl acetate, and methanol. Extraction was carried out using maceration overnight at room temperature (25°C). The extracts were filtered and the residue was extracted again with 500 ml of each solvent. The extraction procedure was applied three times to ensure complete extraction of phenolic compounds. The combined extracts related to each solvent were concentrated under vacuum at 40°C. The dry extracts were weighed to calculate the yield and were stored at 4°C prior to further analyses.
Extraction of Essential Oil
The fresh aerial part of basil was completely immersed in water and hydro-distilled for 4 h in a Clevenger-type apparatus giving greenish-yellow oil. When the condensed material cooled down, the water and essential oil were separated. To improve its recovery, the essential oil was taken up in diethyl ether, dried over anhydrous sodium sulphate until the last traces of water were removed, and stored in a dark glass bottle at 4°C until tested and analyzed. The extraction yield was 0.1% (w/w).
DPPH Radical Scavenging Assay
Determination of antioxidant activity of basil extracts and essential oil was accomplished using the 1,1-diphenyl-2-picrylhydrazyl (DPPH radical) scavenging method according to Bloi et al.[Citation20] Methanolic solution (4 ml) of each sample at different concentrations (25, 50, 100, 150 μg/ml) were added to 10 ml DPPH methanolic solution (1.5 × 10−4 M). After mixing the two solutions gently and leaving for 30 min at room temperature, the optical density was measured at 520 nm using a Shimadzu UV-160A spectrophotometer. The antioxidant activity of each sample was expressed in terms of concentration required to inhibit DPPH radical formation by 50% (IC50 μg/ml) and calculated from the log-dose inhibition curve.
Determination of Antioxidant Activity by- β-Carotene Bleaching Method
In this assay the antioxidant capacity of basil extracts and essential oil was determined in emulsion by the β-carotene bleaching method of Farag et al.[Citation21] consisting in a coupled oxidation of linoleic acid and β-carotene. A stock solution of β-carotene/linoleic acid (Sigma–Aldrich) was prepared as follows. β-carotene (0.5 mg) was dissolved in 1 ml of chloroform (HPLC grade), then 25 μl of linoleic acid and 200 mg of Tween 40 (Merck) were added. The chloroform was subsequently evaporated, then distilled and oxygenated water (100 ml) was added with vigorous shaking. Aliquots (2.5 ml) of the stock solution were transferred to test tubes, and 300 ml portions of the extracts (1 g/l in methanol) were added before incubating for 48 h at room temperature. The antioxidant activity was evaluated by absorbance measurement at 470 nm against a blank containing emulsified linoleic acid without β-carotene.
Estimation of Total Phenolics
Total soluble phenolic compounds in the extracts were measured according to the method of Singleton and Rossi[Citation22] and expressed as gallic acid equivalent (GAE). A sample (0.5 ml) of ethyl acetate or methanolic extract (1 mg/ml) was oxidized with 0.5 ml of Folin–Ciocalteu’s phenol reagent. The reaction was neutralized with 0.5 ml of a saturated solution of sodium carbonate (Na2CO3) (75 g/l) and the volume was adjusted to 5 ml with water. The absorbance of the resulting blue color was measured at 725 nm against a blank after incubation for 90 min at room temperature in the dark. Quantification was done on the basis of the standard curve of gallic acid. Results were expressed as milligram of GAE per g of dry weight (DW).
Gas Chromatography–Mass Spectrometry (GC–MS)
The analysis of the essential oil was performed on a GC–MS HP model 5975B inert MSD (Agilent Technologies, J&W Scientific Products, Palo Alto, CA, USA), equipped with an Agilent Technologies capillary DB-5MS column (30 m length; 0.25 mm i.d.; 0.25 mm film thickness), and coupled to a mass selective detector (MSD5975B, ionization voltage 70 eV; all Agilent, Santa Clara, CA, USA). The carrier gas was He and was used at 1 ml/min flow rate. The oven temperature program was as follows: 1 min at 100°C ramped from 100 to 260°C at 4°C min−1 and 10 min at 260°C. The chromatograph was equipped with a split/splitless injector used in the split mode. The split ratio was 1:100. Identification of components was assigned by matching their mass spectra with Wiley and NIST library data, standards of the main components and comparing their Kovats Retention Indices (KRI) with reference libraries and from the literature.[Citation23] The component concentration was obtained using semi-quantification by peak area integration from GC peaks and by applying the correction factors.
Thermal Stability Evaluation of Methanolic Extract
Methanolic extract, exhibiting the powerful antioxidant activity, was used for further studies. Thermal stability of basil methanolic extract (BME) was evaluated by heating at 185°C in an oven for a period of 120 min in separate crucibles. After each interval, a crucible was removed from the oven, cooled to room temperature and used for antioxidant activity determination following the DPPH radical scavenging assay.
Preparation of SFO Samples for Oxidative Stability Determination
The BME was applied to preheated RBD SFO at different concentrations (200, 500, and 1000 ppm, based on extract weight), in a series of dark brown colored bottles having a volume of 100 ml each, to examine its antioxidative activity. Synthetic antioxidant (BHT) was employed at its legal limit of 200 ppm[Citation18] to compare the efficacy of natural antioxidants. The bottles were completely filled with oil and sealed. A control sample (Ctrl) was prepared by using the same amount of methanol used to dissolve the extracts. The antioxidant-enriched oil samples were evaporated in a vacuum evaporator below 40°C to evaporate the solvent and subjected to accelerated oxidation in the dark in an oven at 70°C for 24 days. Immediately after storage period, oil samples were withdrawn after regular intervals of 4 days for triplicate analyses.
Weight Gain Analysis of SFO Samples
For weight gain analyses, 2 g of each SFO sample supplemented with methanolic extract (in triplicate) were placed in glass Petri dishes, which were kept in a vacuum oven overnight at 35°C to remove any traces of moisture. The samples were reweighed and stored in the oven at 70°C. The rate of oxidation, in terms of weight increase, was recorded at 24 h intervals up to 16 days. The time required for a 0.5% weight increase for oil was taken as the index of stability.
Analytical Procedures for SFO Samples
Peroxide value (PV)
The used method is based on iodometric titration, which measures the iodine produced from potassium iodide by peroxides present in the oil. PV determination was performed according to the method proposed by IUPAC.[Citation24]
Conjugated dienes (CD) and conjugated trienes (CT)
Specific extinctions at 232 nm and 270 nm (i.e., CD and CT) were determined using a spectrophotometer as previously described by Fki et al.[Citation18]
Evaluation of SFO oxidation by the Rancimat
Induction time to primary oxidation of lipid in SFO supplemented with methanolic extract was measured using a Rancimat apparatus (Metrohm, Herisau, Switzerland). A flow of air (20 l/h) was bubbled through 5.0 g of oil heated to 100°C. The volatile oxidation products were stripped from the oil and dissolved in cold water, increasing its conductivity. Induction period (IP), the time elapsed from the beginning until the oil starts to become rancid, was measured by drawing tangents on both sides of the induction curve, the intercept of which meet the time axis.
Statistical Analysis
All analyses were performed in triplicate and data were reported as means ± standard deviation (SD). Differences between experiments were analyzed using Student’s t-test in Microsoft Excel 2000 (Microsoft Corporation, USA). The confidence limits used in this study were based on 95% (P < 0.05).
RESULTS AND DISCUSSION
Extraction
The chemical composition of Ocimum basilicum L. essential oil is presented in . The average yield in essential oil was 0.1% (w/w). GC–MS analysis resulted in the identification of 22 compounds representing 93.74% of the total essential oil which contains 0.26% monoterpene hydrocarbons, 74.64% oxygenated monoterpenes, 12.85% sesquiterpene hydrocarbons, and 6% oxygenated sesquiterpenes. Furthermore, the most abundant components (>4%) of the Ocimum basilicum L. essential oil were linalool (18%), carvone (5.08%), carvacrol (17.81%), and eugenol (24.69%). The identified compounds are known and were reported in previous studies.[7,Citation10] However, the composition of the basil essential oil is different from other Mediterranean basil oils. This could be related to basil variety, climatic conditions, and period of plant collection.
Table 1 Chemical composition of Ocimum basilicum L. essential oil
shows the percentage yield and antioxidant activity of basil extracts in different solvents as well as its essential oil. The range of extraction yields was 0.96–29.57%. The highest yield was obtained with methanol. It has been established that the extraction yield increases with increasing polarity of the extractant solvent.[Citation6] On the other hand, change in solvent polarity alters its efficacy to extract a specific group of antioxidant compounds and influences the antioxidant properties of the extracts.[Citation25] Methanol is the widely used and the effective solvent for antioxidants extraction. Assessment of antioxidant activity using DPPH radical scavenging assay and β-carotene bleaching method, is summarized in . Results show that ethyl acetate and methanolic extracts exhibited strong antiradical and antioxidant activities. Essential oil and hexane extract possessed medium activity. Methanolic extract with IC50 value of 16.43 μg/ml exhibited the highest radical scavenging activity compared with BHT (IC50 = 25.62 mg/ml). Therefore, this extract was used in the assay of SFO stabilization.
Table 2 Percentage yield and antioxidant activity of basil extracts
Thermal Stability of BME
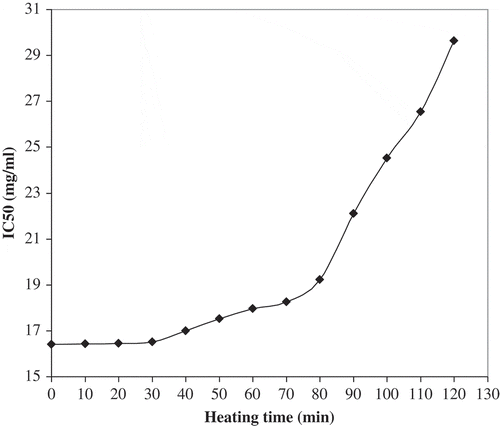
The heating effect on BME (at 185°C) for different intervals is shown in . Thermally treated extract was subjected to antioxidant activity evaluation using DPPH radical scavenging method. Up to 30 min heating time, the extract was almost stable. However, after 40 min a slight gradual decrease in its antioxidant activity (increase in IC50 values) was observed with the increase in heating period. The decrease in antioxidant activity was not significant up to 70 min but became pronounced after 80 min heating time. At the 100th min of heating time, the extract exhibited antioxidant activity (IC50 = 24.53 mg/ml) higher than that of BHT (IC50 = 25.62 mg/ml). After 100 min, the extract lost considerably its antioxidant activity which becomes weaker than that of BHT. This decrease in antioxidant activity, after longer heating times at high temperatures, may be due to various chemical reactions occurring during oxidation, leading to the formation of hydroperoxides, hydrolysis, polymerization, and chemical decomposition. These oxidation reactions induce fats and oils deterioration giving rancidity.[Citation19] These results indicate that basil is a potential source of natural antioxidants which can be used in food systems even at high processing temperatures.
Determination of IP of SFOs with Rancimat
IP provides direct evidence for trends in resistance to oxidative rancidity of vegetable oils. IPs were determined in all cases at 100°C. The SFO oxidative resistance, measured by Rancimat, was greatly improved in the presence of BMEs (). Indeed, the IP of SFO increased significantly from 1.98 (control) to 3.26, 8.23, and 11.63 h by enrichment with 200, 500, and 1000 ppm of BME, respectively (). BME at 500 ppm was significantly more effective (8.23 h) than BHT at 200 ppm (4.65 h). IP (8.23 h) of basil stabilized SFO sample, SFO-500, was higher than that of SFO stabilized with 500 ppm of garlic methanolic extract (2.79 h)[Citation16] or with 500 ppm of pomegranate peel methanolic extract (4.07 h).[Citation17] The total phenolic content of BME was 48.26 mg gallic acid/g of dried herb quantified by Folin–Ciocalteu’s method. The highest stability of SFO supplemented with BME can be explained by its richness in components with powerful antioxidant activity especially polyphenols. In fact, it was reported that antioxidant and antimicrobial activities of basil are due to its phenolic and aromatic compounds.[Citation8,Citation11,Citation14] The main phenolics reported in basil are phenolic acids and flavonol-glycosides.[Citation7,Citation8] Caftaric acid, cinnamylmalic acid, feruloyltartaric acid, caffeic acid, quercetin-rutinoside, cinnamic acid, chicoric acid, and rosmarinic acid were identified in the basil leaves extract.[Citation26] The concentrations of these phenolic compounds are known to have directly impact on the antioxidant properties of Ocimum basilicum.[Citation13,Citation14]
Table 3 Antioxidant effects on sunflower oil measured by Rancimat under 100°C
Weight Gain (WG)
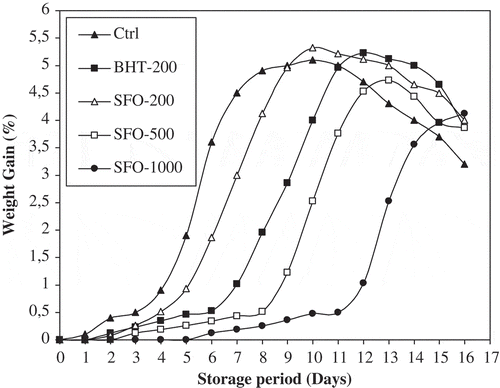
WG technique is very useful for comparing the effect of antioxidants on the oxidative stability of vegetable oils. WG is generally employed for quantitative assessment of the amount of oxygen added to the unsaturated content of lipid molecules and formation of hydroperoxides during oxidation. This amount of oxygen is used as a good parameter for the determination of IP besides extent of oxidation and the evaluation of antioxidants effect on oil stability. Weight remains practically constant in oil samples experiencing little or no oxidation. WG was measured for all the stabilized and control samples after 24 h intervals up to 16 days and results were calculated in percentage (). Initially, WG was not appreciable but it increased very sharply for all the samples reaching a maximum value followed by a sharp decrease during the last days of storage. A significant increase in IP of all the stabilized samples was observed compared to the control. Indeed, the time taken to achieve 0.5% increase in weight was 3, 3.9, 5.7, 7.88, and 11 days for Ctrl, SFO-200, SFO-BHT, SFO-500, and SFO-1000, respectively (). The synthetic antioxidant, BHT has induction time between SFO-200 and SFO-500. It can be deduced that BME at 300–400 ppm has the same effect as BHT at 200 ppm in SFO stabilization. These findings are more interesting than that dealing with sunflower stabilization using garlic[Citation16] or pomegranate peel[Citation17] extracts. Indeed, it was established that garlic or pomegranate peel extracts at 700–800 ppm have the same effect as BHT at 200 ppm in SFO stabilization.
PV
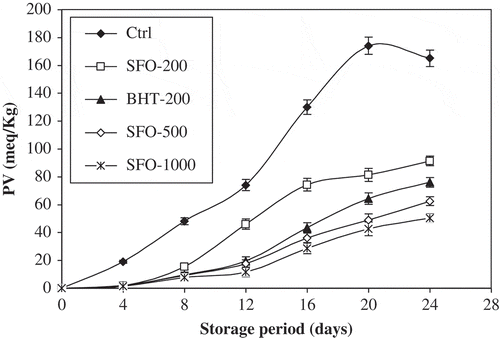
PV represents the concentration of peroxides and hydroperoxides formed in the initial stages of lipid oxidation. PV is one of the most widely-used tests for the evaluation of oxidative rancidity in oils and fats. A continuous increase in PV with the increase in storage period was observed for all the samples (). Initially, the PV was very low for all the samples. It started to increase after three days of storage and went on increasing further with the increase in storage period. The range of PV was 50.23–91.25 meq/kg for the stabilized samples after storage up to 24 days, while the maximum value of PV for control sample was 174 meq/kg (). At all stages, highest PV was observed for control sample followed by SFO-200, SFO-BHT, SFO-500, and SFO-1000, respectively. A significant difference (P < 0.05) in PV was observed between the control and SFO samples containing BME, which slowed the rate of peroxides formation revealing good antioxidant efficacy in oil stabilization. After the fourth day, there was a remarkable increase in PV of control sample which reached its maximum on day 20 of analysis, followed by a sharp decrease on day 24. This may be explained by the findings of Shahidi and co-workers, who suggested that a decrease in PV after long heating times may be due to the volatilization of some breakdown products of lipid hydroperoxides, formed in the primary stages of oxidation.[Citation27] The PV of SFO-500 was comparable to that of BHT at the beginning of storage periods; but it decreased after 12 days, suggesting greater stability of basil extract at 500 ppm than BHT at 200 ppm. This result is in good concordance with that of the previous weight gain analysis. Maximum PV contents for SFO-1000 and SFO-500 were 53.65 and 71.43 meq/kg, respectively, which are far less than those of SFO stabilized with garlic[Citation16] or pomegranate peel extracts.[Citation17] These data suggest the superiority of the BME over synthetic antioxidants, because of their long term effectiveness and stability. All antioxidants remain effective over a specific period of time, and with the passage of time their effectiveness decreases and they finally become ineffective.[Citation28] Such antioxidants inhibit oils and fats deterioration in the early stages and thus, delay the onset of the oxidation reaction. Antioxidants were found to be efficient only up to a specific period.[Citation29]
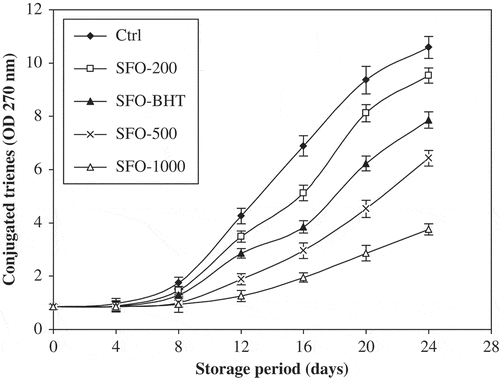
CD and Trienes
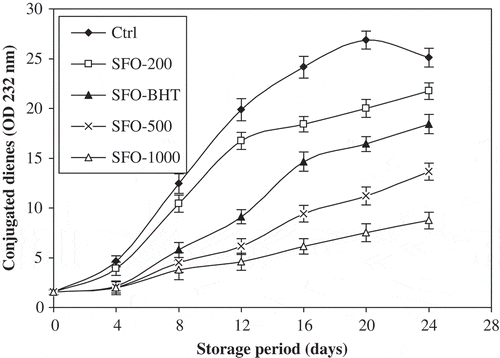
The previous PV estimation gives a clear indication of lipid autoxidation. For further confirmation of these results, other oxidation parameters, i.e., CD and CT values were also measured. The assessment of CD and CT is a good parameter for the measurement of oxidative deterioration of oils, hence indicates the effectiveness of antioxidants in oils.[Citation18] and show the relative increase in CD and CT contents, respectively, of control and stabilized SFO samples as a function of storage time. Initially, the formation rate of CD was higher and went on decreasing with the increase in storage time, whilst the reverse behavior was observed for CT content, i.e., initially, the formation rate was lower and went on increasing with the storage time. The formation of high contents of CD may be related to the presence of higher contents of polyunsaturated fatty acids in SFO.[Citation30] CD and trienes may be produced by dehydration of conjugated diene hydroperoxides.[Citation16] Highest contents were observed for control, indicating greater intensity of oxidation, followed by SFO-200, SFO-BHT, SFO-500, and SFO-1000, respectively. This result is in full agreement with that obtained with the PV estimation. The increase in CD and CT contents is proportional to the uptake of oxygen.
CONCLUSION
From the present investigation, it can be concluded that BME can stabilize SFO very effectively. It inhibits thermal deterioration of oil by improving its hydrolytic stability and inhibiting double bond conjugation. Basil extract at concentration of 200–500 ppm has stabilization efficiency comparable to conventional synthetic antioxidants, i.e., BHT at its legal limit. Therefore, on behalf of this study, BME may act as an alternative to synthetic antioxidants for use in the food industry, especially in the stabilization of vegetable oils. The isolation and identification of the basil active compounds are now in progress.
ACKNOWLEDGMENT
This research was supported by the Ministry of High Education and Scientific Research, Tunisia.
REFERENCES
- Michalczyk, M.; Macura, R. Effect of processing and storage on the antioxidant activity of frozen and pasteurized shadblow serviceberry (amelanchier canadensis). International Journal of Food Properties 2010, 13, 1225–1233.
- Singla, R.; Ganguli, A.; Ghosh, M. Antioxidant activities and polyphenolic properties of raw and osmotically dehydrated dried mushroom (agaricus bisporous) snack food. International Journal of Food Properties 2010, 13, 1290–1299.
- Hou, D.X. Potential mechanism of cancer chemoprevention by anthocyanin. Current Molecular Medicine 2003, 3, 149–159.
- Hamden, K.; Allouche, N.; Damak, M.; Elfeki, A. Hypoglycemic and antioxidant effects of phenolic extracts and purified hydroxytyrosol from olive mill waste in vitro and in rats. Chemico-Biological Interactions 2009, 180, 421–432.
- Herken, E.N.; Guzel, S. Total antioxidant capacity and total phenol contents of selected commercial fruit juices in turkey. International Journal of Food Properties 2010, 13, 1373–1379.
- Allouche, N.; Fki, I.; Sayadi, S. Toward a high yield recovery of antioxidants and purified hydroxytyrosol from olive mill wastewaters. Journal of Agricultural and Food Chemistry 2004, 52, 267–273.
- Lee, S.J.; Umano. K.; Shibamoto, T.; Lee, K.G. Identification of volatile components in basil (Ocimum basilicum L.) and thyme leaves (Thymus vulgaris L.) and their antioxidant properties. Food Chemistry 2005, 91, 131–137.
- Javanmardi, J.; Khalighi, A.; Kashi, A.; Bais, H.P.; Vivanco, J.M. Chemical characterization of basil (Ocimum basilicum L.) found in local accessions and used in traditional medicines in Iran. Journal of Agricultural and Food Chemistry 2002, 50, 5878–5883.
- Darrah, H.H. The Cultivated Basil, Buckeye Printing: Independence, MO, 1988.
- Telci, I.; Bayram, E.; Yilmaz, G.; Avci, B. Variability in essential oil composition of Turkish basils (Ocimum basilicum L.). Biochemical Systematics and Ecology 2006, 34, 489–497.
- Wannissorn, B.; Jarikasem, S.; Siriwangchai, T.; Thubthimthed, S. Antibacterial properties of essential oils from Thai medicinal plants. Fitoterapia 2005, 76, 233–236.
- Deshpande, R.S.; Tipnis, H.P. Insecticidal activity of Ocimum basilicum L. Pesticides 1977, 11, 11–12.
- Petersen, M.; Simmonds, M.S.J. Rosmarinic acid. Phytochemistry 2003, 62, 121–125.
- Javanmardi, J.; Khalighi, A.; Kashi, A.; Bais, H.P.; Vivanco, J.M. Chemical characterization of basil (Ocimum basilicum L.) found in local accessions and used in traditional medicines in Iran. Journal of Agricultural and Food Chemistry 2002, 50, 5878–5883.
- Lee, J.; Scagel, C.F. Chicoric acid levels in commercial basil (Ocimum basilicum) and Echinacea purpurea products. Journal of Functional Foods 2010, 2, 77–84.
- Iqbal, S.; Bhanger, M.I. Stabilization of sunflower oil by garlic extract during accelerated storage. Food Chemistry 2007, 100, 246–254.
- Iqbal, S.; Haleem, S.; Akhtar, M.; Zia-ul-Haq, M.; Akbar, J. Efficiency of pomegranate peel extracts in stabilization of sunflower oil under accelerated conditions. Food Research International 2008, 41, 194–200.
- Fki, I.; Allouche, N.; Sayadi, S. The use of polyphenolic extract, purified hydroxytyrosol and 3,4-dihydroxyphenyl acetic acid from olive mill wastewater for the stabilization of refined oils: A potential alternative to synthetic antioxidants. Food Chemistry 2005, 93, 197–204.
- Juntachote, T.; Berghofer, E.; Siebenhandl, S.; Bauer F. Antioxidative effect of added dried Holy basil and its ethanolic extracts on susceptibility of cooked ground pork to lipid oxidation. Food Chemistry 2007, 100, 129–135.
- Blois, M.S. Antioxidant determinations by the use of a stable free radical. Nature 1958, 181, 1199–1200.
- Farag, R.S.; Badei, A.Z.M.A.; Hewedi, F.M.; El-Baroty, G.S.A. Antioxidant activity of some spice essential oils on linoleic acid oxidation in aqueous media. Journal of the American Oil Chemists Society 1989, 66 (6), 792–799.
- Singleton, V.L.; Rossi, J.A. Jr. Colorimetry of total phenolics with phosphomolybdic–phosphotungstic acid reagents. American Journal of Enology and Viticulture 1965, 16, 144–158.
- Adams, R.P. Quadrupole Mass Spectra of Compounds Listed in Order of Their Retention Time on DB-5. Identification of Essential Oils Components by Gas Chromatography/Quadrupole Mass Spectroscopy, Allured Publishing Corporation: Carol Stream, IL, USA, 2001, pp.456.
- IUPAC. Standard Methods for the Analysis of Oils, Fats, and Derivatives, 7th Ed; Blackwell Scientific Publication: Oxford, 1987.
- Zhou, K.; Yu, L. Effects of extraction solvent on wheat bran antioxidant activity estimation. Lebensmittel-Wissenschaft & Technologie 2004, 37, 717–721.
- Lee, J.; Scagel, C.F. Chicoric acid found in basil (Ocimum basilicum L. ) leaves. Food Chemistry 2009, 115, 650–656.
- Shahidi, F.; Janitha, P.K.; Wanasundara, P.D. Phenolic antioxidants. Critical Reviews in Food Science and Nutrition 1992, 32, 67–103.
- Negi, P.S.; Jayaprakasha, G.K.; Jena, B.S. Evaluation of antioxidant and antimutagenic activities of the extracts from the fruit rinds of garcinia cowa. International Journal of Food Properties 2010, 13, 1256–1265.
- Liu, H.; White, P.J. Oxidative stability of soybean oils with altered fatty acid compositions. Journal of the American Oil Chemists Society 1992, 69, 528–532.
- Yanishlieva, N.V.; Marinova, E.; Pokorny, J. Natural antioxidants from herbs and spices. European Journal of Lipid Science and Technology 2006, 108, 776–793.