Abstract
Major polyphenolic compounds in pineapple peels were identified and quantified. The antioxidant capacities of pineapple peel extracts and these polyphenolic compounds were determined using DPPH• scavenging capacity and phosphomolybdenum method. Effects of these polyphenolics’ interactions on their antioxidant capacity were also evaluated. Gallic acid (31.76 mg/100 g dry extracts), catechin (58.51 mg/100 g), epicatechin (50.00 mg/100 g), and ferulic acid (19.50 mg/100 g) were found to be the main polyphenolics in pineapple peels. The IC50 for DPPH• scavenging assay of the extracts was 1.13 mg/ml and total antioxidant capacity was 0.037 g ascorbic acid equivalents/g. The order of DPPH• scavenging capacity of per mole of these polyphenolic compounds present in pineapple peels was gallic acid > epicatechin = catechin > ferulic acid, but it was different when using phosphomolybdenum method the order of which was epicatechin. > catechin > gallic acid = ferulic acid. Results of polyphenolics’ interactions indicated no synergistic effects. In the combinations of ferulic acid-epicatechin and ferulic acid-gallic acid, additive effects were found using both antioxidant activity assays.
INTRODUCTION
Polyphenolic compounds are secondary plant metabolites that exist ubiquitously in the plant kingdom where they have a wide range of different structures[Citation1] and physiological properties, such as anti-allergenic, anti-artherogenic, anti-inflammatory, anti-microbial, antioxidant, anti-thrombotic, cardioprotective, and vasodilatory effects.[Citation2−Citation6] The interest in polyphenolic antioxidants has increased remarkably in the last decade because of their capacity in scavenging free radicals associated with various diseases. Phenolic acids, flavonoids, and tannins are regarded as the main dietary phenolic compounds. Of these, phenolic acids consist of two subgroups, i.e., the hydroxybenzoic and hydroxycinnamic acids.[Citation7] Flavonoids constitute the largest group of plant phenolics, accounting for over half of the 8000 naturally occurring polyphenolic compounds.[Citation8] Variations in substitution patterns to ring C result in the major flavonoid classes, i.e., flavonols, flavones, flavanones, flavanols (or catechins), isoflavones, flavanonols, and anthocyanidins.[Citation9] Lately, researches on antioxidant capacity of polyphenols from different sources have emerged in large numbers measured by different assays, including DPPH• scavenging capacity, phosphomolybdenum method, β-carotene-linoleate model system, ABTS radical-scavenging ability, superoxide anion radical scavenging activity, etc.[Citation10−Citation13]
Pineapples are one of the leading commercial fruits of the tropics around the world and the major producing areas are Southeast Asia and Latin America.[Citation14] The canning industry is producing large quantities of solid and liquid wastes, which leads to a serious environmental problem for disposal.[Citation15] As waste products of the pineapple cannery, pineapple peel waste could be a potential source for the extraction of beneficial bioactive compounds. One alternative use for value addition of pineapple peels is the isolation of bromelain.[Citation16] So far, there have been reports of nutrient analyses of pineapples, including fiber, protein, carotenoid, mineral composition, total polyphenol content, etc.[Citation17,Citation18] However, only a few researches have concentrated on the antioxidant capacity of pineapples. de Oliveira et al.[Citation13] has studied the total phenolic content and antioxidant activities of methanolic extracts of pineapple (Ananas comosus) residues (including pulp, seeds, and peels from a local juice factory) using DPPH• and superoxide anion scavenging activity. The research of total polyphenols of pineapple (Calendar, India) extracted with different solvents was reported by Hossain et al.[Citation10] and the results showed that the polyphenolic contents of the extracts were found to be highest in methanol corresponding to the highest antioxidant properties. However, to the best of the authors knowledge, there has been no report regarding the types of polyphenolic compounds in pineapple peels and their antioxidant interactions are also not known, both of which are need to be further explored. Therefore, in the present work, pineapple peels were employed as materials and the major polyphenolic compounds and their antioxidant interactions have been studied. The composition and contents of polyphenols in pineapple peels were determined by high performance liquid chromatography-mass spectroscopy (HPLC-MS). The antioxidant capacities of pineapple peel extracts (PPE) and these major polyphenolics were measured and compared using DPPH• scavenging capacity and phosphomolybdenum method. Furthermore, the effects of the polyphenol-polyphenol interactions on their antioxidant capacity were evaluated.
MATERIALS AND METHODS
Materials
For this experiment we used the Bali variety which is one of the most popular pineapple cultivars grown in Hainan, China. Bali pineapples were collected from Wanning, Hainan province of China from January to March, 2011 and purchased from a local market in Nanchang, Jiangxi, China. The peel of pineapples is deep yellow when ripe.
DPPH• (2,2’-diphenyl-2-picrylhydrazyl radical) was purchased from Solarbio (Beijing, China). Standards of (+) –catechin (≥ 98%, 20 mg), (–) –epicatechin (≥ 98%, 20 mg), gallic acid (99.1%, 100 mg), ferulic acid (99.5%, 50 mg) and ascorbic acid (100%, 100 mg) were obtained from National Institutes for Food and Drug Control (Beijing, China). Methanol and acetonitrile were of chromatographic grade and the other reagents were of analytical grade.
Extraction of Polyphenols from Pineapple Peels
Sample preparation and extraction were processed according to the method described by de Oliveira et al.[Citation13] The peels of pineapple were removed and rinsed with water before being processed. The pineapple peels were then dried in a ventilated oven at 60°C for 48 h and ground to a fine powder. Extraction of 25 g of pineapple peels were carried out by reflux, first with 150 ml of n-hexane to remove non-polar compounds and then with 150 ml methanol for 4 h at 60°C. Supernatants were decanted and filtered through Whatman filter paper followed by concentration and drying in a vacuum rotary evaporator (RE 2000, Rongya, Shanghai) at 50 ± 10°C, and the extracts were kept under nitrogen in a refrigerator for 1 h.
Determination of Total Polyphenolic Contents (TPC)
Total polyphenolic content of PPE was determined according to the method of de Oliveira et al.[Citation13] with minor modifications. 0.25 mg extracts were dissolved in 0.5 ml milliQ purified water and 0.50 ml aliquots or deionized water (control) were mixed by manual shaking, for 10–15 s, with 2.50 ml of Folin-Ciocalteau reagent. After 5–8 min, 7.50 ml of saturated sodium carbonate solution was added and solution diluted to 50 ml with deionized water. The reaction mixture was kept in dark for 2 h and its absorbance was measured at 760 nm against water in a UV-Vis spectrophotometer (T6, Puxi, China). The concentration of the total polyphenols was determined as mg of gallic aid equivalents by using an equation obtained from gallic acid calibration curve. The TPC was calculated as mg of gallic acid equivalents/100 g fresh pineapple peels and mg of gallic acid equivalents/g dry pineapple peels powders.
Identification and Quantification of Major Polyphenolic Compounds
HPLC-MS analysis of PPE were performed on an Agilent 1200 series HPLC system (Agilent Technologies, USA), equipped with an autosampler, binary pump, degasser, and a UV-Vis diode array detection (DAD) connected directly to the mass detector (Agilent G6430A mass hunter) with an Electrospray ionization (ESI) source. HPLC analysis was performed according to the method described by Kammerer et al.[Citation19] PPE (20 μL) was injected into an Elipse XDB C-18 column (5 μm, 250 × 4.6 mm, i.d., Agilent Technologies, USA). The HPLC conditions for the polyphenols were as follows:
System 1: The mobile phase consisted of 2% (v/v) acetic acid in water (eluent A) and 0.5% acetic acid in water and acetonitrile (50:50, v/v; eluent B) using a gradient program as follows: from 10–24% B (20 min), from 24–30% B (20 min), from 30–55% B (20 min), from 55–100% B (15 min), 100% B isocratic (8 min), from 100–10% B (2 min). Simultaneous monitoring was performed at 280 nm at a flow rate of 1.0 mL/min.
System 2: The mobile phase consisted of the same eluents as described for system 1 using a gradient program as follows: from 10–15% B (10 min), 15% B isocratic (3 min), from 15–25% B (7 min), from 25–55% B (30 min), from 55–100% B (1 min), 100% B isocratic (5 min), from 100–10% B (0.1 min). Simultaneous monitoring was performed at 320 nm at a flow rate of 1.0 mL/min.
Mass spectra were obtained by electrospray-ionization in negative mode. The ESI parameters were drying gas (N2) flow and temperature, 10 L/min and 350°C, respectively; nebulizer pressure, 30 psi, and capillary voltage, 4000 V. Scan range of the mass spectrometry was m/z 50–1500. The contents of polyphenolic compounds were calculated from their areas with regression equations from standard curves obtained from HPLC analysis, and are expressed as mg per 100 g dry peel powders.
Antioxidant Capacity
DPPH• scavenging capacity
The DPPH• scavenging capacities of PPE and the pure polyphenolic compounds were measured according to the method of Hossain et al.,[Citation10] with some modifications. An aliquot of 0.1 ml of PPE (0.25–1.50 mg/mL) and pure polyephenolic compounds (25–150 μM) at six different concentrations was mixed with 3.9 ml of 100 μM DPPH• solution. The reaction mixture was incubated for 30 min in the darkness at room temperature. The absorbance of resulting solution was measured at 517 nm with a spectrophotometer (T6, Puxi, China). The control was prepared as above without any extracts and methanol was used for the baseline correction. The radical scavenging capacity of the test samples was measured as a decrease in the absorbance of DPPH• and was calculated by using the following equation:
All samples were run in triplicate. The results were expressed as IC50, and obtained by plotting the remaining percentage of DPPH• against the sample concentration to obtain the amount of antioxidant necessary to decrease the initial DPPH• concentration by 50%. The lower value of IC50 indicates higher antioxidant activity.
Evaluation of Total Antioxidant Capacity (TAC) by Phosphomolybdenum Method
The total antioxidant capacities of PPE and the pure polyphenolic compounds were evaluated by the method of Prieto et al.[Citation20] 0.1 ml of each sample solution (1 mg/ml) and ascorbic acid were combined with 1 ml of reagent (0.6 M sulphuric acid, 28 mM sodium phosphate and 4 mM ammonium molybdate). The tubes were capped and incubated in a boiling water bath at 95°C for 90 min. After the samples had cooled to room temperature, the absorbance of aqueous solution each was measured at 695 nm against blank using a UV-Vis spectrophotometer (T6, Puxi, China). A typical blank solution contained 1 ml of reagent solution and the appropriate volume of the same solvent used for the sample and it was incubated under the same conditions. The experiment was performed in triplicates. The antioxidant activity is expressed as equivalents of ascorbic acid (g/g of extract). The higher value of antioxidant activity indicates higher total antioxidant activity.
Antioxidant Interactions of Polyphenolics
In order to evaluate the possible interactions among the polyphenolic compounds of pineapple peels, combinations of two and all of them together were measured using DPPH• scavenging capacity and phosphomolybdenum method. To determine the synergistic, antagonistic, or additive effect for DPPH• scavenging capacity and TAC, the following equations described by Hidalgo et al.[Citation21] were used:
Statistical Analysis
The results were reported as means ± standard deviation (SD) of at least three measurements. One way analysis of variance (ANOVA) was used to compare the means, and the least significant difference (LSD) test showed the values statistically different. Differences were considered significant at P < 0.05. All statistical analyses were performed with SPSS 17.0.
RESULTS AND DISCUSSION
Extraction and Composition of Polyphenolic Compounds from Pineapple Peels
Polyphenols extracted with different solvents showed different yields. Hossain et al.[Citation10] had studied the effect of solvents on the polyphenols extraction in pineapple (Calendar, India) and the results indicated that the yield was found to be highest in methanol (21.50%) followed by ethyl acetate (4.90%) and water extract (4.30%). Furthermore, the extraction of phenolic compounds from the fruits was commonly achieved with methanol or aqueous methanol.[Citation23] Therefore, methanol was chosen for polyphenol extraction in this research and the yield of pineapple (Bali, China) peels extracts was 24.95% which was a little higher than that reported by Hossain et al.,[Citation10] but was lower than 30.2% reported by de Oliveira et al.[Citation13] However, the phenolic content of 31.98 mg gallic acid equivalents (GAE)/g extracts in this study was less than 51.1 mg caffeic acid equivalents/g pineapple extracts reported by Hossain et al.[Citation10] but much higher than 9.1 mg GAE/g dry extracts reported by de Oliveira et al.[Citation13] The TPC of pineapples (Calendar, India) and pineapple (Ananas comosus) residues were 10.99 mg caffeic acid equivalents/g fresh weight (FW) and 2.75 mg GAE/g dry weight (DW), respectively.[Citation10,Citation13] In this study, it was found that the TPC of pineapple peels was 7.98 mg GAE/g DW (148.91 mg GAE/100 g of FW).
Polyphenols content in pineapple (Bali, China) peels was lower than some fruits which have been studied for their high content of polyphenols, including grapes, apples, and teas. Researches indicated that TPC were 201.0 and 296.3 mg GAE/g FW of red grape and apple, respectively.[Citation24] For black tea, its TPC ranged from 62 to 107 mg GAE/g DW.[Citation25] However, TPC of pineapple peels is higher than that of banana (Musa Paradasiaca) (72.2 mg GAE/100 g FW),[Citation26] and it was close to that of starfruit (acidic) (142.9 mg GAE/100 g FW).[Citation27]
Table 1 Identification of polyphenolic compounds in pineapple peels by HPLC–ESIMS
In order to give a full picture of the qualification and quantification of polyphenolic constituents in pineapple peels, the polyphenolic acids and flavonoids in the extracts were determined by HPLC-MS () and the results are presented in . Four peaks in the chromatograms were identified as gallic acid, catechin, epicatechin and ferulic acid by comparing their retention times and pseudomolecular ion [M-H]− with authentic standards. In details, gallic acid was identified with ESI-MS pseudomolecular and fragment ions at m/z 169.0 [M-H]− and 125.0 [M-COOH]−, respectively. Catechin and epicatechin, with the same pseudomolecular ions m/z (289.1 [M-H]−), and fragment ions m/z 245 (a loss of a –CH2CHOH-), were identified by comparing with the authentic standards for their retention times. Ferulic acid was identified with 192.9 [M-H]− and 133.8 [M-H-COO-CH3-]−.
Figure 1 HPLC chromatograph of pineapple peel extracts detected at (A) 280 nm and (B) 320 nm. Peak numbers refer to .
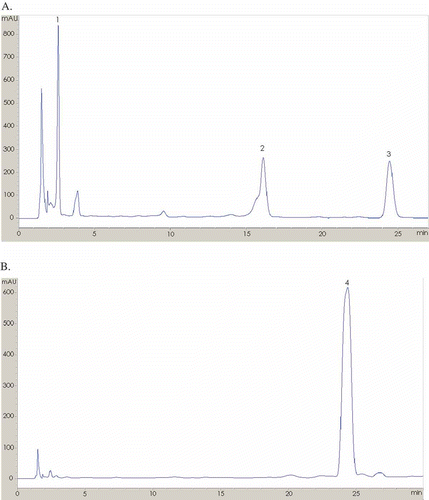
To conduct the quantification for each phenolics, a calibration was constructed by measuring six different concentrations of the standard solution. The content of each phenolic compound could be found in which shows that catechin and epicatechin were the two major monomeric polyphenolics in pineapple peels, followed by gallic acid and ferulic acid.
It has been reported that some fruits and teas are particularly rich in flavanol monomers (catechins).[Citation28] For examples, research on composition and content of grape (Narince) showed that major polyphenolic compounds were gallic acid, (+)-catechin, (−)-epicatechin, and rutin.[Citation29] The main polyphenolic compounds in apples are chlorogenic acid, (+)-catechin, and (−)-epicatechin.[Citation30] These researches as well as the authors results indicate that catechin and epicatechin exist widely in high amount in some fruits with high antioxidant capacity.
Antioxidant Capacity of PPE
The antioxidant capacity of PPE was measured by DPPH• scavenging capacity and phosphomolybdenum method. Our results found that the IC50 value of PPE was 1.13 mg/ml (). Hossain et al.[Citation10] has reported that 0.25 mg/ml methanolic extracts of pineapple (Calendar, India) could inhibit nearly 90% of DPPH•, while we found that only 19.75% of DPPH• was scavenged by 0.25 mg/ml of PPE. Moreover, it has also been reported 0.1 mg/ml pineapple (Ananas comosus) residues extracts quenched approximately 17% of DPPH•.[Citation13] The main reasons accounting for this could be differences of the phenolic contents in the extracts and pineapples’ locations, prevailing climatic conditions, as well as postharvest handling.
Table 2 Antioxidant capacity of PPE and the major polyphenolic compounds
There are wide variations between the antioxidant capacities of different fruits or vegetables. In the present article, the IC50 value of pineapple (Bali, China) peels was 1.13 mg/mL, which was higher than that of red grape pomace (Nerod Avola) (0.039 mg/ml),[Citation31] but lower than that of apple pomace (Reinders) extract (6.33 mg/ml).[Citation32]
The TAC of PPE was measured spectrophotometrically through phosphomoly-bdenum method, which is based on the reduction of Mo (VI) to Mo (V) by the antioxidant agents.[Citation33] One gram of PPE exhibited TAC equivalent to 0.037 g ascorbic acid () which was much lower than 0.34 g ascorbic acid/g pineapple extracts (Calendar, India) reported by Hossainet al.,[Citation10] but close to 0.042 g ascorbic acid/g grape (Vitis vinifera) seed extract.[Citation34]
Antioxidant Capacity of Pure Polyphenolic Compounds
DPPH• scavenging capacity and phosphomolybdenum method were also used to evaluate the antioxidant of the four polyphenolic compounds found in pineapple peels. DPPH• scavenging capacities of the two main polyphenolics in pineapple peels — catechin and epicatechin, were nearly the same (). Catechin and epicatechin are two flavonoid stereoisomers, so they may share the same level of DPPH• scavenging capacity.
Gallic acid was found to be the most active DPPH• radical scavenger while ferulic acid was the least active scavenger (). DPPH• has a single electron and the method has been widely used to evaluate the hydrogen-donating ability of compounds by accepting an electron or hydrogen radical.[Citation35] Gallic acid is one of the hydroxybenzoic acids with the C6–C1 structure, while ferulic acid belongs to hydroxycinnamic acids with a three-carbon side chain (C6–C3).[Citation36] The antioxidant activity of polyphenolic acids increases with increasing degree of hydroxylation, as in the case of the trihydroxylated gallic acid,[Citation7] which exhibited higher antioxidant capacity than ferulic acid with only one hydroxyl group.
The order of DPPH• scavenging capacities of the four polyphenolic compounds present in pineapple peels was in the order gallic acid > epicatechin = catechin > ferulic acid (). Yilmaz and Toledo[Citation37] have studied the proton-donating activity of catechin, epicatechin, and gallic acid by the galvinoxyl method. The result indicated 2.9, 3.1, and 3.2 protons were available for antioxidative donation per mole of catechin, epicatechin, and gallic acid, respectively. In this research, the order of DPPH• scavenging capacity of the four compounds is in general agreement with that expressed in terms of protons available for antioxidative donation, which could be found structurally.
However, when assayed by phosphomolybdenum method, the order of TAC of these polyphenolic compounds was epicatechin > catechin > gallic acid = ferulic acid, which differed from that of the DPPH• scavenging capacity. showed that flavanols (catechin and epicatechin) exhibited higher TAC than phenolic acids (gallic acid and ferulic acid) and among these, epicatechin exhibited the highest antioxidant capacity (1.930 mol ascorbic acid equivalents/mol). It is important to realize that the TAC of the four pure compounds determined by phosphomolybdenum method may be different from a proton donation as determined by DPPH• scavenging assay and a further study is required to better understand the mechanisms involved in these differences.
Effect of Interactions of Four Polyphenolics on Their Antioxidant Capacity
The antioxidant capacities of combinations of two polyphenolic compounds with the molar ratio of 1:1 obtained from DPPH• scavenging capacity and phosphomolybdenum method were compared with their theoretical values. In this way, any statistically significant effects resulting from these combinations could be established whether synergistic, antagonistic or additive. Antioxidant capacities of two phenolics combined in a mixture were reported by Heo et al.,[Citation22] including catechin, chlorogenic acid, and quercetin, etc. Hidalgoet al.[Citation21] has studied the effects of flavonoid-flavonoid interactions on their antioxidant activity by mixing two kinds of flavonoids at different molar ratios. All of the selected phenolics are very common in fruits. Combinations of two phenolics were chosen since they may provide basic information to determine what type of interaction contributes to TAC in the complicated model mixtures of phenolics.
Table 3 Antioxidant activity of polyphenolics combinations measured by DPPH• scavenging assay
The results of DPPH• scavenging capacities are summarized in , and no synergistic effect was revealed for the different polyphenolics combinations. When ferulic acid was mixed with epicatechin or gallic acid, the statistic analysis suggested that there was an additive effect. Antagonistic effects were found in the combinations of catechin-epicatechin, catechin-gallic acid, catechin-ferulic acid, and epicatechin-gallic acid. It has been reported that when catechin combined with epicatechin, there was an antagonistic effect using DPPH• scavenging assay and the difference was –14.1%.[Citation21] The results indicated that this difference was –22.16%.
The reason accounting for these antagonistic effects of DPPH• scavenging capacity may be that a hydrogen-bonding between polyphenolics occurs in these interactions, decreasing the availability of the hydroxyl groups, which may in turn reduce the possibility of interaction with the DPPH•.[Citation21] In this study, it was found that catechin and epicatechin had nearly the same level of DPPH• scavenging capacity. However, when they were paired with the other two phenolic acids respectively, different extents of antagonistic effects were found. To explain the observed variability in inhibition of DPPH• radicals between the two combinations, the steric structure difference between these two isomers could be responsible for the different interactions in these mixtures. In contrast with catechin, epicatechin possesses higher steric hindrance, which may decrease the hydrogen-bonding between polyphenolics. Moreover, the two additive effects were found in the interactions of ferulic acid with epicatechin or gallic acid. So the small number of hydroxyl group of ferulic acid may account for the small quantity of hydrogen-bonding with other polyphenolics.
The results of interactions between the two polyphenolic compounds measured by phosphomolybdenum method also indicated that there was no synergistic effect. However, all the six combinations showed additive effects (), which was different from DPPH• scavenging capacity. Although there was a slight decrease in the combination of catechin-epicatechin when comparing with summation of their antioxidant capacities, no significant difference was found between theoretical and real values. When gallic acid was mixed with ferulic acid, the real value was nearly the same as the theoretical value. It was obvious that additive effects were found in the combinations of ferulic acid-epicatechin and ferulic acid-gallic acid using both antioxidant assays.
Table 4 Theoretical and real values of total antioxidant capacity of polyphenolics combinations measured by phosphomolybdenum method
To further investigate the interactions of two polyphenolics by phosphomolybdenum method, various molar ratios were used according to Hidalgo et al.[Citation21] in the model mixtures. The results () indicated that there was no significant synergistic/antagonistic but an additive effect between the real and theoretical values. Typical example could be found when epicatechin mixed with ferulic acid with the molar ratio of 0.5:1.5 (mol:mol). The real value was only 0.06% higher than its theoretical value. This suggested that TAC was equal to the summation of individual antioxidant capacity of polyphenolics. Finally, the antioxidant capacity of the four polyphenolics mixed at the concentration according to their molar ratio in pineapple peels was determined by phosphomolybdenum method. The results showed the real value was only 6.77% lower than its theoretical value which indicated an additive effect on their antioxidant capacity.
Table 5 Theoretical values (TV) and real values (RV) of total antioxidant activitya of polyphenolics combinations measured by phosphomolybdenum method
CONCLUSIONS
Pineapple (Bali, China) peels were extracted with methanol and the extraction yield was 24.95% with the polyphenols purity of 31.98 mg gallic acid equivalents/g. The major polyphenolic compounds existing in pineapple peels were catechin (58.51 mg/100 g dry extracts), epicatechin (50.00 mg/100 g), gallic acid (31.76 mg/100 g), and ferulic acid (19.50 mg/100 g). These four polyphenolic compounds exhibited their antioxidant capacities with structure-activity relationships. Results of polyphenolics interactions indicated no synergistic effects. However, a more detailed study using other experimental models is required in order to better understand the antioxidant mechanisms involved in these interactions.
REFERENCES
- Kammerer, D.R.; Saleh, Z.S.; Carle, R.; Stanley, R.A. Adsorptive recovery of phenolic compounds from apple juice. European Food Research and Technology 2007, 224, 5–613.
- Benavente-Garcia, O.; Castillo, J.; Marin, F.R.; Ortuno, A.; Del Rio, J.A. Uses and properties of citrus flavonoids. Journal of Agricultural and Food Chemistry 1997, 45, 4505–4515.
- Manach, C.; Mazur, A.; Scalbert, A. Polyphenols and prevention of cardiovascular diseases. Current Opinion in Lipidology 2005, 16, 77–84.
- Middleton, E.; Kandaswami, C.; Theoharides, T.C. The effects of plant flavonoids on mammalian cells: Implications for inflammation, heart disease, and cancer. Pharmacological Reviews 2000, 52, 673–751.
- Puupponen-Pimiä, R.; Nohynek, L.; Meier, C.; Kähkönen, M.; Heinonen, M.; Hopia, A.; Oksman-Caldentey, K.M. Antimicrobial properties of phenolic compounds from berries. Journal of Applied Microbiology 2001, 90, 494–507.
- Samman, S.; Lyons Wall, P.M.; Cook, N.C. Flavonoids and Coronary Heart Disease: Dietary Perspectives, Marcel Dekker Inc. Press: New York, 1998, pp.469–482.
- Balasundram, N.; Sundram, K.; Samman, S. Phenolic compounds in plants and agri-industrial by-products: Antioxidant activity, occurrence, and potential uses. Food Chemistry 2006, 99, 191–203.
- Harborne, J.B.; Baxter, H.; Moss, G.P. 2nd Ed.; Harborne, J.B.; Baxter, H.; Moss, G.P.; Eds.; Taylor & Francis: London, 1999.
- Hollman, P.C.H.; Katan, M.B. Dietary flavonoids: Intake, health effects, and bioavailability. Food and Chemical Toxicology 1999, 37, 937–942.
- Hossain, M.A.; Rahma, S.M.M. Total phenolics, flavonoids, and antioxidant activity of tropical fruit pineapple. Food Research International 2011, 44, 672–676.
- Negi, P.S.; Jayaprakasha, G.K.; Jena, B.S. Evaluation of antioxidant and antimutagenic activities of the extracts from the fruit rinds of Garcinia Cowa. International Journal of Food Properties 2010, 13, 1256–1265.
- Singla, R.; Ganguli, A.; Ghosh, M. Antioxidant activities and polyphenolic properties of raw and osmotically dehydrated dried mushroom (Agaricus Bisporous) snack food. International Journal of Food Properties 2010, 13, 1290–1299.
- de Oliveira, A.C.; Valentim, I.B.; Silva, C.A.; Bechara, E.J.H.; Barros, M.P.; Mano, C.M.; Goulart, M.O.F. Total phenolic content and free radical scavenging activities of methanolic extract powders of tropical fruit peels. Food Chemistry 2009, 115, 469–475.
- Morton, J. Fruits of Warm Climates, Creative Resource Systems Inc. Press: Winterville, USA, 1987, pp.18–28.
- Idris, A.; Suzana, W. Effect of sodium alginate concentration, bead diameter, initial pH, and temperature on lactic acid production from pineapple waste using immobilized Lactobacillus delbrueckii. Process Biochemistry 2006, 41, 1117–1123.
- Ketnawa, S.; Sai-Ut, S.; Theppakorn, T.; Chaiwut, P.; Rawdkuen, S. Partitioning of bromelain from pineapple peel (Nang Lae cultv.) by aqueous two phase system. Asian Journal of Food and Agro-Industry 2009, 2, 457–468.
- Gorinstein, S.; Zemser, M.; Haruenkit, R.; Chuthakorn, R.; Grauer, F.; Martin-Belloso, O.; Trakhtenberg, S. Comparative content of total polyphenols and dietary fiber in tropical fruits and persimmon. Journal of Nutritional Biochemistry 1999, 10, 367–371.
- Alejandra, R.; Emperatriz, P.D.D. Chemical composition and bioactive compounds in pineapple, guava, and soursop pulp. Interciencia 2011, 36, 71–75.
- Kammerer, D.; Claus, A.; Carle, R.; Schieber A., Polyphenol screening of pomace from red and white grape varieties (Vitisvinifera L.) by HPLC-DAD- MS/MS. Journal of Agricultural and Food Chemisty 2004, 52, 4360–4367.
- Prieto, P.; Pineda, M.; Aguilar, M. Spectrophotometric quantitation of antioxidant capacity through the formation of a phosphomolybdenum complex, Specific application to the determination of vitamin E. Analytical Biochemistry 1999, 269, 337–341.
- Hidalgo, M.; Sánchez-Moreno, C.; Pascual-Teresa, S.D. Flavonoid–flavonoid interaction and its effect on their antioxidant activity. Food Chemistry 2010, 121, 691–696.
- Heo, H.J.; Kim, Y.J.; Chung, D.; Kim, D.O. Antioxidant capacities of individual and combined phenolics in a model system. Food Chemistry 2007, 104, 87–92.
- Antolovich, M.; Prenzler, P.; Robards, K.; Ryan, D. Sample preparation in the analysis of phenolic compounds in fruits. Analyst 2000, 125, 989–1009.
- Sun, J.; Chu, Y.F.; Wu, X.; Liu, R.H. Antioxidant and antiproliferative activities of common fruits. Journal of Agricultural and Food Chemisty 2002, 50, 7449–7454.
- Luximon-Ramma, A.; Bahorun, T.; Crozier, A.; Zbarsky, V.; Datla, K.P.; Dexter, D.T.; Aruoma, O.I. Characterization of the antioxidant functions of flavonoids and proanthocyanidins in Mauritian black teas. Food Research International 2005, 38, 357–367.
- Alothman, M.; Bhat, R.; Karim, A.A. Antioxidant capacity and phenolic content of selected tropical fruits from Malaysia, extracted with different solvents. Food Chemistry 2009, 115, 785–788.
- Luximon-Ramma, A.; Bahorun, T.; Crozier, A. Antioxidant actions and phenolic and vitamin C contents of common Mauritian exotic fruits. Journal of the Science of Food and Agriculture 2003, 83, 496–502.
- Fine, A.M. Oligomeric proanthocyanidin complexes: History, structure, and phytopharmaceutical applications. Alternative Medicine Review 2000, 5, 144–151.
- Sagdic, O.; Ozturk, I.; Ozkan, G.; Yetim, H.; Ekici, L.; Yilmaz, M.T. RP-HPLC–DAD analysis of phenolic compounds in pomace extracts from five grape cultivars: Evaluation of their antioxidant, antiradical, and antifungal activities in orange and apple juices. Food Chemistry 2011, 126, 1794–1758.
- Carbone, K.; Giannini, B.; Picchi, V.; Lo Scalzo, R.; Cecchini, F. Phenolic composition and free radical scavenging activity of different apple varieties in relation to the cultivar, tissue type, and storage. Food Chemistry 2011, 127, 493–500.
- Ruberto, G.; Renda, A.; Daquino, C.; Amico, V.; Spatafora, C.; Tringali, C.; De Tommasi, N. Polyphenol constituents and antioxidant activity of grape pomace extracts from five Sicilian red grape cultivars. Food Chemistry 2007, 100, 203–207.
- Ćetković, G.; Čanadanović-Brunet, J.; Djilas, S.; Savatović, S.; Mandić, A.; Tumbas, V. Assessment of polyphenolic content and in vitro antiradical characteristics of apple pomace. Food Chemistry 2008, 109, 340–347.
- Kumaran, A.; Karunakaran, J. In vitro antioxidant activities of methanol extracts of five Phyllanthus species from India. LWT-Food Science and Technology 2006, 40, 344–352.
- Jayaprakasha, G.K.; Selvi, T.; Sakariah, K.K. Antibacterial and antioxidant activities of grape (Vitis vinifera) seed extracts. Food Research International 2003, 36, 117–122.
- Cheng, Y.H.; Wang, Z.; Xu, S.Y. Antioxidant properties of wheat germ protein hydrolysates evaluated in vitro. Journal of Central South University Science and Technology (Chinese, English Edition) 2006, 13, 160–165.
- Bravo, L. Polyphenols:Chemistry, dietary sources, metabolism, and nutritional significance. Nutrition Reviews 1998, 56, 317–333.
- Yilmaz, Y.; Toledo, R.T. Major flavonoids in grape seeds and skins: Antioxidant capacity of catechin, epicatechin, and gallic acid. Journal of Agricultural and Food Chemistry 2004, 52, 225–260.
