Abstract
An inexpensive natural antioxidant, tocotrienol rich fraction, prepared from crude rice bran oil, was developed for enriching the quality of mustard oil. DPPH assay of tocotrienol rich fraction showed that it possesses high antioxidant potential. Efficacy of tocotrienol rich fraction in retarding lipid peroxidation was studied by comparing acid value, iodine value, peroxide value, TBARS value, and fatty acid composition of crude mustard oil with and without TRF, stored under accelerated oxidation conditions, such as exposure to elevated temperature of 60°C, aerial oxygen, and UV radiation. The enhanced peroxidation under accelerated oxidation conditions was found to be retarded by tocotrienol rich fraction addition, thereby it increased the shelf life of the mustard oil by decelerating the process of lipid peroxidation.
INTRODUCTION
Oxidative deterioration is a significant contributing factor to the limited shelf life of lipid-containing foods and is of great economic concern to the food industry. As lipid-containing foods are stored, oxidation causes the development of objectionable flavors and, therefore, decreases the sensory acceptability of the products over time.[Citation1] The shelf life of products can be potentially increased with the addition of antioxidants, thereby reducing the rate of lipid oxidation and hydrolysis by sequestering and stabilizing free radicals.[Citation2] Antioxidants are substances that when added to food products, especially lipids and lipid-containing foods, can increase shelf life by retarding lipid peroxidation, which is one of the major processes causing deterioration of food products during processing and storage. Synthetic compounds, such as butylated hydroxyl anisole (BHA), butylated hydroxyl toluene (BHT), and propyl gallate (PG), have been used as antioxidants since the beginning of the 20th century.[Citation3] But side effects of these synthetic antioxidants used in food processing, such as BHT and BHA, have been proved. For example, these substances exhibit carcinogenic effects in living organisms and are responsible for liver damage.[Citation4] Consequently, the need to identify alternative natural and safe sources of food antioxidants arose, and the search for natural antioxidants, especially of plant origin, has notably increased in recent years.[Citation3] Special attention has been focused on the extraction of natural food additives with antioxidant activity from inexpensive or residual sources from the agricultural and food-processing industries.[Citation5] Recently, consumer health consciousness has led to a demand for “natural” alternatives to synthetic food antioxidants. Natural plant-based antioxidants, such as phenolics, have received much attention for their antioxidant characteristics.[Citation6]
Rice bran is a component of raw rice that is obtained when it is removed from the starchy endosperm in the rice milling process. It is high in oil content (15–25%), has low moisture content (6–7%), and possesses a powdery consistency. Rice bran is a waste product in the milling process; it has been used as a feedstock and has the potential to be used as a food ingredient and oil source.[Citation7] Rice bran oil (RBO) obtained from rice bran is a rich source of tocopherols, tocotrienols, and γ-oryzanol.[Citation8] RBO contains approximately 38% oleic acid, 34% linoleic acid, and 18.6% palmitic acid; however, it contains a negligible amount of α-linolenic acid (n-3 or 18:3 fatty acid).[Citation9]
Two novel tocotrienols were isolated and established as desmethyltocotrienol [3,4-dihydro-2-methyl-2-(4,8,12-trimethyltrideca-3′(E),7′(E),11′-trienyl)-2H-1-benzopyran-6-ol] and didesmethytocotrienol [3,4-dihydro-2-(4,8,12-trimethyltrideca-3′(E),7′(E),11′-trienyl)-2H-1-benzopyran-6-ol].[Citation10] Rice bran oil is widely used in pharmaceutical, food, and chemical industries due to its unique properties and high medicinal value.[Citation11] The RBO contains tocopherols and tocotrienols as part of the unsaponifiable matter, which have been well-studied for their antioxidative properties.[Citation12] The blending of other oils with rice bran oil has also been found to improve the stability of the blend during frying and storage.[Citation13] Rice bran oil antioxidants are very efficient in reducing low density lipoprotein and total serum cholesterol. Tocotrienol possesses antioxidant, hypocholesterolemic, cardioprotective, neuroprotective, anticancer, anti-inflammatory, antidiabetic, and antiadipogenic effects. The mechanism of hypocholesterolemic effect of tocotrienol is exerted through decreased cholesterol absorption in the intestine and downregulation of the expression of 3-hydroxy-3-methyl glutaryl coenzyme A (HMG Co A) reductase.[Citation14] A tocotrienol rich fraction (TRF) has been prepared from crude rice bran oil, and DPPH assay of TRF was done. The present study was undertaken to find whether the addition of TRF to mustard oil could minimize lipid peroxidation of mustard oil under three different oxidation conditions—exposure to 60°C, aerial exposure, and exposure to UV radiation. The parameters measured were acid value, iodine value, peroxide value, and TBARS value. The fatty acid composition analyses of the oil samples with and without TRF were also done. Crude mustard oil free of additives was used as the substrate for oxidation studies.
MATERIALS AND METHODS
Chemicals
2, 2-Diphenyl-1-picrylhydrazyl was procured from Sigma-Aldrich (USA). 2-Thiobarbituric acid (TBA) was bought from Merck (Germany). Potassium iodide and sodium thiosulphate were purchased from Merck Specialities Private Limited (Mumbai). Sodium hydroxide (NaOH) was bought from Hi Media Laboratories (Mumbai). Wij’s solution was obtained from Nice Chemicals Private Limited (Cochin). Crude rice bran oil and crude mustard oil were bought from local oil mills.
Preparation of TRF
The crude rice bran oil was mixed with ethanol and kept in a shaker. The alcohol mixture was then evaporated under vacuum at low temperature (40°C) to obtain a fraction that contains many medicinally beneficial micronutrients of which tocotrienol is a major component.[Citation12] TRF can be utilized for preventing lipid peroxidation by adding it to the oil. The antioxidant potential of TRF was evaluated in edible oils by monitoring acid value, iodine value, peroxide value, and TBARS value. For the study, crude mustard oil without any added antioxidant has been selected.TRF was added as an antioxidant by blending in crude mustard oil. This would enable us to ascertain the TRF’s potential solely. To find the extent to which TRF can offer protection against lipid peroxidation, crude mustard oil was exposed to various conditions under which accelerated oxidation could take place.
The TRF prepared was added to mustard oil (MO) at the concentration of 100 ppm and then subjected to accelerated oxidation conditions. To find the effect of TRF when the oil is heated, oil samples, with and without TRF, were kept at 60°C in an incubator. At an interval of 7 days, samples were taken and analyzed. The parameters measured were acid value, iodine value, peroxide value, and TBARS value. To study the effect of aerial exposure, oil samples, with and without TRF, were kept in two petri dishes separately at room temperature (25°C). The above parameters were measured at 7-day intervals. The oil samples, with and without TRF, were exposed to UV radiation for 15, 30, 60, and 90 min and the above mentioned parameters were measured.
DPPH Assay
The DPPH assay was carried out as described by Cuendet and co-workers.[Citation15] Samples (5, 10, 15, 20, or 25 μl) were added to 5 ml of a 0.004% methanol solution of DPPH. After a 30-min incubation period at room temperature (25°C), the absorbance was read against a blank at 515 nm. The assay was carried out in triplicate, analyses of all samples were done in duplicate, and results were averaged.
Fatty Acid Composition of Oil by Gas Chromatography (GC)
Methyl esters of the oils were prepared by the method described by Litchfield[Citation16] and the fatty acid compositions were determined by GC analysis using an analytical gas chromatography (Agilent 6890 Series gas chromatograph) equipped with a flame ionization detector (FID) and HPd capillary column (J & W Scientific Columns from Agilent Technologies) of 30-m length with 0.25 mm (i.d) and 0.25 mm (film thickness). The GC inlet temperature and FID temperature was maintained at 250°C and oven temperature was maintained at 250°C for 2 min, then temp was increased at 10°C/min, up to 280°C, then a 20 min hold at 280°C. The gas flow was 1, 300, and 30 mL/min for N2, H2, and air, respectively.[Citation17]
The acid value was determined by the AOAC method number 28.030.[Citation18] The iodine value was determined by the AOAC method number 28.021.[Citation18] The peroxide value was determined according to the AOAC official method 28.024.[Citation18]
The thiobarbituric test is based on the color reaction of TBA with malondialdehyde, the secondary oxidation product of lipid peroxidation. The sample mixed with TBA was kept in a water bath (95°C), cooled, and measured spectrophotometrically. During lipid peroxidation, low molecular weight end products, malondialdehyde, are formed by oxidation of polyunsaturated fatty acids. Malondialdehyde can be reacted with two molecules of TBA to give a pinkish red chromagen.[Citation19]
Statistical Analysis
The data are presented as mean ± S.D. (standard deviation) of three replicates (data are not shown). The triplicate data were subjected to Analysis of Variance (ANOVA) using Microsoft Excel 2007. Comparison of means was analyzed by Fischer’s Least Significant Different Test at a significance level p < 0.05.
RESULTS AND DISCUSSION
The effect of addition of TRF to crude mustard oil has been studied by measuring the acid value, iodine value, peroxide value, and TBARS value. shows the changes in DPPH radical scavenging activities of the antioxidants with different concentrations. The scavenging effect of TRF (77–92%) on DPPH radical proportionately increased with increasing concentration. Thus, addition of TRF to oil is expected to reduce lipid peroxidation.
Table 1 Changes in DPPH radical scavenging activities according to different concentrations.
shows the fatty acid composition (% w/w) of the oil samples. When compared to control mustard oil, the percentage of unsaturated fatty acids in mustard oil decreases after exposure to 60°C. However, upon TRF addition, the unsaturated fatty acid percentage is restored close to the control value. The TRF possibly reacts with the highly reactive free radicals, thereby retaining the double bonds of the unsaturated fatty acids. Similar trends in the fatty acid composition have been observed in case of aerial and UV exposures also.
Table 2 Fatty acid composition (% w/w) of the oil samples.
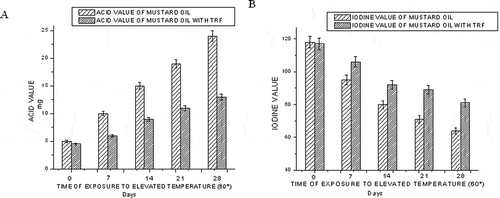
The free fatty acid content is a measure of the acidic components in the oil. They are formed due to breakdown of oil molecules, triglycerides into its components, fatty acids, and glycerol. The effect of TRF addition on the acid value of mustard oil exposed at an elevated temperature (60°C) is shown in . While storing at 60°C, at the end of 28 days, the acid value of mustard oil becomes 24 mg. However, TRF addition has restricted the acid value to 13 mg. Thus, addition of TRF could lower the acid value of mustard oil. Iodine value is a measure of overall unsaturation and is widely used to characterize oils and fats. When the oil was kept at 60°C, the iodine value of mustard oil decreased to 95, 80, 71, and 64 at the end of 7, 14, 21, and 28 days, respectively (). Addition of TRF decreases the rate of lowering the iodine value of mustard oil under the same condition to the values of 106, 92, 89, and 81 at the end of 7, 14, 21, and 28 days, respectively. Possibly, the TRF quenches the free radicals and protects the double bonds from being attacked by the free radicals. Thus, the decrease in lowering of iodine value was consistent with the increasing number of double bonds in oil.
Figure 1 Effect of time of exposure to elevated temperature (60°C) on the acid value (a) and iodine value (b) of mustard oil.
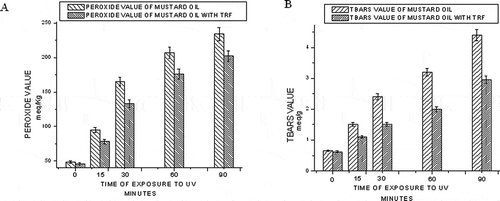
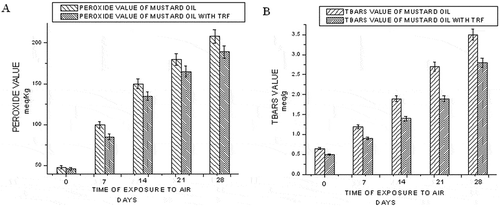
The peroxide value is a common indicator of lipid oxidation, but its use is limited to the early stages of oxidation. Peroxides are primary products of lipid oxidation and play a central role in auto-oxidation of lipids and are decomposed into carbonyls and other compounds. This index accounts for hydroperoxides, labile intermediate compounds that decompose into several secondary oxidation products. The parameter, peroxide value, measures the total peroxide and hydroperoxide content of the edible oil system.[Citation20] The effect of time of exposure to elevated temperature (60°C) on peroxide value of mustard oil with and without TRF is shown in . The peroxide value increases with time for both of the samples studied. But the addition of TRF lowers the peroxide value compared to mustard oil without TRF.
Figure 2 Effect of time of exposure to elevated temperature (60°C) on the peroxide value (a) and TBARS value (b) of mustard oil.
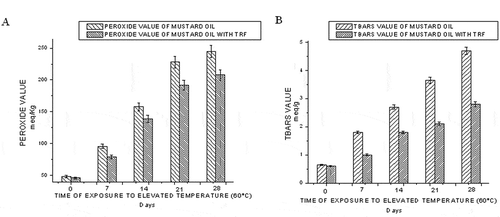
The peroxide decomposition products, present in the oil, may catalyze further oxidation. To measure such secondary oxidation products, TBARS values of mustard oil samples were also recorded during the storage period. The TBARS value is an index of lipid oxidation measuring malondialdehyde content. shows the effect of TRF addition on the TBARS value of mustard oil exposed to 60°C. Initially, the rate of formation of TBARS is lower. But with an increase in time of exposure to elevated temperature (60°C), the degradation of the primary oxidation products increases and formation of secondary oxidation products enhances. So, with an increasing number of days, malondialdehyde concentration increases leading to higher TBARS value.
A higher amount of oxygen is dissolved in the oil when the oxygen partial pressure in the headspace is high. Oxidation of the oil increased with the amount of dissolved oxygen. The solubility of oxygen is higher in oil than in water, and also in crude oil than in refined oil.[Citation21] , , , and show the variation of acid, iodine, peroxide, and TBARS values of mustard oil, with and without the addition of TRF, under aerial oxidation condition (25°C). With an increase in time of exposure to air, lipid peroxidation increases and acid value increases as shown in . TRF possibly quenches the free radicals and inhibits lipid peroxidation. Thus, in mustard oil containing TRF, the increase of acid value is at a comparatively lower rate. shows the variation of iodine value of mustard oil with respect to time of exposure to air. The oxidation is reflected in the loss of double bonds and lowering of iodine values. The antioxidant, TRF, when added, reacts with the reactive free radicals that would have otherwise attacked the electron-rich double bonds. Hence, the iodine value of mustard oil containing TRF is higher than that of only mustard oil. The effect of TRF addition on the peroxide value and TBARS value of mustard oil exposed to air is shown in and , respectively. In the case of mustard oil, the peroxide value increases sharply, whereas in mustard oil containing TRF, the rate of increase is gradual because probably the added antioxidants compete with the unsaturated lipid molecules for lipid peroxy radicals and restricts lipid peroxidation. With an increase in lipid peroxidation, the formation of secondary oxidation products also increases leading to higher TBARS value in both the cases. Here again, oil samples with TRF show lower values of TBARS than mustard oil.
Figure 3 Effect of time of exposure to air on the acid value (a) and iodine value (b) of mustard oil.
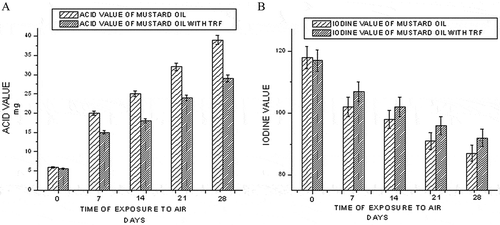
Figure 4 Effect of time of exposure to air on the peroxide value (a) and TBARS value (b) of mustard oil.
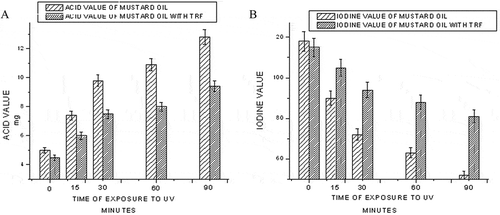
UV radiation induces lipid oxidation and it increases with an increase in UV radiation exposure time. The production of •O2− and •OH by UV radiation is actually responsible for the increased lipid oxidation in oil.[Citation22] When the oil is exposed to UV radiation, oxygen goes into an excited state through an energy transfer mechanism and this singlet oxygen reacts with methyl linoleate faster (by 1500 times) than triplet oxygen (normal oxygen) to form hydroperoxides. Lipid hydroperoxides are very unstable and they undergo cleavage to give free alkoxy radicals, which decompose to give mainly aldehydes, but also ketones and hydrocarbons.[Citation23] With UV light used to mimic sunlight, lipid peroxidation increases steadily with exposure time. The effect of addition of TRF to mustard oil exposed to UV is shown in . Ultraviolet radiation accelerates the formation of free radicals, which leads to an increase in acid value. TRF addition, however, lowers the acid value. In the case of mustard oil with TRF, the antioxidant TRF possibly protects the double bonds and the iodine value decreases gradually in contrast to mustard oil, in which the iodine value decreases sharply at a much faster rate (). UV exposure accelerates lipid peroxidation leading to higher peroxide value and TBARS value as shown in and , respectively. But TRF effectively lowers the peroxide value and TBARS value.
CONCLUSION
It has been found that TRF has the potential to minimize lipid peroxidation. It is evident from the decrease in acid value (from 24 to 13), peroxide value (from 245 to 208), and TBARS value (from 4.7 to 2.8) and a corresponding increase in iodine value (from 64 to 81) for exposure to 60°C. Similar trends have been noticed for aerial oxygen and UV radiation exposure. This is also supported by the fatty acid composition changes. Our results show that the TRF from rice bran oil can be well used as a natural source of strong antioxidants to preserve the quality of mustard oil.
FUNDING
The authors would like to express their sincere gratitude to UGC CAS I for financing the research work.
REFERENCES
- Awah, F.M.; Verla, A.W. Antioxidant activity, nitric oxide scavenging activity and phenolic contents of Ocimum gratissimum leaf extract. Journal of Medical Plant Research 2010, 4 (24), 2479–2487.
- Armitage, D.B.; Hettiarachchy, N.S.; Monsoor, M.A. Natural antioxidants as a component of an egg albumen film in the reduction of lipid oxidation in cooked and uncooked poultry. Journal of Food Science 2002, 67, 631–634.
- Singh, G.; Marimuthu, P.; Heluani, C.S.D.; Catalan, C.A.N. Antioxidant and biocidal activities of carumnigrum (seed) essential oil, oleoresin, and their selected components. Journal of Agricultural and Food Chemistry 2006, 54, 174–181.
- Rahimi-Nasrabadi, M., Nazarian, S., Farahani, H., Koohbijari, G.R.F., Katalinic, V., Ahmadi, F., Batooli, H. Chemical composition, antioxidant, and antibacterial activities of the essential oil and methanol extracts of Eucalyptus largiflorens F. Muell. International Journal of Food Properties 2013, 16, 369–381. http://dx.doi.org/10.1080/10942912.2010.551310
- Katalinic, V.; Mozina, S.S.; Generalic, I.; Skroza, D.; Ljubenkov, I.; Klancnik, A. Phenolic profile, antioxidant capacity, and antimicrobial activity of leaf extracts from six Vitis vinifera L. varieties. International Journal of Food Properties 2013, 16, 45–60. DOI:10.1080/10942912.2010.526274.
- Rupasinghe, H.P.V.; Erkan, N.; Yasmin, A. Antioxidant protection of eicosapentaenoic acid and fish oil oxidation by polyphenolic-enriched apple skin extract. Journal of Agricultural and Food Chemistry 2010, 58, 1233–1239. DOI:10.1021/jf903162k.
- Lakkakula, N.R.; Lima, M.; Walker, T. Rice bran stabilization and rice bran oil extraction using ohmic heating. Bioresource Technology 2004, 92, 157–161.
- Nagendra Prasad, M.N.; Sanjay, K.R.; Shravya Khatokar, M.; Viswamaya, M.N.; Nanjunda Swamy, S. Health benefits of rice bran: A review. Journal of Nutrition & Food Sciences 2011, 1, 108. DOI:10.4172/2155-9600.1000108.
- Chopra, R.; Rastogi, N.K..; Sambaiah, K. Enrichment of rice bran oil with α-linolenic acid by enzymatic acidolysis: Optimization of parameters by response surface methodology. Food and Bioprocess Technology 2011, 4, 1153–1163. DOI:10.1007/s11947-009-0191-1.
- Saini, V.; Fatima, N.; Shivani, B.; Ishaq, F.; Singh, R.N.; Khan, A. Anti-atherogenic and anti-oxidant effect of tocopherol and tocotrienols on Cu++ mediated low-density lipoprotein (LDL) oxidation in normal lipidemic subjects. European Journal of Experimental Biology 2012, 2 (4), 1071–1086.
- Amarasinghe, B.M.W.P.K.; Kumarasiri, M.P.M.; Gangodavilage, N.C. Effect of method of stabilization on aqueous extraction of rice bran oil. Food and Bioproducts Processing 2009, 87, 108–114.
- Ghosh, M. Review on recent trends in rice bran oil processing. Journal of the American Oil Chemists’ Society 2007, 84, 315–324. DOI:10.1007/s11746-007-1047-3.
- Gopalakrishna, A.G.; Khatoon, S.; Babylatha, R. Frying performance of processed rice bran oils. Journal of Food Lipids 2005, 12, 1–11.
- Bardhan, J.; Chakraborty, R.; Raychaudhuri, U. The 21st century form of vitamin E–Tocotrienol. Current Pharmaceutical Design 2011, 17, 2196–2205.
- Cuendet, M.; Hostettman, K.; Potterat, O. Iridoidglucosides with free radical scavenging properties from Fagraea blumei. Helvetica Chimica Acta 1997, 80, 1144–1152.
- Litchfield, C. Analysis of Triglycerides; Academic Press: New York & London, 1972; 32 pp.
- Dhara, R.; Bhattacharyya, D.K.; Ghosh, M. Analysis of sterol and other components present in unsaponifiable matters of mahua, sal and mango kernel oil. Journal of Oleo Science 2010, 59 (4), 169–176.
- Sharma, H.K.; Kaur, B.; Sarkar, B.C.; Singh, C. Thermal behavior of pure rice bran oil, sunflower oil and their model blends during deep fat frying. Grasas Y Aceites 2006, 57 (4), 376–381.
- Kirk, R.S.; Sawyer, R. Pearson’s Composition and Analysis of Foods; Addison Wesley Longman Ltd.: England, 1991; 642 pp.
- Poiana, M.A. Enhancing oxidative stability of sunflower oil during convective and microwave heating using grape seed extract. International Journal of Molecular Sciences 2012, 13 (7), 9240–9259.
- Choe, E.; Min, D.B. Mechanisms and factors for edible oil oxidation. Comprehensive Reviews in Food Science and Food Safety 2006, 5 (4), 169–186.
- Chen, X.; Ahn, D.U. Antioxidant activities of six natural phenolics against lipid oxidation induced by Fe2+ or ultraviolet light. Journal of the American Oil Chemists’ Society 1998, 75, 1717–1721.
- deMan, J.M. Chemical and physical properties of fatty acids. In Fatty Acids in Foods and Their Health Implications; Chow, C.K.; Eds.; CRC Press Taylor and Francis Group: Boca Raton, Florida, 2007; 17–46.