Abstract
Traditional surimi-based products are usually prepared through a heating process, which often causes high energy consumption and increased production costs. Acid-induced surimi gel, as a new preparation technique, were prepared from silver carp surimi by soaking in acetic acid in this study, and their properties and formation mechanism were investigated and compared to heat-induced gel. The moisture content, breaking force, deformation, and whiteness of acid-induced surimi gel were all higher than those of heat-induced gel, irrespective of acid concentration. The textural properties, color values and water holding capacity of acid-induced gel were increased with acetic acid concentration increase from 1 to 3%. On the other hand, the high molecular weight fractions were also formed with myosin heavy chain based on electrophoresis analysis. The data of protein subunits solubilized in various solvents revealed that the most important associative force in acid-induced gel was hydrophobic interactions. Furthermore, the compact and fine fiber microstructure in acid-induced surimi gel was observed by scanning electron microscopy. It is concluded that a tri-dimensional network was formed within the acid induced surimi gel through hydrophobic interactions and disulfide bonds with the presence of myosin heavy chain and actin, resulting in formation of compact fine fiber microstructures.
INTRODUCTION
Over the past decades, aquaculture production in China has continuously increased to 36.7 million tons in 2010, which accounts for more than 60% of the world’s aquaculture production.[Citation1] Silver carp (Hypophthalmichthys molitrix) is one of the most important economic freshwater fish species cultured in China, and by 2011 its production was estimated to be 3.7 million tons.[Citation2] However, silver carp is undervalued due to its product muddy flavor and the lack of value addition. Nevertheless, fish meat offers an immense scope for development of surimi-based products, which represents a form of value addition to low-value fish products. Application of silver carp muscle protein is reported to possess excellent gelling properties and therefore application of its muscle protein in production of surimi-based gel products has received increased attention.[Citation3−Citation5]
Surimi is an intermediate product manufactured by removal of undesirable compounds, such as sarcoplasmic proteins, lipids, pigments, enzymes, and odors.[Citation6] According to the protein gel formation mechanisms, proteins such as fish surimi form a gel when induced physically (using heat and pressure), chemically (using acids and bases), and enzymatically (by gelation reactions).[Citation3,Citation7] Heat-induced fish surimi gel has previously produced at approximately near neutral pH conditions.[Citation3,Citation4,Citation8] Partial unfolded surimi protein with the presence of NaCl forms a continuous matrix during setting process, which changes into a rigid opaque gel by raising the temperature to approximately 90°C, due to thermal aggregation and further protein cross-linking.[Citation9] Therefore, heating processes, such as boiling, steaming, or frying, play an important role in the formation of traditional surimi-based products, but this result is in high energy consumption and increased production cost.
Fish muscle protein gels are prepared under mild acidic conditions without NaCl and heat-treatment.[Citation10] The gel forming mechanism of non-salt protein under acidic conditions might be explained by the fractal aggregation theory, which states that protein molecules moving by Brownian motion aggregate when they encounter each other. The protein aggregation formed clusters by acidic curing conditions result into protein gel blocks.[Citation11] On the other hand, it is reported that unheated salt-ground surimi also forms gel at an optimum pH of approximately 4.0.[Citation12] Furthermore, the low product pH provides non-suitable conditions for microbial spoilage growth, thus better storage stability and lower cost.[Citation13] An acid-induced gel preparation technique is applied in Japan, and the products are prepared by soaking the setting gel in vinegar.[Citation12] An acid-induced gel formation process has been used on marine fish surimi[Citation14] and squid surimi;[Citation15,Citation16] it is, therefore, keen to evaluate the application of this in freshwater fish surimi. Moreover, there is little information about the mechanism of unheated salt-ground surimi protein gel formation under acidic pH. Therefore, we set to compare the properties of silver carp surimi acid-induced gel with the heat-induced gel, and to elucidate the mechanism of acid-induced gel formation.
MATERIALS AND METHODS
Surimi Preparation
Silver carp (Hypophthalmichthys molitrix), with an average weight of 2 kg, were purchased alive from a local fish stall. Surimi preparation was done as described by Chaijan et al.[Citation17] Upon arrival, we thoroughly washed the silver carp immediately and dressed to remove skin, head, viscera, bones, and red muscle. The fillets were washed using cold water, and then minced with a chopped mixer. Using a conventional washing process, we washed the mince with chilled water (fish/water ratio of 1:3 (w/v)) and gently stirred for 10 min. The washed mince was filtered through four layers of gauze. The washing and filtering process was repeated three times, and the obtained mince was dewatered using a wringing machine and chopped with 4% sucrose, 4% sorbitol, 0.2% sodium pyrophosphate, and 0.2% sodium polyphosphate for 5 min. The prepared surimi (50 g) was packed into a polyethylene bag and stored at --37°C until use. This surimi contains moisture of 77.11 ± 0.36%, crude protein of 14.14 ± 0.11%, and ash of 0.42 ± 0.03%.
Protein Solubility and Composition of Surimi
As described by Sato et al.[Citation18] but minor modifications, we determined the surimi protein water solubility with or without 3% NaCl to be in a pH range of 3.0–7.0. Eight grams of previously thawed (at 4°C) surimi were homogenized in 100 mL of distilled water on ice with or without 3% NaCl using a high-speed homogenizer (Fluko FA25, Equipment Shanghai Co., Ltd., Shanghai, China). The pH of the prepared surimi suspension was adjusted to the desired range using 1 M HCl, centrifuged at 15000× g for 30 min and the protein concentration in the supernatant was determined using the Lowry method with bovine serum albumin as a standard.[Citation19] The protein supernant concentration was further quantified using the sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE).[Citation20]
Surimi Gel Preparation
Heat-induced gel
Frozen surimi was thawed at 4°C and grinded in a mortar for 10 min using a pestle and then further grinded for 15 min after an addition of 3% NaCl. The moisture content of the mixture was adjusted to about 80% by adding iced water. The resulting paste was stuffed into stainless steel tubes (inter diameter 30 mm, height 30 mm). Throughout the entire process, the temperature of samples was kept below 10°C. Heat-induced gel was prepared by setting the stuffed surimi pastes in water baths at 30°C for 1 h, followed by heating to 90°C for 15 min. The products were then cooled on ice for 30 min, and kept at 4°C overnight before analysis.
Acid-induced gel
The preparation and setting of the surimi paste was carried out as described in the heat-induced gel. After setting at 30°C for 1 h, the obtained setting gels were put into 1–4% acetic acid solution (Xilong Chemical Co., Guangzhou, China) at 10°C for 24 h, followed by equilibrating at 4°C for 72 h before analysis. The ratio of setting gel and acetic acid solution was 1:4 (w/v), and the corresponding acetic acid concentration was adjusted using distilled water.
Moisture Content and pH Measurement
The moisture content of surimi gels was determined gravimetrically by drying at 105°C. The pH of surimi gels was measured using a pH meter (206-pH meter, Testo, Germany) at five random locations of the gels.
Textural Properties
Textural properties of gel samples (gel column size, diameter 30 mm, height 30 mm) were measured using a texture analyzer (TMS-PRO, Food Technology Co., USA) equipped with a spherical probe (diameter 5 mm) and 10 N load cell for breaking force (g) and deformation (mm) at a penetration speed of 30 mm/min. The textural properties of all the gels were measured at room temperature and every measurement was repeated at least six times.
Expressible Water Content
Expressible water content of the gel was measured as described by Uresti et al.[Citation21] About 3 g of gel samples were placed into the filter paper, pushed to the bottom of the centrifuge tubes, and centrifuged at 1000 × g for 15 min. Expressible drip was determined as percentage of expressible water from gels. All determinations were carried out at least five times.
Color
The surimi gels were cut with a diameter of 26 mm and height of 5 mm, and the color values of L* (lightness), a* (redness), and b* (yellowness) were measured using a colorimeter (WSC-S, Shanghai Precision & Scientific Instrument Co., China) with measurements standardized in relation to the white calibration plate (L* = 91.86; a* = -0.88; b* = 1.42). Whiteness was calculated according to the following formula:
SDS-PAGE
SDS-PAGE was performed according to the method of Laemmli using a PROTEAN II xL and Power Pac 3000 (Bio-Rad Laboratories, Hercules, USA) with a 5% stacking gel and 8% running gel.[Citation20] After electrophoresis, gels were stained with 0.025% Coomassie Brilliant Blue R-250 in 5% methanol and 10% acetic acid and destained with 30% methanol and 10% acetic acid. A protein standard (Fermentas Life Sciences, Hanover, MD, USA) ranging from 10 to 200 kDa was used to estimate the molecular weight of the proteins.
Protein Subunits Solubilized in Various Solvents
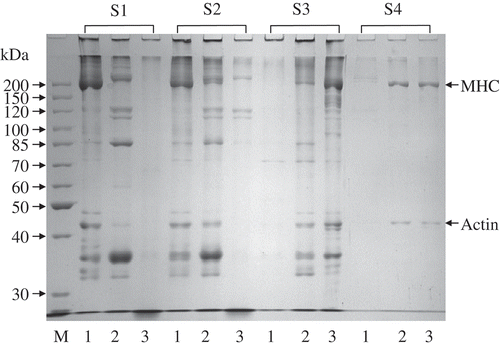
The surimi gels were dissolved in four different solutions at pH 7.0: 0.6 M NaCl (S1); 0.6 M NaCl + 1.5 M urea (S2); 0.6 M NaCl + 8 M urea (S3); and 0.6 M NaCl + 8 M urea + 0.5 M β-mercaptoethanol (βME, S4).[Citation22] Twenty grams of surimi gel were homogenized at 16000 rpm with 50 mL of S1 for 2 min in the ice bath using a high speed homogenizer (Fluko FA25, Shanghai, China). The resulting homogenized solution was centrifuged at 15000× g for 30 min. Following the same process, the obtained pellet was homogenized in S2, S3, and finally in S4. The protein concentration of supernatant was determined using the Lowry method,[Citation19] with a commercial reagent (Bio-Rad Laboratories, Hercules, USA). The protein subunits of supernatants were determined by SDS-PAGE.[Citation20] Five microliters of the samples (about 5 mg/ml) were loaded into sample wells along with a protein standard and subjected to an electrophoresis.
Scanning Electron Microscopy (SEM)
The microstructure of surimi paste and surimi gels was analyzed using the scanning electron microscope (S-4800, Hitachi Co., Tokyo, Japan). Samples with a thickness of 2–3 mm were immersed in 0.1 M phosphate buffer (pH 7.2) containing 2.5% (v/v) glutaraldehyde at 4°C overnight. Then the samples were washed three times with 0.1 M phosphate buffer (pH 7.2) before being dehydrated in ethanol with serial concentrations of 30, 50, 60, 70, 80, 90, and 100% (v/v). After being dehydrated, the samples were dried at the critical-point (Tousimis samdri-PVT-3D, Rockville, MD, USA) using CO2 as a transition fluid. The prepared samples were mounted on aluminum specimen holders and coated with gold (Hitachi E-1010, Tokyo, Japan) and observed at an acceleration voltage of 5 kV.
Statistical Analysis
The obtained data was subjected to variance (one- and two-ANOVA) analysis (Minitab Inc., State College, PA, USA). The probability value of p < 0.05 was used as the criterion for significant differences.
RESULTS AND DISCUSSION
Surimi Protein Solubility
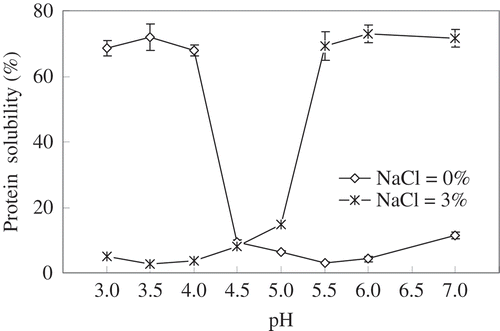
The protein solubility of silver carp surimi was determined at different pH levels with or without 3% NaCl (). In the absence of NaCl, the solubility of surimi proteins in distilled water at pH 7.0 was about 10%, but it decreased to around 5% at a pH range of 5.0–6.0. Below 4.5 pH, there was a sharp increase in surimi protein solubility, and a maximum value was observed at pH 3.0–4.0. However, after the addition of 3% NaCl, an opposite trend of surimi protein solubility was noted. Between pH 5.5 and 7.0, approximately 70% of surimi protein was soluble. As pH decreased below 5.5, a sharp decrease in protein solubility of surimi was observed. These results clearly showed that proteins in silver carp surimi were more soluble at acidic pH, but the presence of NaCl increased protein solubility at neutral pH. Surimi protein molecules become negatively charged above the isoelectric points (pH 5.5) of fish myofibrillar proteins,[Citation23] and the negative net charge of protein is increased by adding neutral salts.[Citation24] As a result of increased electrostatic repulsion between protein molecules, protein solubility was enhanced by adding NaCl at pH 5.5 and 7.0 (). The lowest solubility was noted at acidic conditions resulting in a decrease of the electrostatic repulsions, which is associated to the binding of negatively charged chloride ions to the positive charge of surimi protein below their respective isoelectric points ().
FIGURE 1 Protein solubility of silver carp surimi in water with or without 3% NaCl as related to pHs.
Soluble surimi protein fraction analysis at different pHs with or without salt () clearly depicts that in the absence of NaCl, the myosin heavy chain (MHC) and actin were soluble in distilled water at 3.0–4.0 pH range, but insoluble between pH 4.5 and 7.0, suggesting that MHC and actin could be unfolded by low pH treatment. At pH 3.0 and 3.5, a slight decrease in intensity of actin band was observed along with the appearance of a low molecular fraction, due to the action of endogenous acid proteases in surimi.[Citation25] When pH was increased to 4.5 and above, it was found that only sarcoplasmic proteins were soluble in water, especially just below 60 kDa proteins at pH 5.0 and 5.5. This phenomenon was quite similar to the sarcoplasmic proteins of striped catfish.[Citation26] However, in the case of NaCl addition, MHC and actin were well dissolved at pH 5.5–7.0, but they were not solubilized at pH 3.0–5.0. A good correlation between the protein solubility () and the intensity of MHC/actin bands () was also observed.
TABLE 1 Moisture content, pH, breaking force, and deformation of acid- and heat-induced gels from silver carp surimi
Moisture Content, pH, and Textural Properties of Surimi Gels
Moisture content, pH, breaking force, and deformation analysis of acid- and heat-induced silver carp surimi gel () indicated that the moisture content of acid-induced gel was slightly decreased with increasing acetic acid concentration and higher than heat-induced gel. The pH of acid-induced gel was lowered with increasing acetic acid concentration from 1 to 3%, but no difference was observed when acetic acid concentration was further increased to 4%. Similar trends were observed in the increase of breaking force and deformation of surimi gels induced by acetic acid. Moreover, both breaking force and deformation of acid-induced gel were significantly (p < 0.05) higher than that of heat-induced gel, which had a pH of about 6.8. In the heat-induced fish protein gel, a three-dimensional network structure was formed by random aggregation of initial unfolded proteins minced with salt.[Citation9] Similarly, weaker gel could be formed from shark meat without salt induced by weak organic acids.[Citation27] We demonstrated that the salt-minced surimi protein aggregates at low pH or under acidic conditions (), which resulted in formation of stronger gels as compared to the heat-induced gel (). This demonstrated that weak organic acid-induced gel formation has the potential to replace the conventional heating process in the manufacture of silver carp surimi product.
Color of Surimi Gels
Color characteristic is an important factor to evaluate surimi gel quality. We showed that the L*, a*, b*, and whiteness of surimi gel was induced by the addition of acetic acid at different concentrations (). With increasing acetic acid concentration, there was a slight increase in lightness (L*) and whiteness in the acid-induced surimi gel with no difference being noted in the redness (a*) and yellowness (b*). However, there was a significant difference in L*, b*, and whiteness between the acid- and heat-induced gels from silver carp surimi. It is reported that the higher b* value of suirimi gel is mainly due to the Maillard reaction between sugars, surimi proteins, and water at thermal processing conditions.[Citation28] However, this situation is not easy to occur at low temperature and acidic conditions, resulting in lower b* values of acid-induced surimi gels compared to heat-induced surimi gels. On the other hand, L* value is one of the factors representing for the concentration of total pigments.[Citation29] An increase in L* value of acid-induced gel was noted and this could be attributed to the removal of the pigment during the surimi gel setting acetic acid soaking process. Thus, the whiteness value of acid-induced gels was higher than that of heat-induced gels, which was mainly due to the considerable increase in their L* value, suggesting that excellent color quality of gels could be prepared from silver carp surimi using the acid-induced process.
TABLE 2 Color values (L*, a*, b*) and whiteness of acid- and heat-induced gels from silver carp surimi
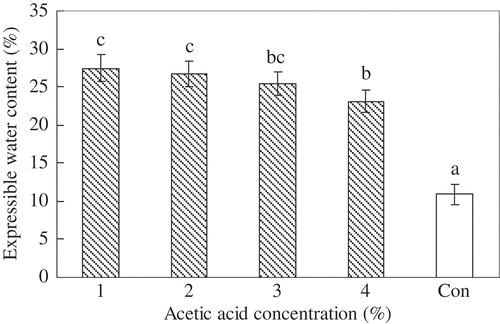
Expressible Water Content of Surimi Gels
The expressible water content analysis () showed a slight decrease in the acid-induced gel, in expressible with increase in acetic acid concentration. Compared to the heat-induced surimi gel, which clearly showed a higher expressible water content irrespective of acid concentration. Generally, the lowered expressible water content correlated well with the increased breaking force and deformation of the heat-induced surimi gel, since water molecules are entrapped by the formed fine compact gel network.[Citation17,Citation30,Citation31] However, a different trend was observed in the acid-induced surimi gel, which not only has a higher expressible moisture content but also higher breaking force and deformation than the heat-induced surimi gel, indicating that there was a distinct difference in the gel-forming mechanism between acid- and heat-induced gels from silver carp surimi. At 3% acetic acid concentration, the highest breaking force (320 g) of silver carp surimi gel was noted (), but there was no distinct change in the additional gel properties. Therefore, surimi gel induced with 3% acetic acid solution was preferably selected for studying the gel-forming mechanism over the heat-induced surimi gel.
Mechanism of Gel Formation
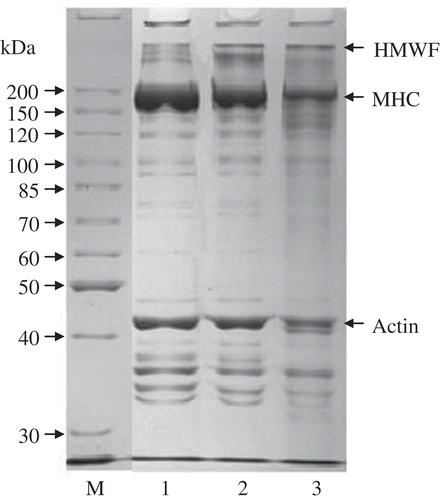
SDS-PAGE patterns of surimi paste, heat-, and acid-induced surimi gels () clearly showed that band intensity of MHC in the surimi paste was higher than that in the heat- or acid-induced surimi gels, while MHC and actin in acid-induced surimi gel were degraded to some extent. Moreover, an increase in the high molecular weight fractions (HMWF) content at the top of running polyacrylamide gel was noted with decreasing MHC content. These results showed that MHC was mainly involved in formation of HMWF whether in the heat- or acid-induced surimi gels.
FIGURE 4 SDS-PAGE patterns of surimi paste, heat-induced surimi gel, and acid-induced gel. M: Standard molecular weight mixture; 1: Surimi paste; 2: Heat-induced surimi gel; 3: acid-induced gel.
The gel formation from surimi proteins is believed to involve a tri-dimensional network of protein molecules by ionic bonds, hydrogen bonds, hydrophobic interactions, and disulfide bonds.[Citation22] Therefore, the protein fractions soluble in four different protein denaturant solutions were determined with SDS-PAGE to reveal their role in the formation of surimi gels (). In the surimi paste, a large amount of protein compositions, including MHC and actin, were completely dissolved in the S1 and S2, indicating that the main associative forces involved in the surimi mince were ionic bonds and hydrogen bonds, which was due to the aggregation of multiple intra- and inter-molecular ionic linkages between positive and negative charged amino acids. In the heat-induced surimi gel, the protein fraction solubility in S3 increased with S1 and S2 decrease, while some protein fractions of MHC and actin were only solubilized in the S4, showing that hydrophobic interactions and disulphide bonds played an important role in the surimi gel formation induced by heating. This result is similar to the heat-induced gels from Atlantic sardine muscle proteins.[Citation32] In contrast, hydrophobic interactions and disulphide bonds were also involved in the acid-induced surimi gels, but the most important associative force was hydrophobic interactions as most of their protein fractions were soluble in S3 (), which was in accordance with the lower water holding capacity of the gels ().
FIGURE 5 SDS-PAGE of soluble protein fractions of surimi paste, heat-, and acid-induced gel in different protein denaturant solutions. M: Standard molecular weight mixture; 1: Surimi paste; 2: Heat-induced surimi gel; 3: acid-induced gel.
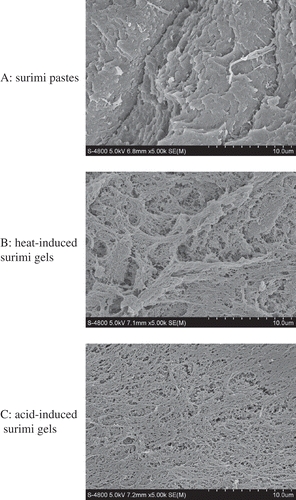
The microstructure of surimi paste and surimi gels was observed by scanning electron microscopy (SEM) (). The heat-induced gel showed a network of interconnected pores with a uniform strand, while the same network was not found in the surimi paste. In comparison to the heat-induced gel, the protein network of acid-induced gel was more compact with smaller clusters of aggregated proteins. Moreover, the regularly ordered and denser strands in acid-induced surimi gels () were responsible for the higher water moisture, breaking force, and deformation (). It was reported by Barbut and Foegeding that fine-stranded gels are usually formed by an ordered association of protein molecules.[Citation33] According to the results obtained in this study, the salt-solubilized surimi protein could be developed gradually to order association of protein molecules through hydrophobic interactions during soaking in acetic acid solution, thus forming a strong structural gel.
CONCLUSIONS
Protein gels were successfully induced from silver carp surimi by acetic acid soaking. On the contrary, the silver carp surimi acid-induced protein gel exhibited superior textural and color properties but with low water holding capacity. We therefore derived the following probable mechanism of acid-induced surimi gel formation: after silver carp surimi proteins were dissolved by grinding in 3% NaCl, aggregated proteins were formed gradually through hydrophobic interactions and disulphide bonds during the acetic acid soaking process; this resulted in formation of fine-stranded and strong structural gels with a slight degradation of MHC and actin through which occurs the gel formation. The breaking force and deformation of acid-induced surimi gel was higher than the heat-induced gel, suggesting that textural properties of acid-induced gels were mainly due to the compact protein network.
FUNDING
This work is sponsored by the Program for New Century Excellent Talents in University of Fujian Province (JA11143), and Xiamen Science and Technology Project (No. 3502Z20123025).
REFERENCES
- FAO. The State of World Fisheries and Aquaculture; Food and Agriculture Organization of the United Nations: Rome, 2012; 3–147.
- Anonymous. China Fisheries Yearbook; China Agricultural Press: Beijing, 2011; 224 pp.
- Fu, X.; Hayat, K.; Li, Z.; Lin, Q.; Xu, S.; Wang, S. Effect of microwave heating on the low-salt gel from silver carp (Hypophthalmichthys molitrix) surimi. Food Hydrocolloids 2011, 27 (2), 301–308.
- Luo, Y.; Shen, H.; Pan, D.; Bu, G. Gel properties of surimi from silver carp (Hypophthalmichthys molitrix) as affected by heat treatment and soy protein isolate. Food Hydrocolloids 2008, 22 (8), 1513–1519.
- Xu, Y.; Xia, W.; Yang, F.; Kim, J.M.; Nie, X. Effect of fermentation temperature on the microbial and physicochemical properties of silver carp sausages inoculated with Pediococcus pentosaceus. Food Chemistry 2010, 118 (3), 512–518.
- Venugopal, V. Mince and mince-based products. In: Seafood Processing: Adding Value through Quick Freezing, Retortable Packaging and Cook-Chilling; Venugopal, V.; Ed.; Taylor and Francis Group: New York, 2005; 281–318.
- Totosaus, A.; Montejano, J.G.; Salazar, J.A.; Guerrero, I. A review of physical and chemical protein-gel induction. International Journal of Food Science and Technology 2002, 37 (6), 589–601.
- Benjakul, S.; Chantarasuwan, C.; Visessanguan, W. Effect of medium temperature setting on gelling characteristics of surimi from some tropical fish. Food Chemistry 2003, 82 (4), 567–574.
- Stone, A.P.; Stanley, D.W. Mechanisms of fish muscle gelation. Food Research International 1992, 25 (5), 381–388.
- Venugopal, V.; Doke, S.N.; Nair, P.M. Gelation of shark myofibrillar proteins by weak organic acids. Food Chemistry 1994, 50 (2), 185–190.
- Lucey, J.A.; Singh, H. Formation and physical properties of acid milk gels: A review. Food Research International 1997, 30 (7), 529–542.
- Niwa, E.; Ueno, S.; Kanoh, S. Mechanism for the gelation of unheated surimi by vinegar curing. Bioscience, Biotechnology, and Biochemistry 1992, 56 (1), 58–61.
- Chawal, S.P.; Venugopal, V.; Nair, P.M. Gelation of proteins from washed muscle of threadfin bream (Nemipterus japonicus) under mild acidic conditions. Journal of Food Science 1996, 61 (2), 362–367.
- Abe, S.; Weng, W.; Tanaka, M.; Limpisophon, K.; Osako, K. Effect of egg white and setting on the gelation of vinegar cured kamaboko. Nippon Suisan Gakkaishi 2009, 75 (4), 695–700 ( in Japanese).
- Techaratanakrai, B.; Nishida, M.; Igarashi, Y.; Watanabe, M.; Okazaki, E.; Osako, K. Effect of setting conditions on mechanical properties of acid-induced kamaboko gel from squid Todarodes pacificus mantle muscle meat. Fisheries Science 2011, 77 (3), 439–446.
- Techaratanakrai, B.; Okazaki, E.; Osako, K. Effect of organic salts on setting gels and their corresponding acids on kamaboko gels prepared from squid Todarodes pacificus mantle muscle. Fisheries Science 2012, 78 (3), 707–715.
- Chaijan, M.; Panpipat, W.; Benjakul, S. Physicochemical properties and gel-forming ability of surimi from three species of mackerel caught in Southern Thailand. Food Chemistry 2010, 121 (1), 85–92.
- Sato, R.; Sawabe, T.; Kishimura, H.; Hayashi, K.; Saeki, H. Preparation of neoglycoprotein from carp myofibrillar protein and alginate oligosaccharide: Improved solubility in low ionic strength medium. Journal of Agriculture and Food Chemistry 2002, 48 (1), 17–21.
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the Folin phenol reagent. Journal of Biological Chemistry 1951, 193 (1), 265–275.
- Laemmli, U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970, 227 (5259), 680–685.
- Uresti, R.M.; López-Arias, N.; González-Cabriales, J.J.; Ramírez, J.A.; Vázquez, M. Use of amidated low methoxyl pectin to produce fish restructured products. Food Hydrocolloids 2003, 17 (2), 171–176.
- Pérez-Mateos, M.; Lourenço, H.; Montero, P.; Borderías, A.J. Rheological and biochemical characteristics of high-pressure- and heat-induced gels from blue white (Micromesistius poutassou) muscle proteins. Journal of Agricultural and Food Chemistry 1997, 45 (1), 44–49.
- Yongsawatdigul, J.; Park, J.W. Effect of alkali and acid solubilization on gelation characteristics of rockfish muscle proteins. Journal of Food Science 2004, 69 (7), 499–505.
- Sousa, R.D.C.S.; Coimbra, J.S.R.; Garcia Rojas, E.E.; Minim, L.A.; Oliveira, F.C.; Minim, V.P.R. Effect of pH and salt concentration on the solubility and density of egg yolk and plasma egg yolk. LWT–Food Science and Technology 2007, 40 (7), 1253–1258.
- Weng, W.Y.; Hamaguchi, P.Y.; Osako, K.; Tanaka, M. Effect of endogenous acid proteinases on the properties of edible films prepared from Alaska pollack surimi. Food Chemistry 2007, 105 (3), 996–1002.
- Tadpitchayangkoon, P.; Park, J.W.; Yongsawatdigul, J. Conformational changes and dynamic rheological properties of fish sarcoplasmic proteins treated at various pHs. Food Chemistry 2010, 121 (4), 1046–1052.
- Venugopal, V.; Kakatkar, A.; Bongirwar, D.R. Gelation of shark meat under mild acidic conditions: Physicochemical and rheological characterization of the gel. Journal of Food Science 2002, 67 (7), 2681–2686.
- Shie, J.S.; Park, J.W. Physical characteristics of surimi seafood as affected by thermal processing conditions. Journal of Food Science 1999, 64 (2), 287–290.
- Gasperlin, L.; Zlender, B.; Abram, V. Colour of normal and high pH beef heated to different temperatures as related to oxygenation. Meat Science 2000, 54 (4), 391–398.
- Rawdkuen, S.; Benjakul, S. Whey protein concentrate: Autolysis inhibition and effects on the gel properties of surimi prepared from tropical fish. Food Chemistry 2008, 106 (3), 1077–1084.
- Tadpitchayangkoon, P.; Park, J.W.; Yongsawatdigul, J. Gelation characteristics of tropical surimi under water bath and ohmic heating. LWT–Food Science and Technology 2012, 46 (1), 97–103.
- Roussel, H.; Cheftel, J.C. Mechanisms of gelation of sardine proteins: Influence of thermal processing and of various additives on the texture and protein solubility of kamaboko gels. International Journal of Food Science and Technology 1990, 25 (3), 260–280.
- Barbut, S.; Foegeding, E.A. Ca2+-induced gelation of preheated whey protein isolate. Journal of Food Science 1993, 58 (4), 867–871.