Abstract
The objectives of this study were to define the phenolic and fatty acid profiles, anticholinesterase, antioxidant, antimicrobial activities, and total phenolic-flavonoid contents of Lycopsis orientalis and Tragopogon latifolius var. angustifolius which have been used as food source and food supplement in Anatolia and have never been examined before. Rosmarinic and quinic acids (21.11 and 11.46 mg g–1 extract, respectively) were found to be the most abundant constituents in L. orientalis and T. latifolius var. angustifolius among the studied 27 compounds by liquid chromatography tandem mass spectrometry. In the fatty acid compositions of L. orientalis and T. latifolius var. angustifolius that were determined by gas chromatography mass spectrometry, oleic (29.1%) and palmitic (28.7%) acids were identified as the major components, respectively. The high antioxidant activity of the methanol extract of L. orientalis shows parallelism to its rosmarinic acid content. Besides, this extract showed medium anticholinesterase activity. The results of the present study proves that the L. orientalis might also be used as a food source due to its high phenolic acid content and strong antioxidant property.
Introduction
Genus Tragopogon L. which belongs to the Asteraceae family, consists of 84 species in the world and 21 species in Turkey.[Citation1] Tragopogon species are known as “Salsify” throughout the world. Tragopogon latifolius Boiss. is grown in south and east Transcaucasia; and its two varieties, T. latifolius Boiss. var. angustifolius Boiss. and T. latifolius Boiss. var. latifolius Boiss, were reported in Turkey.[Citation1,Citation2] T. latifolius var. angustifolius is used to heal wounds and as food source; and are known as Ispınk and Yemlik, in Anatolia.[Citation1,Citation2] T. latifolius var. angustifolius is consumed either raw or cooked.[Citation2] The previous studies on a Tragopogon species show that T. porrifolius L. contains gallic acid, catechin, epigallocatechin, and epigallocatechin gallate.[Citation3] In other reports it was mentioned that the ethanolic extract of T. pratensis L. which contains some polyphenolic compounds, displays antileukemic activity by inducing apoptosis on J-45.01 human acute T leukaemia cell line.[Citation4,Citation5] In some studies, volatile constituents and oleanane triterpenoids of T. porrifolius L. and T. pratensis L. were also determined.[Citation6–Citation8] According to the literature survey, chemical properties and biological activities of T. latifolius var. angustifolius have not been studied yet.
Genus Lycopsis L. which belong to the Boraginaceae family, is represented by a single species (Lycopsis orientalis L.) in Turkey and two species in the world.[Citation9] Being common in Balkan Peninsula, Anchusa arvensis L. Bieb. subsp. orientalis L. Nordh. is the synonym of L. orientalis in Norsk Flora.[Citation10] It is reported that A. arvensis subsp. orientalis, known as Frez, Kara dinding and Mıjık, is used as diuretic and food supplement.[Citation2] In Anatolia, same as T. latifolius var. angustifolius, L. orientalis is also consumed extensively as raw and cooked.[Citation2] In a prior study, the effects of the aqueous extract of L. orientalis on algaesthesis and skin ulcer in mice were investigated.[Citation11] Additionally, pyrrolizidine alkaloids were isolated from the different parts of A. arvensis (Syn. Lycopsis arvensis).[Citation12]
Recently, several scientific studies have been focused on the phenolic compounds of the edible plants having a number of pharmacological effects and the biological activities.[Citation13] From this point of view, in the current study, it was aimed to investigate and compare the results of L. orientalis and T. latifolius var. angustifolius as their cooking and consumption patterns are similar.[Citation2] To the best of our knowledge, there are no reports about the chemical properties and biological activities of L. orientalis and T. latifolius var. angustifolius in literature. In the present study, total phenolic-flavonoid contents, anticholinesterase, antioxidant, and antimicrobial activities of L. orientalis and T. latifolius var. angustifolius were studied. Besides, the fatty acid compositions of L. orientalis and T. latifolius var. angustifolius petroleum ether extracts were examined by gas chromatography mass spectrometry (GC-MS) technique. Finally, the chemical composition of their methanol extracts were determined qualitatively and quantitatively using liquid chromatography tandem mass spectrometry (LC-MS/MS).
MATERIAL AND METHODS
Chemicals and Instruments
Phenolic contents and fatty acid compositions of L. orientalis and T. latifolius var. angustifolius were determined by LC-MS/MS (Shimadzu, Kyoto, Japan) and GC-MS (Thermo Scientific Polaris Q) instruments, respectively. A Shimadzu UV spectrophotometer and BioTek Power Wave XS microplate reader (USA) were used for the activity assays. 2,2′-Azinobis (3-ethylbenzothiazoline-6-sulfonic acid) diammonium salt (ABTS; purity: 97.5%) and butylated hydroxytoluene (BHT; ≥99 %) were purchased from Merck (Germany); (L)-malic acid (95–100%), quercetin (95%), protocatechuic acid (97%), chrysin (97%), rutin (94%), hesperetin (95%), naringenin (95%), rosmarinic acid (96%), vanillin (99%), p-coumaric acid (98%), caffeic acid (98%), chlorogenic acid (95%), hyperoside (≥97%), myricetin (≥96%), coumarin (≥99%), kaempferol (≥97%), formic acid (≤100%), 2,2-diphenyl-1-picrylhydrazyl (DPPH; ≥95%), β-carotene (≥93%), linoleic acid (≥99%), Tween 40, pyrocathecol (≥99%), 5,5-dithiobis-(2-nitro benzoic acid; DTNB; ≥98%), copper (II) chloride dihydrate (CuCl2.2H2O; ≥99%), neocuproine (2,9-dimethyl-1,10-phenanthroline; ≥98%), Ethylenediaminetetraacetic acid (EDTA; ≥98%), acetylcholinesterase (AChE: from electric eel; Type-VI-S, EC 3.1.1.7, 425.84 U/mg), and butyrylcholinesterase (BChE: from horse serum; EC 3.1.1.8, 11.4 U/mg) were obtained from Sigma (Germany); quinic acid (98%), tr-aconitic acid (98%), 4-hydroxybenzoic acid (≥99%), fisetin (≥98%), α-tocopherol (≥95.5%), and acetylthiocholine iodide (≥98%) were from Aldrich (Germany); gallic acid (≥99%), tannic acid (puris), salicylic acid (≥99%), galanthamine hydrobromide (≥94%) were from Sigma-Aldrich (Germany); Folin Ciocalteu Phenol reagent was from Applichem (Germany); hesperidin (≥97%), luteolin (≥97%), apigenin (≥99%), rhamnetin (≥99%), and (butyrylthiocholine iodide (≥99%) were from Fluka (Germany).
Plant Material
The whole plants of Lycopsis orientalis L. and Tragopogon latifolius Boiss. var. angustifolius Boiss. were collected from south eastern part of Turkey (Malatya) in June 2012 and identified by Dr. Yeter Yeşil. These specimens have been stored at the Herbarium of Istanbul University (ISTE 9803 and ISTE 10012, respectively).
Preparation of Plant Extracts for LC-MS/MS
Ten grams of the powdered plants were extracted three times with MeOH (50 mL each) at room temperature for 24 h. Afterward, the extracts obtained were combined, filtered, and evaporated under low pressure. Dry filtrates were reconstituted in methanol at a concentration of 250 mg L–1 and filtered through the 0.2 µm PTFE filter prior to LC-MS/MS analysis.
Preparation of Plant Extracts for Biological Activities and GC-MS
Powdered form of the whole plant material was weighed (100 g) and sequentially macerated three times with petroleum ether (250 mL each), acetone (250 mL each), methanol (250 mL each), and water (250 mL each) at 25ºC for 24 h. After filtration, the solvent was evaporated to get the crude extracts. The yields of the petroleum ether extracts were calculated as T latifolius var. angustifolius petroleum ether extract (TLAP) 1.50%, L. orientalis petroleum ether extract (LOP) 0.80%, the acetone extracts as TLAA 1.65%, LOA 0.87%, the methanol extracts as TLAM 2.89%, LOM 2.60%, and the water extracts as TLAW 2.01%, LOW 1.29% (w/w).
GC/MS Conditions and Esterification of the Fatty Acids
Esterification of the petroleum ether extract and GC/MS procedure described by Ertas et al.[Citation14] were applied. Thermo Scientific Polaris Q GC-MS/MS was used.
Determination of Total Phenolic and Flavonoid Contents
Total phenolic and flavonoid contents expressed as pyrocatechol and quercetin equivalents, respectively, were determined as reported in the literature.[Citation15,Citation16] The following equations were used to calculate total phenolic and flavonoid contents of the extracts:
Antioxidant Activity (AA) Assays
β-Carotene-linoleic acid test system, DPPH free radical scavenging activity, ABTS cation radical decolorization and cupric reducing antioxidant capacity (CUPRAC) assays were carried out to determine the AA.[Citation17–Citation20]
β-Carotene bleaching method
One-half of a milligram of β-carotene in 1 mL of chloroform was added into linoleic acid (25 μL) and Tween 40 emulsifier (200 mg) mixture. After evaporating chloroform, 100 mL of distilled water saturated with oxygen was added followed by shaking, 160 μL of this mixture was transferred into different test tubes containing 40 μL of the sample solutions at different concentrations. The emulsion was added to each tube, the zero time absorbances of the values were read at 470 nm. The mixture was incubated for 2 h at 50°C.[Citation17] A blank, devoid of β-carotene, was prepared for back ground subtraction. α-Tocopherol was used as a standard. The bleaching rate (R) of β-carotene was calculated according to the following equation:
where, ln = natural log, a = absorbance at time zero, b = absorbance at time t (120 min).
The AA was calculated in terms of percent inhibition relative to the control, using following equation:
DPPH free radical scavenging activity method
DPPH solution, 0.1 mM 160 µL in methanol was added to 40 µL of sample solutions in methanol at different concentrations. After 30 min, the absorbance values were read at 517 nm. The DPPH free radical scavenging potential was calculated using the following equation:
AControl is the initial concentration of the DPPH•; and ASample is the absorbance of the remaining concentration of DPPH• in the presence of the extracts or positive controls.[Citation18]
ABTS cation radical decolorization assay
Seven milimolar ABTS in H2O was added to 2.45 mM potassium persulfate to produce ABTS•+ and solution was stored in the dark at 25°C for 12–16 h. The prepared solution was diluted with ethanol to get an absorbance of 0.700 ± 0.025 at 734 nm. ABTS•+ solution (160 µL) was added to each sample solution (40 μL) at different concentrations. After 30 min, the percentage inhibition at 734 nm was read for each concentration relative to a blank absorbance (methanol). The following equation was used to calculate the scavenging capability of ABTS•+:[Citation19]
CUPRAC method. Aliquots of 61 μL of 1.0 × 10−2 M copper (II) chloride, 61 μL of NH4OAc buffer (1 M, pH 7.0), and 61 μL of 7.5 × 10−3 M neocuproine solution were stirred, x μL sample solution (2.5, 6.25, 12.5, and 25 μL) and (67 − x) μL distilled water were added to reach the final volume 250 μL. The tubes were left to stand for 1 h. Afterward, the absorbance at 450 nm was measured against a reagent blank.[Citation20]
Anticholinesterase Activity
A spectrophotometric method developed by Ellman et al.[Citation21] was established to indicate the acetyl- and butyryl-cholinesterase inhibitory effects. Aliquots of 150 µL of 100 mM sodium phosphate buffer (pH 8.0), 10 μL of sample solution and 20 μL BChE (or AChE) solution were stirred and incubated for 15 min at 25ºC, then DTNB (10 μL) is added to mixture. In the next step, by the addition of butyrylthiocholine iodide (or acetylthiocholine iodide; 10 μL) the reaction was started. At the end, final concentration of the tested solutions was 200 μg/mL. BioTek Power Wave XS at 412 nm was used to monitor the hydrolysis of these substrates. The experiments were carried out in triplicate. Galanthamine was used as a reference compound. The percentages of inhibition was calculated by using the following equation:
Determination of Antimicrobial Activity and Minimum Inhibition Concentration (MIC)
The antimicrobial activities of extracts against different microorganisms were assessed according to inhibition zone diameter and MIC value.[Citation22,Citation23] Five different microorganisms including gram positive bacteria (Streptococcus pyogenes ATCC 19615 and Staphylococcus aureus ATCC 25923), gram negative bacteria (Pseudomonas aeruginosa ATCC 27853, Escherichia coli ATCC 25922), and yeast (Candida albicans ATCC 10231) which were purchased from Refik Saydam Sanitation Center (Turkey) were used for detecting the antimicrobial activity of the samples. The disc diffusion method was employed for this purpose. The minimum inhibitory concentration determined by the broth macrodilution method according to the National Committee for Clinical Laboratory Standards (NCCLS). Ampicillin and fluconazole were used as positive controls for bacteria and yeast, respectively.
Method Validation Parameters
In this study, 24 phenolic compounds (flavonoids, flavonoid glycosides, phenolic acids, a phenolic aldehyde, coumarin) and three non-phenolic organic acids that are widespread in edible plant materials were qualified and quantified in L. orientalis and T. latifolius var. angustifolius. Rectilinear regression equations and the linearity ranges of the studied standard compounds were given in . Correlation coefficients were found to be higher than 0.99. The limit of detection (LOD) and the limit of quantitation (LOQ) of the reported analytical method were shown in . For the studied compounds, LOD ranged from 0.05 to 25.8 µg/L and LOQ ranged from 0.17 to 85.9 µg/L ().[Citation24] Moreover, the recoveries of the phenolic compounds ranged from 96.9 to 106.2%.
Table 1 Analytical parameters of LC-MS/MS method
Estimation of Uncertainty
Ertas et al. report was used to evaluate and quantify the uncertainty sources of the applied method.[Citation25,Citation26] Purity of reference standards (pur), sample weights (w), calibration curves (cal), repeatability (rep), stock solutions (Css), and recovery (rec) were the sources used to calculate the uncertainties. To calculate the standard combined uncertainty the following Eq. (1) was applied:
Calibration curve and the purity of standards were defined as the main sources of uncertainty. In order to calculate expanded uncertainties at 95% confidence level (k:2), standard combined uncertainties were multiplied by two. Calculated uncertainties were given in .
Statistical Analysis
The total phenolic-flavonoid contents, antioxidant,anticholinesterase and antimicrobial activities results were shown as means ± standard deviation. The results were evaluated using an unpaired t-test and one way analysis of variance ANOVA. The differences were regarded as statistically significant at p < 0.05.
Result and Discussion
Through the consumption of edible plants, various phenolic acids take part in the protection of human body from oxidative damage diseases like coronary heart disease, cancers, and stroke, inflammation and cardiovascular diseases. There has been an increasing interest on phenolic compounds due to such properties of them. Not only the researchers but also the food manufacturers give importance to phenolic compounds owing to their strong antioxidant properties, ampleness in the human diet, and their probable role in the prevention of various diseases associated with oxidative stress.[Citation27] Therefore, the interest to the natural antioxidants sourced from fruits, vegetables, spices, and other plants has been increased.
Quantitative Analysis of Phenolic and Flavonoid Compounds by LC-MS/MS
In literature, the LC-MS/MS technique is extensively used in quantitative analysis of phenolic compounds.[Citation28,Citation29] Thus, an accurate quantitative method was developed on a mass spectrometer equipped with a triple quadrupole analyzer for the analyses of 27 compounds (). The methanol extracts of L. orientalis and T. latifolius var. angustifolius were analyzed to quantify these compounds by the mentioned method. For the detection of the studied compounds by multiple reaction monitoring (MRM), the specific fragmentation reactions were selected. These compounds were monitored by the transition from the specific deprotonated and protonated pseudomolecular ions to the corresponding fragment ions. Molecular ions and fragments observed in MS/MS, collision energies of these fragments and the quantified results for L. orientalis and T. latifolius var. angustifolius were presented in .
TABLE 2 Identification and quantification of compounds methanol extracts of T. latifolius var. angustifolius (TLAM) and L. orientalis (LOM) by LC-MS/MS
In the methanol extracts of L. orientalis and T. latifolius var. angustifolius, rosmarinic and quinic acids were found to be the most abundant compounds (21.11 and 11.46 mg g–1 extract, respectively; and –). Furthermore, rutin, hesperedin, hyperoside, and apigenin were also detected in the methanol extract of the studied plants ( and , ). Additionally, while quercetin, luteolin, and kaempferol were specified in the methanol extracts of T. latifolius var. angustifolius, they were not identified in the methanol extract of L. orientalis ( and , ). Malic, tr-aconitic, chlorogenic, protocatechuic, trans-caffeic, p-coumaric, 4-OH benzoic, and salicylic acids were determined in both studied methanol extracts. Rosmarinic acid was found in high amounts in the methanol extract of L. orientalis. Nevertheless, in the methanol extract of T. latifolius var. angustifolius, rosmarinic acid was not detected ( and ). It is the first time that the examined compounds were identified in Lycopsis species. Besides, the existance of most of these compounds were not reported in Tragopogon species before. In literature, there are few studies about chemical profile of Tragopogon species with HPLC. In a previous study, gallic acid (1.35 mg g–1), ferulic acid (0.20 mg g–1), rutin (0.09 mg g–1), resveratrol (0.014 mg g–1), sinapic acid (0.11 mg g–1), and caffeic acid (0.28 mg g–1) were detected in T. pratensis.[Citation4] In the current study, rutin (0.28 mg g–1) and caffeic acid (0.24 mg g–1) were identified in T. latifolius var. angustifolius. Moreover, while in the study of Kucekova et al.[Citation4] p-coumaric acid and quercetin were not detected in T. pratensis, they were detected in our study. Another report indicated the existence of gallic acid, catechin, epigallocatechin, and epigallocatechin gallate in T. porrifolius.[Citation3] In this respect, this report is significant in determining the chemical profile of Lycopsis and Tragopogon species.
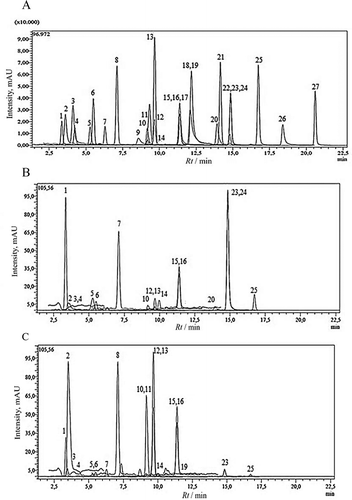
FIGURE 1 LC-MS/MS chromatogram of A: 250 ppb standard mix; B: T. latifolius var. angustifolius methanol extract; C: L. orientalis methnol extract. 1: Quinic acid; 2: Malic acid; 3: tr-Aconitic acid; 4: Gallic acid; 5: Chlorogenic acid; 6: Protocatechuic acid; 7: Tannic acid; 8: tr- caffeic acid; 9: Vanillin; 10: p-Coumaric acid; 11: Rosmarinic acid; 12: Rutin; 13: Hesperidin; 14: Hyperoside; 15: 4-OH Benzoic acid; 16: Salicylic acid; 17: Myricetin; 18: Fisetin;19: Coumarin; 20: Quercetin; 21: Naringenin; 22: Hesperetin; 23: Luteolin; 24: Kaempferol; 25: Apigenin; 26: Rhamnetin; 27: Chrysin.
Fatty Acid Composition by GC-MS
The fatty acid composition of the petroleum ether extracts were determined by GC-MS analyses. As it can be seen in , 14 components were identified; constituting 99.8% of the petroleum ether extract of T. latifolius var. angustifolius. The major constituents of the fatty acids obtained from the petroleum ether extract were identified as palmitic (C16:0; 28.7%), oleic (C 18:1 omega-9; 21.0%), linoleic (C18:2 omega-6; 20.6%) and linolenic acids (C18:3 omega-3; 12.1%). Additionally, 15 components were identified, constituting 99.9% of the petroleum ether extract of L. orientalis. The major constituents of the fatty acid composition obtained from the petroleum ether extract were characterized as oleic (29.1%), palmitic (17.6%), linoleic acids (17.2%), and 1-octadecanol (8.2%; ). When the fatty acid compositions of T. latifolius var. angustifolius and L. orientalis were compared, they contain palmitic acid (28.7 and 17.6%, respectively), oleic acid (21.0 and 29.1%), 1-octadecanol (and 8.2%), and arachidic acid (1.9 and 6.3%). This is the first report on the fatty acid compositions of L. orientalis and T. latifolius var. angustifolius. Also, according to our knowledge there is no report on fatty acid composition of Tragopogon and Lycopsis species in literature.
TABLE 3 GC-MS Analysis of the petroleum ether of T. latifolius var. angustifolius (TLAP) and L. orientalis (LOP)
There are several studies on fatty acid composition of Asteraceae and Boraginaceae families in literature. The major components of the fatty acid composition were identified as palmitic and linoleic acids for Asteraceae family;[Citation30,Citation31] and oleic, linoleic, and linolenic acids for Boraginaceae family.[Citation32,Citation33] Our results are in consistent with literature. In the current study, palmitic and oleic acids were found to be the main components of the fatty acid profile of T. latifolius var. angustifolius, a member of Asteraceae family, and L. orientalis, a member of Boraginaceae family, respectively.
AA and Total Phenolic-Flavonoid Content
The AA of the petroleum ether (TLAP and LOP, respectively), acetone (TLAA and LOA), methanol (TLAM and LOM), and water (TLAW and LOW) extracts prepared from the whole plants of T. latifolius var. angustifolius and L. orientalis were studied using β-carotene bleaching, DPPH free radical scavenging, CUPRAC, and ABTS cation radical decolorization assays. To our knowledge, there is no study about L. orientalis and T. latifolius var. angustifolius regarding these antioxidant activities and total phenolic-flavonoid contents in literature. In the crude methanol extracts prepared from the whole plants of L. orientalis and T. latifolius var. angustifolius, total phenolic and flavonoid amounts were determined expressing as pyrocatechol and quercetin equivalents, respectively (y = 0.0164 pyrocatechol (μg) + 0.0266, R2 = 0.9969 and y = 0.1519 quercetin (μg) – 0.1294, R2 = 0.9986). The total phenolic and flavonoid contents of the LOM and TLAM extracts were determined as 124.70 ± 1.74 mg PEs per g dry extract and 49.04 ± 0.09 mg QEs per g dry extract, respectively. The total phenolic contents were detected to be higher than total flavonoid contents ().
TABLE 4 Antioxidant activity*, total phenolic-flavonoid contents* and anticholinesterase activity* of T. latifolius var. angustifolius and L. orientalis extracts, BHT, α-TOC, and galantamine
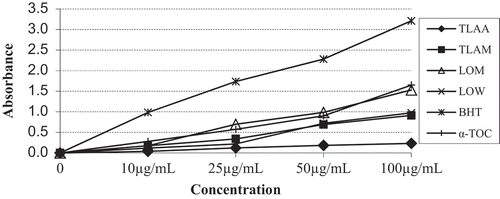
As indicated in , while the LOM extract showed good lipid peroxidation activity (IC50: 38.46 ± 1.30 µg mL–1) in β-carotene bleaching method, the TLAA, TLAM, LOP, and LOA extracts exhibited moderate lipid peroxidation activity (IC50: 105.32 ± 0.98, 112.61 ± 0.67, 109.39 ± 0.31, and 89.49 ± 1.38 µg mL–1, respectively) in the same method. The other tested extracts indicate weak lipid peroxidation activity in β-carotene bleaching method. As it can be observed in , the LOM and LOW extracts showed good activity (IC50: 50.82 ± 0.33 and 59.31 ± 1.18 µg mL–1, respectively) in DPPH free radical scavenging method. Besides, the TLAM, TLAW, and LOA extracts showed moderate activity (IC50: 117.72 ± 0.91, 102.60 ± 1.26, and 89.27 ± 1.36 µg mL–1, respectively) in DPPH free radical scavenging method. Other studied three extracts had weak activities in DPPH free radical scavenging activity test. As shown in , the TLAA, TLAM, TLAW, LOA, LOM, and LOW extracts showed the following IC50 values in ABTS cation radical scavenging assay; 92.61 ± 0.37, 55.51 ± 0.62, 16.61 ± 0.69, 27.81 ± 0.17, 7.62 ± 0.09, and 8.51 ± 0.12 µg mL–1, respectively. Particularly, the LOM and LOW extracts indicate very strong activity in ABTS cation radical scavenging assay. Also, these extracts exhibited higher activity than α-tocopherol (IC50: 9.54 ± 0.09 µg mL–1) and BHT (IC50: 10.31 ± 0.14 µg mL–1), which were used as standards in the ABTS cation radical scavenging assay. The other tested two extracts had weak activity in ABTS cation radical scavenging assay. The LOM extract and α-tocopherol exhibited 1.53 and 1.65 absorbance in CUPRAC at 100 µg mL–1, respectively ().The other tested extracts showed weak or no activity in CUPRAC (data not shown). After examining the antioxidant properties of the eight extracts, the LOM extract showed highest activity among the studied methods. This high AA of LOM extract might be stemmed from high total phenolic content or high rosmarinic acid amount that is known with its high antioxidant capacity.[Citation34]
Anticholinesterase Activity
All of the extracts showed no or weak inhibitory activity against acetyl- and butyryl-cholinesterase enzymes, except from LOM extract which had moderate effect, at 200 µg mL–1 (). According to the literature research, this is the first study on the anticholinesterase activity of Tragopogon and Lycopsis species.
Determination of Antimicrobial Activity and MIC
The antimicrobial activities of extracts against different microorganisms were assessed according to inhibition zone diameter and MIC value. Results were presented in . The petroleum ether and water extracts showed no activity against the five tested microorganisms (data was not shown). The acetone and methanol extracts were active on all microorganisms with different zone diameters indicating weak antimicrobial activity (inhibition zone <12). Only the TLAM extract possesses moderate activity (inhibition zone <20–12) against C. albicans (14 mm inhibition zone diameter and 110 µg mL–1 MIC value). The lowest MIC value was recorded by the LOM extract against C. albicans (25 ± 0.4 µg mL–1).
TABLE 5 Zones of growth inhibition (mm) and MIC values showing the antimicrobial activities of the extracts compared to positive controls
Conclusion
This report represents the first study on chemical compositions and biological activities of L. orientalis and T. latifolius var. angustifolius. The antioxidant capacity of LOM extract was higher than the other seven extracts. The reason why LOM was the most active of all eight extracts tested for four antioxidants methods used, could be related to its high total phenolic content or high amount of rosmarinic acid that have strong AA.[Citation34] According to our study, quinic and rosmarinic acids (11.46 and 21.11 mg g–1 extract, respectively) were found to be the most abundant phenolic compounds in TLAM and LOM extracts. Although the total flavonoid content of TLAM extract was the richest, rutin (0.28 mg g–1 extract), hesperedin (0.29 mg g–1 extract), hyperoside (0.96 mg g–1 extract), apigenin (0.036 mg g–1 extract), quercetin (0.032 mg g–1 extract), luteolin (0.52 mg g–1 extract), and kaempferol (0.53 mg g–1 extract) were in low amount and the other flavonoids were not detected in this extract. Thus, the high total flavonoid content of this extract could be related to some other flavonoids that we have not studied yet. As a result, rich total phenolic content and high antioxidant capacity of the LOM necessitates further studies in this field. The results of the present study showed that LOM extract can also be used as a food source due to its high phenolic acid content and strong antioxidant properties.
Acknowledgments
The authors are thankful to Dicle University Science and Technology Research and Application Center (DUBTAM) for its support in this study.
REFERENCES
- Coskuncelebi, K.; Goztepe, M.; Tragopogon, L. In: The Plant List of Turkey/Vascular Plants; Guner, A.; Aslan, S.; Ekim, T.; Vural, M.; Babac, M.T.; Eds.; Nezahat Gokyigit Botanic Garden and The Publication of Flora Researches Association: Istanbul, Turkey, 2012, 211–212 pp.
- Yesil, Y.; Akalin, E. The Use of Wild Edible Plants in Kürecik (Akçadağ/Malatya). Journal of Faculty of Pharmacy of Istanbul 2010–2011, 41, 90–103.
- Spina, M.; Cuccioloni, M.; Sparapani, L.; Acciarri, S.; Eleuteri, A.M.; Fioretti, E.; Angeletti, M. Comparative Evaluation of Flavonoid Content in Assessing Quality of Wild and Cultivated Vegetables for Human Consumption. Journal of The Science of Food and Agriculture 2008, 88, 294–304.
- Kucekova, Z.; Mlcek, J.; Humpolicek, P.; Rop, O.; Valasek, P.; Saha, P. Phenolic Compounds from Allium schoenoprasum, Tragopogon pratensis, and Rumex acetosa and Their Antiproliferative Effects. Molecules 2011, 16, 9207–9217.
- Wegiera, M.; Smolarz, H.D.; Jedruch, M.; Korczak, M.; Kopron, K. Cytotoxic Effect of Some Medicinal Plants from Asteraceae Family on J-45.01 Leukemic Cell Line-Pilot Study. Acta Poloniae Pharmaceutica 2012, 69, 263–268.
- Miyase, T.; Kohsaka, H.; Ueno, A. Tragopogonosides-A-I, Oleanane Saponins from Tragopogon Pratensis. Phytochemistry 1992, 31, 2087–2091.
- Formisano, C.; Rigano, D.; Senatore, F.; Bruno, M., Rosselli, S. Volatile Constituents of the Aerial Parts of White Salsify (Tragopogon porrifolius L., Asteraceae). Natural Product Research 2010, 24, 663–668.
- Riu-Aumatell, M.; Vargas, L.; Vichi, S.; Guadayol, J.M.; Lopez-Tamames, E.; Buxaderas, S. Characterization of Volatile Composition of White Salsify (Tragopogon porrifolius L.) by Headspace Solid-Phase Microextraction (HS-SPME) and Simultaneous Distillation-Extraction (SDE) Coupled to GC-MS. Food Chemistry 2011, 129, 557–564.
- Koruklu, S.T. Lycopsis L., In: The Plant List of Turkey/Vascular Plants. Guner, A.; Aslan, S.; Ekim, T.; Vural, M.; Babac, M.T.; Eds.; Nezahat Gokyigit Botanic Garden and The Publication of Flora Researches Association: Istanbul, Turkey, 2012, 230 p.
- Nordhagen, R. Lycopsis L. In: Lid, J.; Lid, D.T.; Eds.; Norsk Flora, Oslo: Aschehoug, 1940, 526 p.
- Hai-Gang, Z.; Wen-Xiao, S. Effects of Aqueous Extract from Lycopsis orientalis linn on Algaesthesis and Skin Ulcer in Mice. Journal of Pharmacological Sciences 2009, 109, 149.
- El-Shazly, A.; Domiaty, M.; Witte, L.; Wink, M. Pyrrolizidine Alkaloids in Members of the Boraginaceae from Sinai (Egypt). Biochemical Systematics and Ecology 1998, 26, 619–636.
- Ramos-Escudero, F.; Morales, M.T.; Agustín, G. Characterization of Bioactive Compounds from Monovarietal Virgin Olive Oils: Relationship Between Phenolic Compounds-Antioxidant Capacities. International Journal of Food Properties 2015, 18, 348–358.
- Ertaş, A.; Boğa, M.; Haşimi, N.; Yeşil, Y.; Gören, A.C.; Topcu, G.; Kolak, U. Antioxidant, Anticholinesterase, Antimicrobial Activities, and Fatty Acid Constituents of Achillea cappadocica Hausskn. et Bornm. Turkish Journal of Chemistry 2014, 38, 592–599.
- Slinkard, K.; Singleton, V.L. Total Phenol Analyses: Automation and Comparison With Manual Methods. American Journal of Enology and Viticulture 1977, 28, 49–55.
- Moreno, M.I.N.; Isla, M.I.; Sampietro, A.R.; Vattuone, M.A. Comparison of the Free Radical-Scavenging Activity of Propolis from Several Regions of Argentina. Journal of Ethnopharmacology 2000, 71, 109–114.
- Miller, H.E. A Simplified Method for the Evaluation of Antioxidants. Journal of the American Oil Chemists’ Society 1971, 48, 91.
- Blois, M.S. Antioxidant Determinations by the Use of a Stable Free Radical. Nature 1958, 181, 1199–1200.
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant Activity Applying An Improved ABTS Radical Cation Decolorization Assay. Free Radical Biology and Medicine 1999, 26, 1231–1237.
- Apak, R.; Guçlu, K.; Ozyurek, M.; Karademir, S.E. Novel Total Antioxidant Capacity Index for Dietary Polyphenols and Vitamins C and E, Using Their Cupric Ion Reducing Capability in the Presence of Neocuproine: CUPRAC Method. Journal of Agricultural Food Chemistry 2004, 52, 7970–7981.
- Ellman, G.L.; Courtney, K.D.; Andres, V.; Featherstone, R.M. A New and Rapid Colorimetric Determination of Acetylcholinesterase Activity. Biochemical Pharmacology 1961, 7, 88–95.
- NCCLS (National Committee for Clinical Laboratory Standards). Performance Standards for Antimicrobial Disk Susceptibility Test, 6th Ed.; Approved Standard, Wayne, PA, M2-A6, 1997.
- NCCLS (National Committee for Clinical Laboratory Standards). Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically; Approved Standard, 8th Ed.; Wayne, PA, M08-A8, 2009.
- Ertas, A.; Yilmaz, M.A.; Firat, M. Chemical Profile by LC–MS/MS, GC/MS and Antioxidant Activities of the Essential Oils and Crude Extracts of Two Euphorbia Species. Natural Product Research 2015, 29, 529–534. DOI:10.1080/14786419.2014.954113.
- Ertas, A.; Boga, M.; Yılmaz, M.A.; Yesil, Y.; Hasimi, N.; Kaya, M.S.; Temel, H.; Kolak, U. Chemical Compositions by Using LC-MS/MS and GC-MS and Biological Activities of Sedum sediforme (Jacq.) Pau. Journal of Agricultural and Food Chemistry 2014, 62, 4601–4609.
- Goren, A.C.; Bilsel, G.; Bilsel, M.; Yenisoy-Karakas, S.; Karakas, D. Simple High-Performance Liquid Chromatographic Method for Determination of Atropine and Obidoxime in a Parenteral Injection Device. Journal of Chromatography A 2004, 1057, 237–239.
- Choe, J.H.; Kim, H.Y.; Kim, Y.J.; Yeo, E.J. Antioxidant Activity and Phenolic Content of Persimmon Peel Extracted with Different Levels of Ethanol. International Journal of Food Properties 2014, 17, 1779–1790.
- Sulaiman, C.T.; Thushar, K.V.; George, S.; Balachandran, I. Phenolic Characterisation of Selected Salacia Species Using LC-ESI-MS/MS Analysis. International Journal of Food Properties 2014, 25, 1021–1024.
- Koubaier, H.B.H.; Snoussi, A.; Essaidi, I.; Chaabouni, M.M.; Thonart, P.; Bouzouita, N. Betalain and Phenolic Compositions, Antioxidant Activity of Tunisian Red Beet (Beta Vulgaris L. Conditiva) Roots and Stems Extracts. International Journal of Food Properties 2014, 17, 1934–1945.
- Tel, G.; Ozturk, M.; Duru, M.E.; Dogan, B.; Harmandar, M. Fatty Acid Composition, Antioxidant, Anticholinesterase, and Tyrosinase Inhibitory Activities of Four Serratula Species from Anatolia. Records of Natural Products 2013, 7, 86–95.
- Tekeli, Y.; Sezgin, M.; Aktumsek, A.; Guler, G.O.; Sanda, M.A. Fatty Acid Composition of Six Centaurea Species Growing in Konya, Turkey. Natural Product Research 2010, 24, 1883–1889.
- Ozcan, T. Analysis of the Total Oil and Fatty Acid Composition of Seeds of Some Boraginaceae Taxa from Turkey. Plant Systematics and Evolution 2008, 274, 143–153.
- Bagcı, E.; Bruehl, L.; Aitzetmuller, K.; Altan, Y. Fatty Acid and Tocochromanol Patterns of Some Turkish Boraginaceae—A Chemotaxonomic Approach. Nordic Journal of Botany 2003, 22, 719–726.
- Erkan, N.; Ayranci, G.; Ayranci, E. Antioxidant Activities of Rosemar (Rosmarinus Officinalis L.) Extract, Blakseed (Nigella Sativa L.) Essential Oil, Carnosic Acid, Rosmarinic Acid, and Sesamol. Food Chemistry 2008, 110, 76–82.