Abstract
A highly reliable high-performance liquid chromatography method was developed and validated for determining quality of extracts from the leaves of Dipsacus sativus (Linn.) Honck. Under optimum conditions, the chromatography profile of eight compounds was collected, with compound saponarin as marker. The analysis results indicated that saponarin and isovitexin were the main active components. Pharmacological experiments proved that extracts from the leaves showed significant antioxidant activity. This work could serve to validate the usefulness of high-performance liquid chromatography analyses in traditional Chinese medicine.
INTRODUCTION
Traditional Chinese medicine (TCM), based on medicinal use of traditional herbs, is still used by a large population of Chinese people for the treatment of various diseases. Despite its continued use over many centuries, TCM still cannot be officially recognized by many countries due to lack of awareness about what active compounds are in it and quality control, as well as lack of sufficient safety and efficacy data.[Citation1] Even for those active components that have already been identified, they vary greatly in both their content as well as physical and chemical properties in different herbs.[Citation2,Citation3]
High-performance liquid chromatography (HPLC) analysis is widely used in the identification and quantitation of components in different kinds of samples.[Citation4,Citation5] During the past decade, the HPLC analysis technique has been accepted as a useful tool for identification and quantitation of components in herbal medicines.[Citation6] Multiple chromatographic techniques have also been used to well-characterize the total chemical characteristics of a given TCM.[Citation7,Citation8] The HPLC analysis technique has attracted great attention in recent years as an important quality control method due to its advantages of versatility, simultaneous multi-component analysis, high speed, precision, high reliability, automation, many choices of detectors, small sample size requirement, and reasonable cost.[Citation9]
The complexity of TCM presents a great challenge for ensuring quality control.[Citation6,Citation10,Citation11] Future research should focus on its analytical and pharmacological studies to allow for better control. D. sativus (Linn.) Honck, a plant of the Dipsacus family, is a promising herbal medicine source. It was originally cultured in Europe and first imported to China from Japan in 1929. In folk medicine, its fruits are used for cotton fleece production and its leaves are used as tea for treatment of cardiovascular disease. Currently there are no reports on the characteristics of D. sativus (Linn.) Honck. In the current study, we established the first HPLC analysis of D. sativus (Linn.) Honck. We then sought to investigate the pharmacological activity of its active components and to explore its potential values in TCM.
MATERIALS AND METHODS
Chemicals and Materials
D-galactose was purchased from Sinopharm Chemical Reagent Co., Ltd. (Shanghai, China). Commercial kits used for detecting superoxide dismutase (SOD) activity and malonaldehyde (MDA) content were purchased from Jiancheng Institute of Biotechnology (Nanjing, China). HPLC grade water was prepared using a Milli-Q50 SP Reagent Water System. Institute for Cancer Research (ICR) outbred mice (two-months old, 20 ± 2 g) were obtained from Zhejiang Academy of Medical Science (Zhejiang, China). Animal housing and all experimental procedures were performed by following the Provisions and General Recommendations of Chinese Experimental Animal Administration Legislation. D. sativus (Linn.) Honck was obtained from Yuyao, Zhejiang province, China (with moist climatic condition), and the leaves and stems were collected from 2-year-old D. sativus (Linn.) Honck plants in September 2011. A voucher specimen was deposited in the Herbarium of the College of Biomedical Engineering and Instrument Sciences in Zhejiang University (Zhejiang, China).
HPLC Analysis
The HPLC system consisted of a Waters 2695 separation module and a Waters 2998 photodiode array detector was used. Chromatographic separation was performed using a Waters column C18 (250 mm, 4.6 mm, 5 µm) with a sample injection volume of 20 µL, and an elution flow rate of 1.0 mL/min using gradient run. The mobile phase consisted of methanol and acetic acid in water at a ratio of 15:85 (v/v) was used during equilibration. The gradient profile was established as follows: The ratio of methanol and acetic acid was 25:75 (v/v) for the first 10 min, and then increased from 40:60 (v/v) to 70:30 (v/v) over 11–31 min.
Extraction Procedure
Two grams of dried D. sativus (Linn.) Honck leaves were extracted to remove their pigment first using 100 mL of petroleum (65°C). This extract was refluxed and further extracted using 100 mL methanol until colorless. It was then stored at –20°C until later analysis. When analyzed, the sample was dissolved in methanol and centrifuged (10,000 rpm, 10 min) before being injected into the HPLC system.
Pharmacological Experiments
D-galactose was dissolved in 0.9% saline (5%, w/v). ICR mice were housed five per cage at room temperature (25 ± 2°C) with a standard (12/12 h) light/dark cycle. They were allowed ad libitum access to food and water during the entire experiment. After a 1-week acclimatization phase to their cage, mice were randomly divided into three groups: control (n = 10), model (n = 10), and D. sativus (Linn.) Honck (n = 15) groups. The model and D. sativus (Linn.) Honck groups were subcutaneously injected with D-galactose at a dose of 1250 mg/kg once daily for 6 weeks. The control group was treated with the same volume of vehicle (0.9% saline) once daily for 6 weeks. From the first day after the injection of D-galactose, the mice in the D. sativus (Linn.) Honck group were also orally given extracts of D. sativus (Linn.) Honck leaves at a dose of 300 mg/kg per day. The control and model groups were given the same volume of vehicle (0.9% saline). Twelve h after the last dose, the retro-orbital blood samples were harvested and centrifuged (3000 r/min for 15 min) using a hypothermia super centrifuge, and serum was collected. Serum SOD and MDA levels were then measured using commercially available kits by following the manufacturer’s instructions.
RESULTS AND DISCUSSION
Chromatographic Separation and Selection of Reference Peak
Ultra-violet (UV) chromatograms obtained from D. sativus (Linn.) Honck leaf extracts were obtained by scanning from 254–365 nm. Our results showed that the peaks were moderate and adequately separated, while the baseline was steady and stable at 337.1 nm (). After examining the solvent effects on the chromatographic peaks using various binary mobile phases with different gradients, we found that the mobile phase of methanol and acetic acid in water was the most appropriate one for this chromatographic analysis and 40°C was found to be the optimum temperature for the procedure.
FIGURE 1 Fingerprint chromatogram of the extract from D. sativus obtained from Zhejiang (saponarin was used as the standard reference compound).
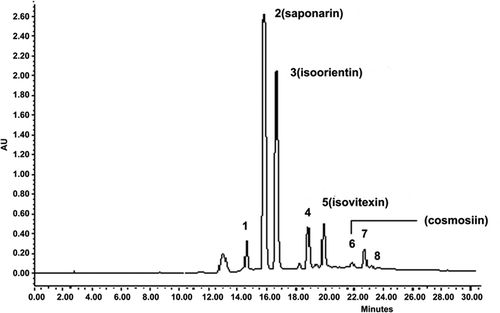
Saponarin is one of the active components in D. sativus (Linn.) Honck and the retention time and peak area of saponarin were stable and reproducible, so it was selected as the reference standard in our study. Under our experimental chromatographic conditions, the reference marker peak for saponarin had an area approximately 38% of the total peak area (). The marker peak, relative retention time, and relative peak area can be calculated more reliably than simply assessing the retention time and area of a single peak. By carefully analyzing the HPLC profiles of these samples, eight peaks with reasonable heights and good resolutions were selected as characteristic peaks for the identification of D. sativus (Linn.) Honck ().
Validation and Quantitative Analysis
After carefully optimizing the HPLC conditions, we analyzed two pharmacologically active marker compounds saponarin and isovitexin. HPLC chromatograms of quantification of the two compounds are shown in . The results showed a regression coefficient over 0.9998. The peak areas of saponarin and isovitexin occupied 65% of the total area. In other words, they are the main components of D. sativus (Linn.) Honck and possibly the active components.
TABLE 1 Regression data of the two main components
Experimental Stability, Precision, Reproducibility, and Recovery
The stability of the HPLC analysis was assessed by analyzing the same samples at 0, 2, 4, 6, 8, and 12 h. The precision of the HPLC analysis method was examined by five repeated extractions of D. sativus (Linn.) Honck leaves. The reproducibility of the HPLC analysis was evaluated by assaying the five samples according to the protocol described. Saponarin was added to the D. sativus (Linn.) Honck samples (1:1) and the recovery of the HPLC analysis was repeatedly (6×) measured as previously described. As shown in , the results indicated that the relative standard deviation (RSD) was less than 3%, demonstrating that this quantification method is suitable for the quantitative analysis of D. sativus (Linn.) Honck. The HPLC method was thus validated for its limits of detection (LOD) and quantification (LOQ). The LOD and LOQ values were calculated based on the standard deviation of the response and the slope obtained from the linearity plot of each marker compound. The LOD was established as a signal six times higher than noise. The LODs of saponarin and isovitexin were determined to be 0.45 and 0.51 mg/mL, respectively. Meanwhile, the LOQs of saponarin and isovitexin were determined to be 1.21 and 0.93 mg/mL, respectively.
TABLE 2 Stability, precision, repeatability, and recovery data of the proposed high-performance liquid chromatography method
Antioxidant Activities
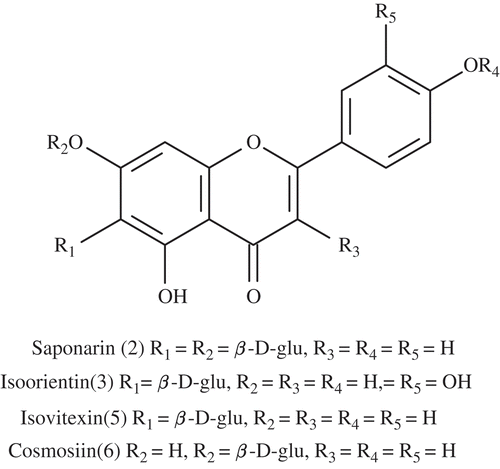
presents the levels of SOD and MDA measured in peripheral blood. The results showed that the content of SOD in the D. sativus (Linn.) Honck group was significantly higher (p < 0.01) than those in control and the model groups. While the MDA levels were decreased after treatment with extracts of D. sativus (Linn.) Honck. These results suggest that extracts of D. sativus (Linn.) Honck could significantly alter the levels of SOD and MDA in mice, indicating an antioxidant role for these extracts. We obtained four compounds from the leaves of D. sativus (Linn.) Honck and their structures are depicted in . Three of them are flavone-C-glycosides and they occupied more than 65% of the total peak area. The compounds also have flavonoids properties, which might be the reason for their effectiveness.
TABLE 3 Effects of D. sativus (Linn.) Honck extract on the contents of SOD and MDA in the blood plasma of mice (mean ± SD)
Both flavones and flavone-C-glycosides are effective free-radical scavengers and antioxidants.[Citation12,Citation13] Flavonoids are widely distributed in plants and have been referred to as “nature’s biological response modifiers.” Flavonoids are well known for their antioxidant activity.[Citation14–Citation16] Several lines of experimental evidence have demonstrated their inherent ability to enhance the body’s defensive system to fight against viruses and carcinogens.[Citation17,Citation18] It has been suggested that flavonoids may delay or prevent the onset of diseases induced by free radicals, such as cancer.[Citation19] They could also inhibit low density lipoprotein (LDL) oxidation by free radicals[Citation20] and have negative correlation with incidence of coronary heart disease.[Citation21] Our study opens a new gate for investigation of D. sativus (Linn.) Honck, especially for its pharmacological activities study with a focus on its flavonoids components, which might shed new light on TCM study.
CONCLUSION
In this study, we developed a highly reliable HPLC analysis approach to analyze the components of D. sativus (Linn.) Honck with saponarin as a marker compound. The validation of precision, stability, reproducibility, and recovery confirmed that this HPLC analysis method used for analysis of D. sativus (Linn.) Honck components is adequate, valid, and applicable. The results in this study indicated that extracts from D. sativus (Linn.) Honck leaves have significant antioxidant activities. Our study might shed new light on TCM investigation using HPLC analyses technique.
FUNDING
This work was supported by the National Science Foundation of China (grant no. U1303122), Lishui Science and Technology Innovation team (grant no. 2012CTXD09), National Science Program for Zhejiang Leading Team of S&T Innovation (grant no. 2012R10044-10), and Technology Major Project of China (Grant No. 2013ZX09103002-005).
Additional information
Funding
REFERENCES
- Zhang, L.; Yan, J.; Liu, X.; Ye, Z.; Yang, X.; Meyboom, R.; Chan, K.; Shaw, D.; Duez, P. Pharmacovigilance Practice and Risk Control of Traditional Chinese Medicine Drugs in China: Current Status and Future Perspective. Journal of Ethnopharmacology 2012, 140(3), 519–525.
- Yang, C.S.; Landau, J.M.; Huang, M.-T.; Newmark, H.L. Inhibition of Carcinogenesis by Dietary Polyphenolic Compounds. Annual Review of Nutrition 2001, 21(1), 381–406.
- Wang, M.; Lamers, R.J.A.; Korthout, H.A.; van Nesselrooij, J.H.; Witkamp, R.F.; van der Heijden, R.; Voshol, P.J.; Havekes, L.M.; Verpoorte, R.; van der Greef, J. Metabolomics in the Context of Systems Biology: Bridging Traditional Chinese Medicine and Molecular Pharmacology. Phytotherapy Research 2005, 19(3), 173–182.
- Li, W.; Xu, K.; Xiao, R.; Yin, G.; Liu, W. Development of An HPLC-Based Method for the Detection of Aflatoxins in Pu-Erh Tea. International Journal of Food Properties 2014, 18(4), 842–848.
- Zareen, S.; Ismail, I.S.; Shaari, K.; Yusof, K.N.; Akhtar, M.N. Quantitative HPLC Analysis of Benzene Derivatives of Melicope Ptelefolia Leaves. International Journal of Food Properties 2013, 16(8), 1830–1838.
- Zhang, Q.; Ye, M. Chemical Analysis of the Chinese Herbal Medicine Gan-Cao (Licorice). Journal of Chromatography A 2009, 1216(11), 1954–1969.
- Li, Y.; Hu, Z.; He, L. An Approach to Develop Binary Chromatographic Fingerprints of the Total Alkaloids from Caulophyllum Robustum by High Performance Liquid Chromatography/Diode Array Detector and Gas Chromatography/Mass Spectrometry. Journal of Pharmaceutical and Biomedical Analysis 2007, 43(5), 1667–1672.
- Fan, X.-H.; Cheng, Y.-Y.; Ye, Z.-L.; Lin, R.-C.; Qian, Z.-Z. Multiple Chromatographic Fingerprinting and Its Application to the Quality Control of Herbal Medicines. Analytica Chimica Acta 2006, 555(2), 217–224.
- Goyal, S.S. Use of High Performance Liquid Chromatography for Soil and Plant Analysis. Communications in Soil Science and Plant Analysis 2002, 33(15–18), 2617–2641.
- Su, J.; Zhang, C.; Zhang, W.; Shen, Y.-H.; Li, H.-L.; Liu, R.-H.; Zhang, X.; Hu, X.-J.; Zhang, W.-D. Qualitative and Quantitative Determination of the Major Coumarins in Zushima by High Performance Liquid Chromatography with Diode Array Detector and Mass Spectrometry. Journal of Chromatography A 2009, 1216(11), 2111–2117.
- van Beek, T.A.; Montoro, P. Chemical Analysis and Quality Control of Ginkgo Biloba Leaves, Extracts, and Phytopharmaceuticals. Journal of Chromatography A 2009, 1216(11), 2002–2032.
- Okawa, M.; Kinjo, J.; Nohara, T.; Ono, M. DPPH (1, 1-Diphenyl-2-Picrylhydrazyl) Radical Scavenging Activity of Flavonoids Obtained from Some Medicinal Plants. Biological and Pharmaceutical Bulletin 2001, 24(10), 1202–1205.
- Lu, Y.; Foo, L.Y. Antioxidant Activities of Polyphenols from Sage (Salvia Officinalis). Food Chemistry 2001, 75(2), 197–202.
- Wei, H.; Li, L.; Song, Q.; Ai, H.; Chu, J.; Li, W. Behavioural Study of the D-Galactose Induced Aging Model in C57BL/6J Mice. Behavioural Brain Research 2005, 157(2), 245–251.
- Enayat, S.; Banerjee, S. Comparative Antioxidant Activity of Extracts from Leaves, Bark, and Catkins of Salix Aegyptiaca sp. Food Chemistry 2009, 116(1), 23–28.
- Schwarz, M.; Rodríguez, M.; Martínez, C.; Bosquet, V.; Guillén, D.; Barroso, C.G. Antioxidant Activity of Brandy de Jerez and Other Aged Distillates, and Correlation with Their Polyphenolic Content. Food Chemistry 2009, 116(1), 29–33.
- Sanchez, I.; Gómez‐Garibay, F.; Taboada, J.; Ruiz, B. Antiviral Effect of Flavonoids on the Dengue Virus. Phytotherapy Research 2000, 14(2), 89–92.
- Moon, Y.J.; Wang, X.; Morris, M.E. Dietary Flavonoids: Effects on Xenobiotic and Carcinogen Metabolism. Toxicology in Vitro 2006, 20(2), 187–210.
- Galati, G.; O’Brien, P.J. Potential Toxicity of Flavonoids and Other Dietary Phenolics: Significance for Their Chemopreventive and Anticancer Properties. Free Radical Biology and Medicine 2004, 37(3), 287–303.
- Hirano, R.; Sasamoto, W.; Matsumoto, A.; Itakura, H.; Igarashi, O.; Kondo, K. Antioxidant Ability of Various Flavonoids Against DPPH Radicals and LDL Oxidation. Journal of Nutritional Science and Vitaminology 2001, 47(5), 357–362.
- Hertog, M.G.; Feskens, E.J.; Kromhout, D.; Hollman, P.; Katan, M. Dietary Antioxidant Flavonoids and Risk of Coronary Heart Disease: The Zutphen Elderly Study. The Lancet 1993, 342(8878), 1007–1011.