 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Effects of various roasting conditions on antioxidant properties of five Theobroma cacao L. varieties were investigated. The cocoa beans were roasted at four different temperatures (110–150°C) and three different air humidities (0.3–5.0%). The raw cocoa beans were characterized by high antioxidant activities. The antioxidant properties of the roasted cocoa beans varied markedly among the analyzed cultivars and geographical regions and were affected by roasting conditions. Generally, cocoa beans of the cv. Forastero from Brazil exhibited higher total phenolic content, free radical scavenging activity, and metal chelating ability than samples of the other analyzed cocoa varieties. Roasting at 110°C caused negligible changes in total phenolics content and antioxidant activity of cocoa beans, while almost all samples tended to have lower antioxidant potential when roasting temperature increased. The air humidity used in roasting did not affect the total phenolics content and antioxidant activity for lowest roasting temperature (110°C). Moreover, the obtained results revealed that thermal processing at the higher temperatures and elevated air humidity resulted in the higher antioxidant capacities. It was also found that the ferrous ion chelating activity of cocoa beans increased with the roasting temperature (in the range from 110 to 150°C), with the exception of cv. Trinitario from Papua New Guinea. The data showed that roasting at lower temperatures with humid air are more favorable in terms of preserving the bioactivity of roasted cocoa beans.
Introduction
Cocoa beans (Theobroma cacao L.) are used for manufacturing of products, which are highly popular and widely consumed around the world, such as cocoa powder, chocolate, and other cocoa-derived products.[Citation1,Citation2] It is known that cocoa seeds are a rich source of bioactive substances that have the beneficial impact on human health.[Citation3,Citation4] Because of the characteristic composition of phytochemicals and their high concentrations, raw cocoa beans are characterized by very strong antioxidant activity. The most important antioxidants of cocoa beans are polyphenols. They concentration is around 12–18% on a raw cocoa beans dry weigh.[Citation1,Citation5] The phenolic compounds play an important role in shaping of the sensory properties of cocoa beans and products derived by their processing, due to interactions with polysaccharides, proteins, other polyphenols, and Maillard reaction products. The flavonoids constitute the largest and the most diverse group of phenolic compounds found in cocoa beans. The high antioxidant properties of raw cocoa beans and flavonol-rich cocoa products are generally connected with high content of flavon-3-ols, mainly (-)-epicatechin and (+)-catechin, as well as oligomeric and polimeric procyanidins.[Citation5–Citation7] Other phenolic compounds, that can be also found in cocoa beans are mainly anthocyanins (cyanidin-3-O-arabinoside and cyanidin-3-O-galactoside), as well as flavonols (quercetin aglycone and its glycosides), flavones, phenols (clovamide and deoxyclovamide), phenolic acids, and hydroxylated stilbene derivatives (trans-resveratrol and trans-piceid).[Citation5,Citation6] It is known that many flavonoids, particularly procyanidins, exhibit high antioxidant activity, due to scavenging free radicals, chelating metal ions, binding proteins, and inhibiting enzymes that generate superoxide radicals.[Citation6,Citation8–Citation11] Antioxidant properties of these compounds depends strongly on the degree of the polymerization and nature of hydroxyl groups substitution.[Citation11–Citation13] High concentrations of antioxidant compounds in cocoa beans are responsible for their important role in prevention of civilization diseases. Several epidemiological studies have associated flavanol-rich cocoa intake with a wide range of biological effects, such as anti-inflammatory, anti-atherosclerotic, and anti-platelet aggregation activities, improved insulin sensitivity, as well as blood pressure and immune function modulation.[Citation2,Citation14]
The diversity of Theobroma cacao L. varieties and regions of cultivation, climatic conditions, postharvest manipulations, and storage conditions may affect the antioxidant properties of cocoa beans.[Citation15,Citation16] Jonfia-Essien et al.[Citation17] reported a wide range of antioxidant capacities in cocoa beans from different varieties and genotypes. They found that the hybrid species, especially the Amazon/Trinitario (HV1) and the Amazon/Amazon (HV2 and HV3) had higher antioxidant activity in comparison to the traditional variety (TV) grown in the same region and climatic conditions.[Citation17] Furthermore, Othman et al.[Citation18] showed that polyphenol content and antioxidant activity of cocoa beans are highly dependent on the geographical region of cultivation.
Roasting is the principal technological operation affecting the quality of both roasted cocoa beans and their derived products. The thermal processing of cocoa beans plays an important role in formation of the mild aroma and characteristic taste of cocoa beans. It also causes changes in their texture and increases the intensity of brown color.[Citation19] In general, cocoa beans are roasted at temperatures varying from 100 to 150°C. Many previous studies revealed that the temperature and duration of thermal processing strongly affected the character of physical and chemical changes occurring in cocoa beans during roasting. However, only a few authors studied changes in the antioxidant activity of cocoa beans caused by their roasting.[Citation9,Citation19–Citation21] It is thought that some thermal processes used in the technology of chocolate production, including the roasting of cocoa beans, may contribute to the substantial decrease in the antioxidant activity of cocoa products, mainly due to degradation of phenolic compounds, especially flavonoids.[Citation6,Citation16,Citation20] The main factors responsible for the degradation of these compounds are the length of time and high temperatures of roasting. Additionally, flavonoids interact with proteins, amino acids, polysaccharides, and Maillard reaction products, giving insoluble complexes.[Citation7,Citation16] Although natural antioxidant compounds are lost during heating, the overall antioxidant properties of roasted cocoa beans can be maintained or enhanced by the formation of new antioxidants, such as the Maillard reaction products.[Citation19] However, none of the authors determined the dependence of the antioxidant properties of cocoa beans as a function of the cocoa cultivar and roasting conditions, including temperature and relative air humidity (RH). In addition, data on metal chelating properties of raw and roasted cocoa beans of different Theobroma cacao L. cultivars are scarce. Therefore, investigations of the effect of thermal processing conditions on changes in the bioactivity of cocoa beans of different cultivars during roasting are necessary. The aim of this study was to investigate the effect of roasting conditions, such as temperature and RH, on the total phenolic content, free radical scavenging activity, and metal chelating ability of cocoa beans of the different Theobroma cacao L. cultivars originating from various geographical regions.
Materials and Methods
Chemicals and Reagents
Gallic acid, 6-Hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid (Trolox), 2,2-azino-bis (3-ethylbenzthiazoline-6-sulfonic acid; ABTS), 1,1-diphenyl-2-picrylhydrazyl (DPPH), and ferrozine were purchased from Sigma-Aldrich (Poznan, Poland). Folin–Ciocalteu’s reagent and ferrous chloride were obtained from Chempur (Piekary Slaskie, Poland). Ultrapure water was obtained from a Milli-Q water purification system (Millipore Corp., Bedford, MA, USA). All the chemicals used in this study were of analytical grade and reagents were prepared according to standard analytical procedures.
Cocoa Samples
Seven samples of fermented and dried cocoa beans of different Theobroma cacao L. cultivars originating from Brazil (Forastero), Ecuador (Nacional), Papua New Guinea (Trinitario), Venezuela (Trinitario), Ghana (Upper Amazon Forastero hybrid, UAF), Indonesia (Trinitario×Upper Amazon Forastero hybrid, T×UAF), and Cameroon (Trinitario×Upper Amazon Forastero hybrid, T×UAF) were analyzed. All cocoa beans were harvested in 2011 and bought from commercial sources.
Roasting of Cocoa Beans
Broken and damaged seeds, as well as foreign materials were removed prior to roasting. Cocoa beans were convectively roasted in triplicate batches (about 200 g) in a tunnel with the forced air flow without circulation (adapted to processing with either dry or humid air) at four different air temperatures: 110, 120, 135, and 150°C, and three levels of RH of air: 0.3, 2.0, and 5.0%. The humidity of roasting air was increased using saturated steam (pressure of 0.2 MPa) that was produced using steam generator. Measurements of the temperature and RH of roasting air were conducted with precision of ±1°C and ±0.5%, respectively, using a Rotronic HygroPalm HP22 measuring device (Rotronic AG, Bassersdorf, Switzerland). In performed experiments air velocity along the material layer was 1 m s–1, measured with an accuracy of ±0.05 m s–1. The roasting process was terminated when whole seeds reached the moisture content of approximately 2%. The roasting times were determined experimentally for each batch of cocoa beans based on their initial water content and size. Roasting was conducted for 25, 40, 75, and 85 min at temperatures 150, 135, 120, and 110°C, respectively. Application of air with the higher RH prolonged the time of roasting process. After roasting, cocoa beans were immediately cooled to room temperature and stored in plastic containers (500 g) at −20°C prior to analysis.
Extraction
Samples were extracted in triplicate according to the method described by Summa et al.[Citation21] with some modifications. To extract phenolics, the deshelled and ground cocoa seeds were first defatted with petroleum ether. Then, 10 g of defatted cocoa sample was extracted three times with 100 mL of water at 70°C in an orbital shaker for 30 min. After centrifugation of the resulting extracts at 4000 × g for 15 min at 4°C, the supernatants were collected and pooled. The combined supernatants were filtered through a 0.45 μm nylon filter membranes and freeze-dried (−50°C, 0.9 MPa) using a DELTA 1-24LSC Christ freeze drier (Osterode am Harz, Germany). All lyophilized samples were than stored in plastic bags in a desiccators at 4°C for the later experiments.
Determination of Total Phenolic Content
Total phenolic content was determined using the Folin–Ciocalteu method and gallic acid as a standard, according to Belscak et al.,[Citation22] with some modifications. Briefly, 0.5 mL of the suitably diluted cocoa sample with high-purity deionized water (1.5 mg mL–1) or blank was mixed with 0.5 mL of high-purity deionized water and 500 μL of the Folin–Ciocalteu reagent. After 3 min, 1 mL of 20% (w/v) Na2CO3 solution was added to the mixture. The final volume was adjusted to 10 mL with high-purity deionized water. The solution was then mixed vigorously and allowed to stand at room temperature in the dark for 60 min. The absorbance of reaction mixture was measured at 765 nm using a UV-1800 spectrophotometer (Shimadzu, Japan). For each sample, experiments were conducted in triplicate. The results were expressed as mg gallic acid equivalents (GAE) per gram of lyophilized cocoa dry weight (mg GAE g−1 DW).
Determination of Free Radical Scavenging Capacity
Antioxidant capacity of the lyophilized cocoa extracts was determined by ABTS assay according to the method described by Belscak et al.,[Citation22] with some modifications. Briefly (ABTS+) radical was produced by reacting 7 mM ABTS and 2.45 mM potassium persulfate and then allowing the mixture to stand in the dark at room temperature for 12–16 h before use. The lyophilized cocoa extracts solutions were prepared at concentration 100 µg mL–1 in high-purity deionized water. The sample solutions (10 µL) were added to 4 mL of ABTS+ solution and the resulting mixtures were shaken and left for 20 min at room temperature in the dark. The absorbance of the remaining ABTS+ was measured at 734 nm using a UV-1800 spectrophotometer (Shimadzu, Japan) against the appropriate reagent blank of 10 µL of high-purity deionized water instead of the sample. The measurements were carried out in triplicate and the results were expressed as micromole of Trolox equivalents per gram of lyophilized cocoa DW (µmole TE g–1 DW).
The DPPH radical scavenging activity of cocoa samples was determined according to the method of Schinella et al.,[Citation2] with some modifications. Briefly, the lyophilized cocoa extracts solutions were prepared at concentration 20 µg mL–1 in high-purity deionized water). The sample solution (20 µL) was diluted with ethanol to a volume of 1 mL. Then, 2 mL of a 0.12 mM DPPH solution in ethanol was added. The resultant solution was shaken vigorously and allowed to stand for 30 min at room temperature in the dark. The absorbance of the mixture was measured at 517 nm using a UV-1800 spectrophotometer (Shimadzu, Japan). The blank was prepared in the same manner except that high-purity deionized water was used instead of the sample solution. The same reaction mixture without DPPH solution served as the control. The percentage of DPPH radical-scavenging activity was calculated from the equation:
where Asample is the absorbance of the sample, Acontrol is the absorbance of the control and Ablank is the absorbance of the blank. All analyses were repeated three times, and the results were expressed as micromole of Trolox equivalents per gram of lyophilized cocoa DW (µmole TE g–1 DW).
Determination of Metal Chelating Activity
The chelating activity of cocoa samples for ferrous ions Fe2+ was measured according to the method previously described by Abbès et al.,[Citation23] with slight modification. Briefly, 1 mL of the suitably diluted lyophilized cocoa extracts in high-purity deionized water (100 µg mL–1) were added to 1.85 mL of high-purity deionized water and 50 μL of 2.0 mM FeCl2. After mixing, the solution was allowed to stand at room temperature for 30 s, followed by the addition of 100 μL 5 mM ferrozine. The reaction mixture was then vortexed and left to stand at room temperature for 15 min. The absorbance of the solution was measured spectrophotometrically at 562 nm with UV-1800 UV-VIS Spectrophotometer (Shimadzu, Japan). A low absorbance of the resulting solution indicated a strong ferrous ion chelating ability. A reaction mixture containing 1 mL high-purity deionized water instead of sample solution served as the blank Samples were analyzed in triplicate and results are expressed as chelating activity (%). The percentage of chelating activity was calculated from the equation:
where Asample is the absorbance of the sample and Ablank is the absorbance of the blank.
Statistical Analysis
All analyses were carried out in triplicate. Results are presented as mean ± standard deviation (SD). Experimental data were evaluated using analysis of variance (one-way and two-way ANOVA) and significant differences among the means of three replicates (p < 0.05) were determined by post-hoc multiple mean comparison Tukey’s honestly significant difference (HSD) test using STATISTICA 11 software (StatSoft, Inc., Tulsa, USA). The correlation coefficients between investigated parameters were assessed by means of the Pearson Correlation test using Microsoft Office Excel 2013 (Microsoft Corporation, Redmond, WA, USA).
Results and Discussion
Effect of Cultivar and Geographical Region, As Well As Roasting Conditions on Total Phenolic Content
In this study, the total phenolic content in cocoa beans of the five Theobroma cacao L. cultivars was evaluated using Folin–Ciocalteu reagent. The levels of phenolic compounds in samples of raw and roasted cocoa beans of the tested cocoa cultivars are presented in . The total phenolic content obtained in this study was similar to those reported by other authors for lyophilized extracts of raw, pre-roasted, and roasted cocoa beans, as well as polyphenol-rich cocoa powder samples.[Citation17,Citation20,Citation24] The results indicated that the total phenolic content of raw beans varied significantly among the different cocoa varieties and geographical regions. In general, cv. Forastero from Brazil contained the highest amount of phenolic compounds (173.58 mg GAE g−1 DW), followed by cv. Trinitario from Venezuela (167.23 mg GAE g−1 DW), cv. Nacional from Ecuador (140.53 mg GAE g−1 DW), T×UAF hybrid clone from Indonesia and Cameroon (129.37 and 126.71 mg GAE g−1 DW, respectively), and UAF hybrid clone from Ghana (105.18 GAE g−1 DW). The lowest levels of phenolics (76.14 mg GAE g−1 DW) was found in the cv. Trinitario from Papua New Guinea, showing more than two-fold lower content of phenolics in comparison to the same variety cultivated in Venezuela. These distinct differences may be attributed to the diversity of varieties and genotypes. This is in agreement with the findings of other authors. For example, Tomas-Barberan et al.[Citation1] reported that the total phenolic content in the raw cocoa seeds of the Amazon hybrid variety (Clone CCN51) from Ecuador was higher than in the samples of the Trinitario cultivars from Venezuela (1.3-fold) and Criollo variety from Dominican Republic (2.1-fold). Moreover, other studies have shown that the environmental factors, agricultural practices, and storage conditions may also influence the variability in phenolic compounds concentration of cocoa beans.[Citation1,Citation7,Citation16]
TABLE 1 Effects of roasting conditions on the total phenolics of cocoa beans of Forastero, Nacional, Trinitario, UAF, and T×UAF cultivars originating from various geographical regions
Significant differences (p < 0.05) were observed in the total phenolic content amongst the studied cocoa beans of different cultivars subjected to roasting at various conditions. The obtained results indicated that the total phenolic content in roasted cocoa beans remained unchanged or only slightly decreased after roasting at a temperature of 110°C as compared to raw cocoa beans. There was no effect of air humidity on phenolic content when roasting temperature was 110°C. It can be observed that the thermal treatment conducted at higher temperatures (120 and 150°C) caused a gradual decrease in the total phenolic level in almost all analyzed cocoa cultivars (except cv. Forastero from Brazil). We found that at high temperatures (120–150°C) these losses depended on the roasting air humidity and were slightly less advanced when the air humidity was increased from 0.3 to 5.0%. The reduction of phenolic compounds has been previously observed in the roasted cocoa beans and was found to be negatively related to the temperature and duration of roasting process.[Citation9] This phenomenon is generally attributed to the oxidation and thermal degradation of the naturally occurring phenolic compounds, such as monomeric flavan-3-ols and procyanidins, to corresponding semiquinones and quinones, and formation of the polymer structures, that take place at elevated temperatures.[Citation5,Citation9]
Conversely, a significant rise in phenolic compounds (2.4–4.8% of the initial content) was observed in the case of cv. Forastero from Brazil after roasting at 120 and 135°C. The increase in the phenolic content observed in this study could be explained by the cellular structure degradation during heat treatment and in consequence release of the bound phenolic compounds.[Citation25,Citation26] In fermented and dried cocoa beans, phenolic compounds may be bound to proteins and/or cell wall polysaccharides, which hinder their release and extractability from the cocoa matrix.[Citation7,Citation15] Moreover, increase in the phenolic content after roasting could be also explained by the polymerization or condensation of the monomeric flavan-3-ols and anthocyanins with other phenols, and formation of the high molecular weight proanthocyanidins.[Citation9] The Foline–Ciocalteau assay is based on the oxidation/reduction reaction, and the phenolics content measured by this method may be disturbed by the presence of other reducing non-phenolic compounds, such as carbohydrates, pigments, and Maillard reaction products. Thus, also formation of reductones and melanoidins in Maillard reaction between amino acids and reducing sugars could contribute to the increase in the total phenolic content upon heat treatment.[Citation9,Citation27–Citation29]
Roasting of the cocoa beans at 150°C led to a considerable decrease in the total phenolics content in all studied cocoa varieties. Regarding the effect of the RH on phenolic content, our data suggest that the application of humid air for roasting of cocoa beans effectively reduced the losses of phenolic compounds. This phenomenon can be explained by the formation of the protective layer of water (present in humid air) on the surface of samples that reduced the access of oxygen into the seeds. This assumption agrees with observations of Zzaman et al.,[Citation21] who noticed that roasting with superheated steam caused the lower loss of total phenolics than conventional roasting of cocoa beans, due to the absence of oxygen in superheated steam. Similarly, Tamrin et al.[Citation30] found that during vacuum roasting of cocoa powder (45.6 and 60.8 cmHg) at temperatures ranging from 100 to 120°C the limited content of oxygen in the roasting space slowed down the oxidative degradation of catechins.
In our study, the greatest decrease in total phenolics content was observed when cocoa beans were roasted at 150°C and the lowest RH (RH = 0.3%). This finding is in accordance with the fact that phenolic compounds are easily degraded and/or become bound to polymer structures at high temperatures in the presence of oxygen. Roasting under mentioned above conditions resulted in total phenolics losses ranging from 2.4 to 15.2%. Similar results were obtained by Jolic et al.,[Citation31] who showed that roasting of cocoa beans at a temperatures of 140–150°C for 20 min reduced phenolic content by 14%. Zzaman et al.[Citation21] reported that superheated steam roasting at temperatures of 150–250°C for 10–50 min resulted in 2.9–24.6% losses of the total phenolic content in cocoa beans of the Forastero variety cultivated in Malaysia. However, Arlorio et al.[Citation9] showed greater decrease (33–55%) in the level of phenolic compounds after pre-roasting treatment at 100°C and roasting at 130°C of cocoa beans of the Forastero variety originating from different areas (Ecuador, Ivory Coast, and Ghana). The discrepancies between the results presented by various authors may be ascribed to different roasting methods usage or diverse type and variety of cocoa beans. This study showed that the level of changes in the total phenolic content during roasting was influenced by the variety and the origin of applied cocoa beans. We observed the largest losses of phenolic compounds in case in case of cv. Trinitario from Papua New Guinea and T×UAF hybrid clone from Cameroon, while the lowest changes were found for the samples of cv. Forastero from Brazil. These results were expected due to the intrinsic and acquired characteristics of different cocoa cultivars.
Effect of Cultivar and Geographical Region As Well As Roasting Conditions on Antioxidant Potential
The effect of roasting conditions on the antioxidant capacity of different Theobroma cacao L. cultivars and geographical region was also determined. The free radical scavenging ability of raw and roasted cocoa beans was evaluated in the ABTS and DPPH assays. The results of both methods showed that the analyzed cocoa varieties have high free radical scavenging activities. The ABTS and DPPH values for the raw cocoa bean samples varied from 482.00 to 1406.72 µmole TE g−1 DW, and from 323.80 to 1370.07 µmole TE g−1 DW, respectively ( and ). The values of antioxidant potential obtained in ABTS and DPPH assays were equal or higher than the results described in literature.[Citation20,Citation24,Citation26,Citation32,Citation33] Among the five cocoa cultivars analyzed in this study, the cv. Forastero from Brazil exhibited the highest ABTS and DPPH radical scavenging capacities, while cv. Trinitario from Papua New Guinea displayed the poorest antioxidant potential in both assays. There were no significant (p > 0.05) differences in the ABTS radical scavenging capacity between samples of T×UAF hybrid clones grown in different geographical regions. Variations in the antioxidant capacity of the five investigated cocoa cultivars can be related to differences in the levels of polyphenols and other antioxidants. However, the variability in concentrations of these compounds were not only related to the genetic diversity, but can be explained by the different growing conditions of the cocoa fruit, time of harvest, bean maturity, and post-harvest treatment conditions (fermentation and drying).[Citation5,Citation15,Citation26] It is well-know that the levels of phenolic compounds decreased during fermentation and drying of cocoa beans. In addition, some authors have reported that total phenolics content and antioxidant activity were higher in the unfermented cocoa beans than in the fermented samples. This reduction is mainly related to the non-enzymatic or enzymatic oxidation of catechins to o-quinones, and the condensation of oxidized compounds with other polyphenols to highly polymerized substances such as proanthocyanidins (condensed tannins).[Citation5,Citation15,Citation26] Moreover, during fermentation and drying proanthocyanidins may form insoluble complexes with proteins, amino acids, cell wall polysaccharides, and other phenolics. These phenolic reactions, both enzymatic and non-enzymatic, initiate the characteristic chocolate flavor precursors formation, brown color development, and a significant reduction in astringency and bitterness of raw cocoa beans.[Citation15,Citation26] Fermentation and drying techniques depends primarily on the cocoa bean cultivar and regional practices in the country of origin. For example, due to the milder and less bitter taste the “fine or flavor” cocoa beans of the Criollo, Trinitario, and Nacional groups are often less fermented than “bulk” Forastero beans. An exception are well fermented cocoa beans of the Trinitario type grown in Papua New Guinea.[Citation5,Citation34] The results of this study are consistent with previous reports, and indicated that the free radical scavenging activity was significantly influenced by the cocoa variety.[Citation5,Citation7,Citation9,Citation17] Our results also showed that for the tested cocoa cultivars, the scavenging activity for ABTS radical was higher than for DPPH. These differences could be attributed either to the stereoselectivity of interactions with the radicals or different solubility of the cocoa extracts in the applied reaction media, it means; aqueous solutions and organic solvents for ABTS and DPPH methods, respectively.[Citation2] Antioxidant activity of phenolic compounds largely depends on the molecular weight, structure, and concentration of these compounds. The configuration and total number of hydroxyl groups in the flavonoid molecules influence their radical scavenging efficiency.[Citation35–Citation37] The presence of the ortho-dihydroxy (catechol) structure in the ring B, 2,3-double bond in conjugation with a 4-oxo function in the ring C, and hydroxyl groups at positions 3 and 5 enhances radical scavenging activity.[Citation35] Furthermore, the antioxidant activity of procyanidins also depends on their degree of polymerization.[Citation36] Thus the nature (hydrophilic or lipophilic) of distinct compounds present in the cocoa samples may affect the results of antioxidant activity assays, as was previously reported.[Citation2,Citation20]
TABLE 2 Effects of roasting conditions on the antioxidant capacity measured by ABTS assay of cocoa beans of Forastero, Nacional, Trinitario, UAF, and T×UAF cultivars originating from various geographical regions
TABLE 3 Effects of roasting conditions on the antioxidant capacity measured by DPPH assay of cocoa beans of Forastero, Nacional, Trinitario, UAF, and T×UAF cultivars originating from various geographical regions
Our data showed that the cocoa cultivar and thermal processing conditions significantly affect the ABTS radicals scavenging activity of roasted cocoa beans. The ABTS scavenging activities of the roasted samples varied from 405.10 to 1448.44 µmole TE g−1 DW. We observed a slight increase of ABTS scavenging activity in cocoa beans of cv. Nacional from Ecuador, cv. Forastero from Brazil, and cv. Trinitario from Venezuela during roasting at 110°C. However, in the case of the cv. Forastero from Brazil, greater increase in the antioxidant capacity was observed when the RH was elevated to 2.0 and 5.0%. On the other hand, the ABTS scavenging activity of the cocoa seeds of UAF hybrid variety from Ghana decreased significantly during processing at 110°C. Meanwhile, no significant (p > 0.05) changes in scavenging activity was observed for the other investigated cocoa cultivar after thermal treatment at the same temperature. However, compared to unroasted samples, higher roasting temperatures (120–150°C) caused a clear decrease in the ABTS antioxidant capacity of all analyzed cocoa cultivars. The results are in accordance with those reported earlier.[Citation20] The lowest ABTS antioxidant capacity was found in cocoa beans roasted at 150°C and the RH of 0.3%. Moreover, after roasting under these conditions, the losses of the radical scavenging activity varied greatly between the studied cocoa cultivars and ranged from 5.9 to 20.1%. The greatest decline in the radical scavenging activity was observed for the UAF hybrid clone from Ghana, while the lowest losses were found in the samples of the cv. Forastero from Brazil and cv. Trinitario from Venezuela. The discrepancies in the results might be explained by various initial contents of antioxidant compounds in beans of different cocoa cultivars. Correlation analysis () showed a highly significant positive correlation between total phenolic content and the ABTS antioxidant capacity (R2 = 0.989; p < 0.001). These findings are in agreement with the results of other authors who showed relationship between the phenolics content and antioxidant activity of cocoa samples.[Citation20,Citation18,Citation32]
TABLE 4 Correlation between total phenolic content and antioxidant activities in raw and roasted seeds of different cocoa cultivars
We assumed that the increase in the free radical scavenging activity during heat treatment was primarily due to the release of bound phenolic compounds from the cell matrix and/or the formation of new antioxidants, such as Maillard reaction products.[Citation26] Furthermore, it is know that phenolic compounds in an intermediate state of oxidation can exhibit higher radical scavenging potential than the non-oxidized polyphenols.[Citation25] However, the decrease of antioxidant activity observed in roasted cocoa beans may be attributed to the oxidative degradation of phenolic compounds during heating.[Citation26] Oliviero et al.[Citation19] showed that roasting of cocoa bean model systems at 180°C for 10, 30, and 50 min resulted in a considerable decreases in their antioxidant capacity for ABTS. Summa et al.[Citation24] noticed that pre-roasted (at 80–90°C for 10 min) and roasted (130–160°C for 15–20 min) cocoa beans had a lower scavenging activity against the water soluble stable free radical Fremy’s salt determined by electron paramagnetic resonance (EPR) method than the raw samples. In our study, after thermal treatment, the ABTS radical scavenging activity progressively decreased with a rise in roasting temperature from 120 to 150°C. Moreover, when the RH was increased to 2.0 or 5.0% the activity for ABTS was slightly higher in comparison to that achieved at “dry” air (RH = 0.3%), but in almost all samples these differences were insignificant (p < 0.05). These slight differences could be caused by reduced oxygen access into the cocoa kernel. Also the study of Zzaman et al.[Citation21] on the effects of superheated steam roasting on the antioxidant capacity confirmed this observation.
The DPPH antioxidant capacity varied from 191.29 to 1395.97 µmole TE g−1 DW for the roasted cocoa beans and depended on the cocoa variety and processing conditions. We found a slight increase in the DPPH radical scavenging activity in the cocoa beans of cv. Nacional from Ecuador caused by roasting at 110 and 120°C, while their processing at higher temperatures (135 and 150°C) reduced this activity, compared to the raw beans. Also, it was noticed that in the case of the cv. Forastero from Brazil, roasting at temperatures in the range of 110 to 135°C caused a gradual increase in the DPPH radical scavenging capacity (from 0.5 to 1.9% of initial value), while a slight decrease was observed after processing at 150°C compared to the unroasted samples. These results are consistent with findings of Summa et al.[Citation20] who showed that the DPPH radical scavenging activity of the cocoa beans first increased during the pre-roasting and then decreased after roasting. This fluctuation could be caused by the release of bound phenolic compounds during roasting and formation and accumulation of Millard reaction products. This suggested that an increase in melanoidins content can also contribute to the observed enhancement of the DPPH antioxidant activity of roasted cocoa beans. In addition, during processing at higher temperatures the structure of some phenolic compounds are altered, which could change their antioxidant properties.[Citation34]
However, our study showed that in case of almost all other tested cocoa cultivars roasting at temperatures ranging from 110 to 150°C leads to a clear decrease in the DPPH scavenging capacity. Moreover, there was no effect of air humidity on the changes in the DPPH scavenging activity. Although, an increase in the RH from 0.3 to 5.0% slightly reduced the losses of DPPH antioxidant capacity, but in almost all cocoa samples these differences were not statistically significant (p > 0.05). In this study, the lowest reduction in the DPPH scavenging activity, as compared with raw samples, was observed when roasting was conducted at 110°C with humid air (RH = 5.0%). The greatest decrease of the DPPH antioxidant capacity in the cocoa beans of the cv. Nacional from Ecuador, and cv. Trinitario from Papua New Guinea was caused by roasting at 150°C, while in the samples of cv. Trinitario from Venezuela this reduction was the most pronounced during thermal processing at 135°C. The losses in the DPPH antioxidant capacity observed under these conditions varied from 4.5 to 40.9% of the initial antioxidant activity. Nevertheless, in the case of the all investigated hybrid varieties, cocoa beans after roasting at 120°C exhibited the lowest activity in DPPH scavenging. These samples showed 1.1–1.6 lower antioxidant capacities than the unroasted cocoa beans. The reduction in the DPPH scavenging capacity was more drastic in the cv. Trinitario from Papua New Guinea and UAF hybrid clone from Ghana, while the lowest changes were observed in the cv. Forastero from Brazil and cv. Nacional from Ecuador. These variations imply that roasting may affect the antioxidant properties of cocoa beans in different ways depending on the variety and roasting conditions. The antioxidant activity losses differed among analyzed cultivars, probably due to the intrinsic characteristics of the cocoa beans.
As mentioned above, the decrease of the antioxidant activity during roasting may be caused by the degradation of highly thermolabile phenolic compounds. Similarly, Arlorio et al.[Citation9] observed a decrease in the DPPH antioxidant activity of cocoa beans caused by their roasting at 130°C after thermal pretreatment at 100°C. Moreover, some authors concluded that formation of high molecular weight brown pigments via polymerization reactions in the advanced steps of the Maillard reaction can involve the antiradical groups, leading to a depletion of free radical scavenging activity upon high temperature processing.[Citation29]
The previous study showed that the DPPH radical scavenging capacity and the total phenolic content in extracts derived from cocoa beans were not always correlated.[Citation17–Citation19] Therefore, the antioxidant capacity of the cocoa extracts may be also attributed to the presence of bioactive components other than polyphenols, such as methylxanthines and Maillard reaction products, as well as interactions between these compounds.[Citation9,Citation20] Nevertheless, in the present study, a strong linear correlation between the DPPH radical scavenging activity and the total phenolic content (R2 = 0.968, p < 0.001) as well as ABTS antioxidant capacity (R2 = 0.954, p < 0.001) was obtained for all tested cultivars, which confirms the leading role of phenolic compounds in the overall scavenging activity of cocoa bean water extracts. Our results are consistent with literature data; however, other authors studied only the effect of temperature and duration of roasting, as well as the application of superheated steam on changes in the free radical scavenging activity of cocoa beans subjected to different roasting treatments while the influence of various RH conditions has not been investigated so far.
Effect of Cultivar and Geographical Region, As Well As Roasting Conditions on Metal Chelating Activity
The changes in metal chelating activity caused by roasting of cocoa beans of different Theobroma cacao L. cultivars are shown in . The results indicated that all tested cocoa samples were able to chelate ferrous ion (Fe2+), which is the most powerful pro-oxidant among metal ions and can catalyze generation of potentially toxic reactive oxygen species (ROS) by Fenton reactions, such as hydroxyl radical (•OH) that initiate lipid peroxidation.[Citation23,Citation37] This is particularly important because the dysfunctions of cells caused by free radicals and ROS is one of factors contributing to aging process and cancer development. The ferrous ion chelating activities of raw cocoa bean samples were variety dependent and ranged from 35.37 to 63.04% (at 100 µg mL–1 concentration of lyophilized cocoa extracts). Amongst the analyzed cocoa varieties, cv. Forastero from Brazil exhibited the highest ferrous ion chelating potential, while UAF hybrid clone from Ghana possessed the lowest chelating ability.
FIGURE 1 Changes in the ferrous ion chelating activity of the cocoa bean samples of Forastero, Nacional, Trinitario, UAF, and T×UAF cultivars roasted at different temperatures and relative air humidities. B: Brazil; E: Ecuador; V: Venezuela; PNG: Papua New Guinea; G: Ghana; I: Indonesia; C: Cameroon. Results are presented as the mean ± SD from triplicate determinations. Bars that share the same letter within the cultivars are not significantly different according to Tukey’s HSD test at p > 0.05.
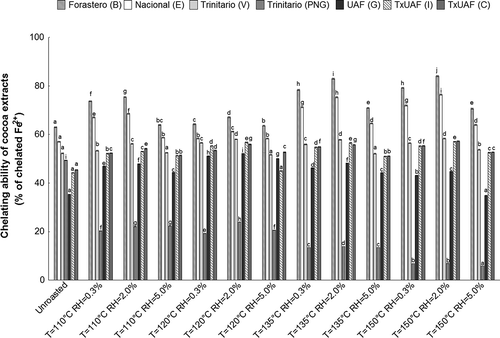
The results demonstrate that ferrous ion chelating activities of roasted cocoa beans depended on the cultivar and roasting conditions. The metal chelating activity of roasted samples varied from 5.90 to 84.12% (at the concentration of lyophilized cocoa extracts of 100 µg ml−1). It was also observed that the ferrous ion chelating activity of cv. Trinitario from Papua New Guinea decreased significantly (from 2.1- to 8.4-fold, compared to raw cocoa beans), as temperature of roasting air increased from 110 to 150°C, probably due to thermal degradation of phenolic compounds. However, the opposite effect was observed in beans of the other studied cocoa cultivars, for which roasting at temperatures of 110–150°C increased the metal chelating activity. This was consistent with the finding of other authors who reported that the metal chelating properties of oats increased after sand roasting at 280°C for 15 s compared to control samples.[Citation38] Among the cocoa phenolic compounds, procyanidins, monomeric flavan-3-ols, and quercetin exhibit high activity in ferrous ions chelating.[Citation39,Citation40] The position, substitution, and number of hydroxyl groups in the flavonoid molecules affect their ability to chelate trace metal ions. The metal chelating properties of different flavonoids are thought to result from the presence of the catechol group in the ring B, the 3-hydroxyl and 4-oxo groups in the heterocyclic ring C, and the 4-oxo and 5-hydroxyl groups between the C and A rings.[Citation35] Processing at elevated temperatures can alter the structure of phenolic compounds and could enhance their ferrous ion chelating activity.[Citation38] A recent study revealed that thermal treatment of cocoa beans increases the content of catechin and quercetin. The rise in catechin content can be attributed to the epimerization of epicatechin and/or decomposition of procyanidins due to the high temperature of roasting, whereas the quercetin levels increase as a result of deglycosylation of its glycosides.[Citation6,Citation8] According to Olennikov et al.[Citation41] glycosylation of the hydroxyl group at C-3 apparently inhibits the ferrous chelating ability of flavonols. These authors noticed that the chelating activity of quercetin was significantly higher that of the quercetin 3-O-glucoside. Moreover, the polymerization of procyanidins during roasting may be responsible for the increased chelating activity, because their ability to chelate metals and bind proteins depends on their structure and molecular weight distribution, more polymerized compounds had a stronger effect.[Citation40] The increase of ferrous ion chelating activity described in this work might be also caused by generation of high molecular weight brown polymers by the heat-induced Maillard reactions. These polymers are known to have the ability to chelate metal ions. As can be seen from our results, the changes in the ferrous ion chelating properties of cocoa beans greatly depend on the RH (0.3–5.0%). Compared to the raw cocoa beans, the highest increase in the metal chelating activity (by 3.7–47.3%) was caused by thermal treatment when the RH was increased to 2.0%. This intense rise could be explained by the fact that the application of elevated air humidity during thermal processing of cocoa beans leads to the loosening of the husk structure by steam presented in the air, and thus facilitates the heat penetration into the kernel. Therefore, the roasted cocoa beans may have higher ferrous ion chelating activity than the unroasted ones as a result of alterations in the structure of the phenolic compounds or generation of polymeric proanthocyanidins and Maillard reaction products. Significant correlation between the ferrous ion chelating ability and total phenolics content, as well as antioxidant activities for ABTS and DPPH of cocoa samples was observed (R2 = 0.778, p < 0.001; R2 = 0.726, p < 0.001; R2 = 0.814, p < 0.001, respectively).
This is first report on the dependence of the total phenolic content, free radical scavenging activity, and metal chelating ability of cocoa beans on the cultivar and different roasting conditions, such as temperature and RH. It may be concluded that thermal processing at elevated temperature and humidity, can significantly affect the metal binding properties of raw materials. Furthermore, changes in the antioxidant properties caused by roasting of different cocoa varieties during roasting may depend on the initial content of bioactive compounds. This finding agrees with the results of other authors who reported that the composition of raw cocoa beans depends on several factors, including variety and genotype, geographical region of cultivation, cultivation conditions, and post-harvest processing treatments.[Citation9]
Conclusion
The results obtained in this study provide a detailed overview of the effect of different roasting conditions, such as temperature and RH, on the antioxidant properties of five different Theobroma cacao L. varieties. We confirmed the significant impact of cocoa variety and cultivation region on the total polyphenol content, antioxidant capacity, and metal chelating activity of cocoa beans. Interestingly, cocoa beans of cv. Forastero from Brazil exhibited higher free radical scavenging activity and metal chelating ability, that can be explained by the total phenolics content than samples of the others cocoa varieties tested. The results revealed that raw cocoa beans have potential health benefits because of their high phenolics content and antioxidant properties. During roasting, significant changes were observed in the total phenolics content, free radical scavenging capacity, and metal chelating activity of all the analyzed varieties. Our data showed that the antioxidant properties of roasted cocoa beans depended on the cocoa cultivar and roasting conditions, such as the temperature and RH. Moreover, we showed that the thermal processing at lower temperatures using humid air can be used to increase the phenolics content and antioxidant activity of roasted cocoa beans. The effect of roasting on the antioxidant properties of cocoa beans depends on the balance between the thermal degradation of naturally occurring phenolic compounds and the formation of new antioxidants, such as polymeric pigments and Maillard reaction products. The results of this study provide evidence that cocoa beans are a good source of antioxidant compounds even after roasting.
Funding
The financial support for this study was provided by the National Science Centre in Poland (NCN), project no. UMO-2012/05/N/NZ9/01399.
Additional information
Funding
References
- Tomas-Barberan, F.A.; Cienfuegos-Jovellanos, E.; Marin, A.; Muguerza, B.; Gil-Izquierdo, A.; Cerda, B.; Zafrilla, P.; Morillas, J.; Mulero, J.; Ibarra, A.; Pasamar, M.A.; Ramón, D.; Espín, J.C. A New Process to Develop a Cocoa Powder with Higher Flavonoid Monomer Content and Enhanced Bioavailability in Healthy Humans. Journal of Agricultural and Food Chemistry 2007, 104, 3926–3935.
- Schinella, G.; Mosca, S.; Cienfuegos-Jovellanos, E.; Pasamar, M.A.; Muguerza, B.; Ramón, D.; Ríos, J.L. Antioxidant Properties of Polyphenol-Rich Cocoa Products Industrially Processed. Food Research International 2010, 43, 1614–1623.
- Visioli, F.; Bernaert, H.; Corti, R.; Ferri, C.; Heptinstall, S.; Molinari, E.; Poli, A.; Serafini, M.; Smit H.J.; Vinson, J.A.; Violi, F.; Paoletti, R. Chocolate, Lifestyle, and Health. Critical Reviews in Food Science and Nutrition 2009, 49, 299–312.
- Rusconi, M.; Conti, A. Theobroma cacao L., the Food of the Gods: A Scientific Approach Beyond Myths and Claims. Pharmacological Research 2010, 61, 5–13.
- Oracz, J.; Zyzelewicz, D.; Nebesny, E. The Content of Polyphenolic Compounds in Cocoa Beans (Theobroma Cacao L.), Depending on Variety, Growing Region, and Processing Operations: A Review. Critical Reviews in Food Science and Nutrition 2015, 55(09), 1176–1192.
- Ortega, N.; Romero, M.-P.; Macia, A.; Reguant, J.; Angles, N.; Morello, J.-R. Obtention and Characterization of Phenolic Extracts from Different Cocoa Sources. Journal of Agricultural and Food Chemistry 2008, 56, 9621–9627.
- Niemenak, N.; Rohsius, C.; Elwers, S.; Omokolo Ndoumou, D.; Lieberei, R. Comparative Study of Different Cocoa (Theobroma Cacao L.) Clones in Terms of Their Phenolics and Anthocyanins Contents. Journal of Food Composition and Analysis 2006, 19, 612–619.
- Kothe, L.; Zimmermann, B.F.; Galensa, R. Temperature Influences Epimerization and Composition of Flavanol Monomers, Dimers, and Trimers During Cocoa Bean Roasting. Food Chemistry 2013, 141(4), 3656–3663.
- Arlorio, M.; Locatelli, M.; Travaglia, F.; Coisson, J.; Grosso, E.; Minassi, A.; Appendino, G.; Martelli, A. Roasting Impact on the Contents of Clovamide (N-Caffeoyl-L-DOPA) and the Antioxidant Activity of Cocoa Beans (Theobroma Cacao L.). Food Chemistry 2008, 106, 967–975.
- Counet, C.; Collin, S. Effect of the Number of Flavanol Units on the Antioxidant Activity of Procyanidin Fractions Isolated from Chocolate. Journal of Agricultural and Food Chemistry 2003, 51, 6816–6822.
- Corcuera, L.A.; Amézqueta, S.; Arbillaga, L.; Vettorazzi, A.; Touriño, S.; Torres, J.L.; López de Cerain, A. A Polyphenol-Enriched Cocoa Extract Reduces Free Radicals Produced by Mycotoxins. Food and Chemical Toxicology 2012, 50, 989–995.
- Hammerstone, J.F.; Lazarus, S.A.; Mitchell, A.E.; Rucker, R.; Schmitz, H.H. Identification of Procyanidins in Cocoa (Theobroma Cacao) and Chocolate Using High-Performance Liquid Chromatography/Mass Spectrometry. Journal of Agricultural and Food Chemistry 1999, 47, 490–496.
- Lagouri, V.; Prasianaki, D.; Krysta, F. Antioxidant Properties and Phenolic Composition of Greek Propolis Extracts. International Journal of Food Properties 2014, 17(3), 511–522.
- Katz, D.L.; Doughty, K.; Ali, A. Cocoa and Chocolate in Human Health and Disease. Antioxidants and Redox Signaling 2011, 15, 2779–2811.
- Di Mattia, C.; Martuscelli, M.; Sacchetti, G.; Scheirlinck, I.; Beheydt, B.; Mastrocola, D.; Pittia, P. Effect of Fermentation and Drying on Procyanidins, Antiradical Activity, and Reducing Properties of Cocoa Beans. Food and Bioprocess Technology 2013, 6, 3420–3432.
- Wollgast, J.; Anklam, E. Review on Polyphenols in Theobroma Cacao: Changes in Composition During the Manufacture of Chocolate and Methodology for Identification and Quantification. Food Research International 2000, 33, 423–447.
- Jonfia-Essien, W.A.; West, G.; Alderson, P.G.; Tucker, G. Phenolic Content and Antioxidant Capacity of Hybrid Variety Cocoa Beans. Food Chemistry 2008, 108, 1155–1159.
- Othman, A.; Ismail, A.; Ghani, N.A.; Adenan, I. Antioxidant Capacity and Phenolic Content of Cocoa Beans. Food Chemistry 2007, 100, 1523–1530.
- Oliviero, T.; Capuano, E.; Cämmerer, B.; Fogliano, V. Influence of Roasting on the Antioxidant Activity and HMF Formation of Cocoa Bean Model System. Journal of Agricultural and Food Chemistry 2009, 57, 147–152.
- Summa, C.; Cordeiro Raposo, F.; McCourt, J.; Lo Scalzo, R.; Wagner, K.-H.; Elmadfa, I.; Anklam, E. Effect of Roasting on the Radical Scavenging Activity of Cocoa Beans. European Food Research and Technology 2006, 222, 368–375.
- Zzaman, W.; Bhat, R.; Yang, T.A. Effect of Superheated Steam Roasting on the Phenolic Antioxidant Properties of Cocoa Bean. Journal of Food Processing and Preservation 2013, 38, 1932–1938.
- Belšcak, A.; Komes, D.; Horzic, D.; Kovacevic, K.; Karlovic, G.D. Comparative Study of Commercially Available Cocoa Products in Terms of Their Bioactive Composition. Food Research International 2009, 42, 707–716.
- Abbès, F.; Kchaou, W.; Blecker, C.; Ongena, M.; Lognay, G.; Attia, H.; Besbes, S. Effect of Processing Conditions on Phenolic Compounds and Antioxidant Properties of Date Syrup. Industrial Crops and Products 2013, 44, 634–642.
- Summa, C.; McCourt, J.; Cämmerer, B.; Fila, A.; Probst, M.; Kun, S.; Anklam, E.; Wagner, K.-H. Radical Scavenging Activity, Anti-Bacterial, and Mutagenic Effects of Cocoa Bean Maillard Reaction Products with Degree of Roasting. Molecular Nutrition & Food Research 2008, 52, 342–351.
- Dorta, E.; Lobo, M.G.; González, M. Using Drying Treatments to Stabilise Mango Peel and Seed: Effect on Antioxidant Activity. LWT–Food Science and Technology 2012, 45, 261–268.
- Suazo, Y.; Davidov-Pardo, G.; Arozarena, I. Effect of Fermentation and Roasting on the Phenolic Concentration and Antioxidant Activity of Cocoa from Nicaragua. Journal of Food Quality 2014, 37, 50–56.
- Prior, R.L.; Wu, X.; Schaich, K. Standardized Methods for the Determination of Antioxidant Capacity and Phenolics in Foods and Dietary Supplements. Journal of Agricultural and Food Chemistry 2005, 53, 3101–3113.
- Ioannone, F.; Di Mattia, C.D.; De Gregorio, M.; Sergi, M.; Serafini, M.; Sacchetti, G. Flavanols, Proanthocyanidins, and Antioxidant Activity Changes During Cocoa (Theobroma Cacao L.) Roasting As Affected by Temperature and Time of Processing. Food Chemistry 2015, 174, 256–262.
- Coghe, S.; Gheeraert, B.; Michiels, A.; Delvaux, F.R. Development of Maillard Reaction Related Characteristics During Malt Roasting. Journal of the Institute of Brewing 2006, 12(2), 148–156.
- Tamrin, K.; Harijono, Yuwono, S.S.; Estiasih, T.; Santoso, U. Various Temperature of Vacuum and Conventional Roasting on Color Alteration and Polyphenols Content of Cocoa Powder. Journal of Food Science and Engineering 2012, 2, 642–651.
- Jolic, S.M.; Redovnikovic, I.R.; Markovic, K.; Sipusic, D.I.; Delonga, K. Changes of Phenolic Compounds and Antioxidant Capacity in Coco Beans Processing. International Journal of Food Science and Technology 2011, 46, 1793–1800.
- Gu, L.; House, S.E.; Wu, X.; Ou, B.; Prior, R.L. Procyanidin and Catechin Contents and Antioxidant Capacity of Cocoa and Chocolate Products. Journal of Agricultural and Food Chemistry 2006, 54, 4057–4061.
- Cienfuegos-Jovellanos, E.; Quiñones, M.; Muguerza, B.; Moulay, L.; Miguel, M.; Aleixandre, A. Antihypertensive Effect of a Polyphenol-Rich Cocoa Powder Industrially Processed to Preserve the Original Flavonoids of the Cocoa Beans. Journal of Agricultural and Food Chemistry 2009, 57(14), 6156–6162.
- Afoakwa, E.O.; Paterson, A.; Fowler, M.; Ryan, A. Flavor Formation and Character in Cocoa and Chocolate: A Critical Review. Critical Reviews in Food Science and Nutrition 2008, 48, 1–18.
- Procházková, D.; Boušová, I.; Wilhelmová, N. Antioxidant and Pro-Oxidant Properties of Flavonoids. Fitoterapia 2011, 82(4), 513–523.
- Lin, C.-C.; Li, C.-W.; Shih, Y.-T.; Chuang, L-T. Antioxidant and Anti-Inflammatory Properties of Lower-Polymerized Polyphenols in Oolong Tea. International Journal of Food Properties 2014, 17(4), 752–764.
- Sarikurkcu, C.; Zengin, G.; Aktumsek, A.; Ceylan, O.; Uysal, S. Screening of Possible in Vitro Neuroprotective, Skin Care, Antihyperglycemic, and Antioxidative Effects of Anchusa undulata L. Subsp. Hybrida (Ten.) Coutinho from Turkey and Its Fatty Acid Profile. International Journal of Food Properties 2015, 18(7), 1491–1504.
- Gujral, H.S.; Sharma, P.; Rachna, S. Effect of Sand Roasting on Beta Glucan Extractability, Physicochemical, and Antioxidant Properties of Oats. LWT–Food Science and Technology 2011, 44, 2223–2230.
- Deng, W.; Fang, X.; Wu, J. Flavonoids Function As Antioxidants: By Scavenging Reactive Oxygen Species Or by Chelating Iron? Radiation Physics and Chemistry 1997, 50(3), 271–276.
- Ojwang, L.O.; Yang, L.; Dykes, L.; Awika, J. Proanthocyanidin Profile of Cowpea (Vigna Unguiculata) Reveals Catechin-O-Glucoside As the Dominant Compound. Food Chemistry 2013, 139, 35–43.
- Olennikov, D.N.; Kashchenko, N.I.; Chirikova, N.K. A Novel HPLC-Assisted Method for Investigation of the Fe2+-Chelating Activity of Flavonoids and Plant Extracts. Molecules 2014, 19, 18296–18316.
