Abstract
Twenty different varieties of Capsicum pepper cultivars belonging to four species (Capsicum chinense, Capsicum annuum, Capsicum frutescens, and Capsicum baccatum) were characterized in terms of their capsaicinoid and total phenolic content. The peppers were sown in a farm in the southeastern region of São Paulo State. The determination of capsaicinoids was performed by ultra-performance liquid chromatography. The total phenolic content was determined spectrophotometrically with the Folin-Ciocalteu reagent. Results were expressed as µg capsaicinoid/g fresh pepper and as Scoville heat unit. A wide variation was observed among the compositions of capsaicinoids. Capsaicin and dihydrocapsaicin were the most abundant peaks. Capsaicinoids were not identified in the pepper varieties Cheiro Verde, Cambuci Verde, Cambuci Vermelha, and Biquinho. The spiciest pepper was Naga Jolokia (119,016 Scoville heat unit). Regarding the phenolic contents, a large variability was observed. Total phenolic content ranged from 0.35 mg gallic acid equivalent/g in Cambuci Verde to 3.06 mg gallic acid equivalent/g in Naga Jolokia. The current study may benefit consumers, the food, and pharmaceutical industries due to the increasing interest in pharmacological compounds present in hot and sweet Capsicum peppers.
Introduction
The Capsicum genus, native from tropical and humid zones of Central and Southern America, belongs to the Solanaceae family and includes peppers of important economic value.[Citation1] In Brazil, peppers are grown throughout the country, from Rio Grande do Sul to Roraima, with a wide variation in sizes, colors, flavors, and pungency, which is characteristic of Capsicum peppers.[Citation2]
The main domesticated species of Capsicum peppers in Brazil are Capsicum annuum, Capsicum frutescens, and Capsicum chinense, which are consumed fresh or processed as colorants, paste, paprika, and oleoresin.[Citation3] Peppers are good sources of different phytochemicals, including vitamin C, phenolic compounds, flavonoids, and carotenoids.[Citation4,Citation5] Capsicum peppers are the only plant genus able to produce alkaloids belonging to the family of capsaicinoids, which are responsible for their typical pungency. Capsaicin (C) and dihydrocapsaicin (DHC) are the predominant molecules, reaching around 90% of total capsaicinoids in the peppers. Other related capsaicinoids, such as nordihydrocapsaicin (n-DHC), homocapsaicin (h-C), and homodihydrocapsaicin (h-DHC), are also present in lower amounts.[Citation6–Citation8] Capsaicinoids have strong pharmacological effects, which may be used for pain relief, cancer prevention, weight reduction, besides providing gastrointestinal and cardiovascular benefits.[Citation9] Additionally, Capsicum peppers are rich in phenolic compounds, which also show beneficial properties to the human organism. Many studies have revealed protective roles of phenolic compounds against coronary heart diseases, stroke, and some forms of cancer. The protective effects of phenolic compounds are attributed to their antiradical and signaling activities in the cells.[Citation10] Other minor components that greatly affect the taste and flavor of Capsicum peppers are alcohols, terpenes, aldehydes, ketones, fatty acids, and fatty acid esters.[Citation11–Citation13]
The quantification of pungency of peppers is very important for consumers and industrial purposes, since it is required to make peppers and their products applicable as ingredients for food production.[Citation14] Thus, the aim of this work was to characterize 20 different varieties of Capsicum cultivars sown in southeastern Brazil. The total phenolic content of the peppers was determined and their capsaicinoid content was analyzed by ultra-performance liquid chromatography (UHPLC-DAD), in order to determine the pungency level. This work presents interesting results regarding the capsaicinoid composition of peppers native from Brazil, such as Biquinho, Cumari, and Murupi, since data about their capsaicinoid composition are not found in the current databases of international scientific literature. Additionally, the current study may benefit consumers, food and pharmaceutical industries due to the increasing interest in the pharmacological compounds present in hot and sweet Capsicum peppers.
Materials and Methods
Chemicals
Folin-Ciocalteu reagent (Dinâmica, SP, Brazil), sodium carbonate (Synth, SP, Brazil) and gallic acid (Sigma-Aldrich, St. Louis, MO, USA) were used for the analysis of total phenolics. For the chromatographic analysis, C and DHC standards were purchased from Cayman Chemical (Cayman Chemical, USA, Purity >95%). Water was obtained from a Milli-Q water deionization system (Millipore, Bedford, MA, USA). Methanol and acetic acid of high-performance liquid chromatography (HPLC) grade were acquired from Merck (Darmstadt, Germany).
Peppers
The 20 different varieties Capsicum peppers analyzed in this work belong to four species: C. chinense, C. annuum, C. frutescens, and C. baccatum. All the evaluated pepper samples were sown in October 2012 and harvested in mid-March 2013, on a farm in the southeastern state of São Paulo, 120 km far from Campinas/SP. The popular name of each pepper variety in Brazil, the stage of maturation and their respective colors are shown in . Care was taken in the selection of fruits with the same generation, maturation, and of similar size, and the fruits were chosen considering their physical integrity. Samples were stored at –20°C until being analyzed.
TABLE 1 Different varieties of Capsicum peppers analyzed: Popular Brazilian name; stage of maturation (respective color)
Pungency Determination
Capsaicinoids were extracted from the Capsicum peppers with methanol, following the method proposed by Barbero et al.[Citation15] To obtain the extract, fresh peppers were peeled and the stems and seeds were separated. Only the pericarp and the placenta of the peppers were used. Both pericarp and placenta were ground using a domestic blender until a homogeneous sample was obtained for the analyses. About 1 g of milled peppers was diluted in 25 mL methanol and homogenized with help of an ultrasonic bath (model TTUSC1450, Unique, Campinas, Brazil) for 15 min. The mixture was then filtered through a 0.20 µm filter before the injection in the UHPLC system. All extractions were performed in triplicate.
UHPLC-DAD Analysis
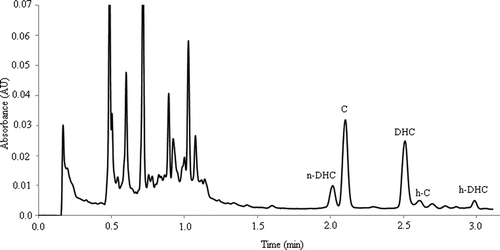
The UHPLC analyses were carried out in an Acquity UPLC System (Waters, Milford, MA, USA) equipped with a PDA detector Model 2996. Empower 2 software (Waters) was used to control the equipment and for data acquisition. Capsaicinoids were analyzed on a Waters BEH C18 column (50 × 2.1 mm I.D., particle size 1.7 µm). The injection volume was 3 µL. The gradient of elution used acidified water (0.1% acetic acid, solvent A) and acidified methanol (0.1% acetic acid, solvent B). The gradient was (min, % solvent B): 0.85 min, 55%; 1.60 min, 55%; 1.95 min, 60%; 2.45 min, 63%; 2.80 min, 70%; 3.00 min, 70%; 4.00 min, 100%. For the analyses, capsaicinoids were measured at a wavelength of 280 nm and the column oven was set at 50ºC for the chromatographic separation. A typical chromatogram of the extract from Malagueta Verde is shown in .
Identification of Capsaicinoids by Liquid Chromatography Coupled to Mass Spectrometry
The pepper extracts were analyzed by UHPLC-DAD coupled to a PDA detector Model 2996 and a quadrupole-time-of-flight mass spectrometry (Q-ToF-MS; Synapt G2, Waters Corp., Milford, MA, USA). The injection volume was 2.5 µL. The chromatographic separation was performed on a reverse-phase C18 analytical column (Acquity UPLC BEH C18, Waters) of 2.1 × 50 mm and 1.7 µm particle size. For the PDA analysis, capsaicinoids were measured at a wavelength of 280 nm. The determination of the analytes was carried out using an electrospray source operating in positive ionization (ESI+) mode under the following conditions: desolvation gas flow = 850 L/h, desolvation temperature = 450ºC, cone gas flow = 5 L/h, source temperature = 130ºC, capillary voltage = 1500 V, cone voltage = 40 V and collision energy = 4 eV. Full-scan mode was used (m/z = 50–600). The software Masslynx 4.1 was used for the control of the equipment, the acquisition, and treatment of data.
A gradient method using acidified water (0.1% acetic acid, solvent A) and acidified methanol (0.1% acetic acid, solvent B), working at a flow rate of 0.5 mL/min, was used for the chromatographic separation. The applied gradient was: 0 min, 0% B; 0.85 min, 55% B; 1.60 min, 55% B; 1.95 min, 60% B; 2.45 min, 63% B; 2.80 min, 70% B; 3.00 min, 70% B; 4.00 min, 100% B; 5.00 min, 100% B. The total run time was 8 min, including 2 min for re-equilibration. The temperature of the column was kept constant at 50ºC. A reverse phase separation column (Acquity UPLC BEH C18, 2.1 × 100 mm, 1.7 µm particle size, Waters) was used.
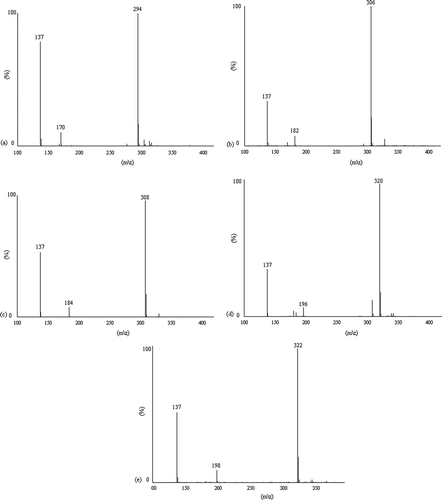
The capsaicinoids identified in the extracts from Capsicum peppers were n-DHC, C, DHC, h-C, and h-DHC. The molecular ions [M+H]+ for the capsaicinoids found presented the following m/z ratios: n-DHC, 294; C, 306; DHC, 308; h-C, 320; and h-DHC, 322. In the mass spectra of these five capsaicinoids the m/z peak (137), characteristic of the fragmentations of capsaicinoids, appears clearly, as shown in .[Citation16,Citation17] This ion is derived from the vanillyl ring fragment of capsaicinoids and appears in all the mass spectra. Other obtained m/z peaks are: 170 fornordihydrocapsaicin; 182 for C; 184 fordihydrocapsaicin; 196 forhomocapsaicin; 198 forhomodihydrocapsaicin. These peaks correspond to the side chain of each capsaicinoid.[Citation18] The fragmentation for these fragments occurs between the vanillyl ring and the side chain. These m/z ratios verified the structures of the five mentioned capsaicinoids.
UHPLC Calibration
To use the UHPLC-DAD method, calibration curves were prepared for C and DHC (y = 2161.10x + 115.61 for C and y = 2329.61x + 73.02 for DHC). Six standards dilutions, from 0.05 to 100 ppm, have been prepared for both capsaicinoids to build the calibration curves. Regression equations and correlation coefficients (RCitation2; 0.9999 for C and 0.9999 for DHC), limits of detection (0.209 mg/L for C and 0.135 mg/L for DHC) and quantification (0.697 mg/L for C and 0.450 mg/L for DHC) were calculated by linear regression using the software Microsoft Office Excel 2010. All analyses were performed in triplicate.
Quantification of Capsaicinoids
The five major capsaicinoids (n-DHC, C, DHC, h-C, and h-DHC) found in the peppers were quantified. C and DHC were quantified from the calibration curves obtained with the standard solutions. Since there are no commercial standards for n-DHC, h-C, and h-DHC, these compounds were quantified from the calibration curves of DHC (for n-DHC and for h-DHC) and C (for h-C), based on the structural similarities between these molecules and taking into account their molecular weights. All analyses were run in triplicate.
Scoville Heat Unit (SHU)
Pungency was also expressed as SHU, and for this determination the amount of each capsaicinoid, expressed as μg/g, was multiplied by the following respective coefficients: 16.1 for C and DHC, 9.3 for n-DHC, 6.9 for h-C, and 8.1 for h-DHC.[Citation19]
Determination of Total Phenolic Content
The total phenolic content of Capsicum peppers was determined spectrophotometrically using the Folin–Ciocalteu method, according to the methodology proposed by Singleton et al.[Citation20] with modifications. Briefly, 2.5 mL of the diluted Folin–Ciocalteu reagent (1:10 v/v) was added to 0.5 mL of extract solution in methanol (the same solution prepared for the chromatographic analysis). After 5 min, 2.0 mL of sodium carbonate solution (7.5%) was added. The mixture remained 2 h in dark at room temperature, and then its absorbance was measured in a spectrophotometer (model 800XI, Femto, São Paulo, Brazil) at 760 nm. Gallic acid was used as standard, and results were expressed as milligrams of gallic acid equivalent (GAE) per gram of fresh pepper. All analyses were run in triplicate
Statistical Analysis
The variance (ANOVA) of the results was evaluated using the software Statistica for Windows 6.0 (Statsoft Inc., USA). This analysis allowed the detection of significant differences between the capsaicinoid profiles of the evaluated pepper varieties. The significant differences at level of 5% (p < 0.05) were analyzed through the Tukey’s test.
Results and Discussion
Pungency Determination
Regarding the capsaicinoid composition, a wide variation was observed between the studied peppers and five capsaicinoids (n-DHC, C, DHC, h-C, and h-DHC) were identified. and show the chromatogram of the separation of these capsaicinoids for the pepper Malagueta Verde with their respective mass spectra. C and DHC were predominant capsaicinoids in most evaluated peppers. C was detected in 16 of the 20 cultivars analyzed, and was the major capsaicinoid in 14 of them, followed by DHC. The highest concentration of C was found in the pepper Naga Jolokia (4457 µg/g fresh pepper), followed by Murupi (1753 µg/g fresh pepper), and Cumari (1315 µg/g fresh pepper). DHC was the predominant capsaicinoid in two varieties: Jalapeño (75.9%) and Fatalli (55.8%). Among the peppers containing DHC in their composition, the lowest DHC concentration (10.7 µg/g fresh pepper) was found in Jalapeño, whereas the highest value (2358 µg/g fresh pepper) was detected in Naga Jolokia ().
TABLE 2 Individual capsaicinoid content (μg/g ± SD) of Brazilian Capsicum peppers and their percentage in terms of the total capsaicinoids (%)
n-DHC was observed in low amounts, ranging from 0.6% of the total capsaicinoids in Pimenta Preta and Murupi to 9.2% in the variety Pimenta de Mesa. The concentrations of h-C and h-DHC were even lower and occurred in fewer varieties than the other capsaicinoids. The highest concentrations were 11.87% for h-C (Dedo-de-Moça Pequena) and 2.99% for h-DHC (Malagueta Verde). The varieties Cheiro Verde, Cambuci Vermelha, Cambuci Verde, and Biquinho did not show capsaicinoids in their compositions. This result was expected, since these peppers are popularly known in Brazil as sweet peppers because they do not have the typical pungency of the Capsicum genus. This property contributes to their characteristic flavor and also makes them suitable for culinary preparation.
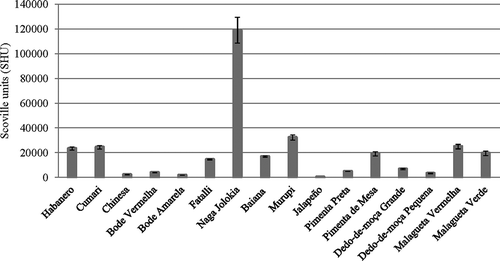
The reported results indicate that the identified capsaicinoids are present in different ratios among the 20 examined pepper varieties, revealing the diversity in the amount of capsaicinoids found in Capsicum peppers grown in Brazil. It is important to remark that the occurrence of these compounds in peppers is highly variable, and depends strongly on the cultivar, but also on parameters such as ripening stage, season, irrigation, among others.[Citation21] shows the results of SHUs of the analyzed peppers. As expected, the varieties that contain higher C and DHC amounts result in higher SHU values. Consequently, the SHU values of the peppers Naga Jolokia and Murupi were higher than those of other pepper varieties.
No relation was observed between the Capsicum species and the capsaicinoid contents of the evaluated peppers. This observation is consistent with those made by Zewdie and Bosland,[Citation22] who found that capsaicinoid profiles are not good chemotaxonomic indicators for Capsicum species. The results obtained in this work showed variations when compared to the data available in the literature regarding the pungency of peppers grown in Brazil, and in general, the peppers analyzed in this study had lower SHU levels. According to Lopez,[Citation23] the SHU values of peppers cultivated in Brazil are: Biquinho (0), Cheiro verde (0–100,000), Jalapeño (37,000), Dedo-de-Moça (46,000), Bode (53,000), Malagueta (164,000), Cumari (210,000), and Murupi (223,000). Despite the numerical difference in the observed values of SHU, the order of pungency (from the most to the least spicy) of the analyzed peppers was practically the same: Murupi, Cumari, Malagueta, Dedo-de-moça, Bode, Jalapeño, and Biquinho e Cheiro Verde. The pepper variety with the highest value of SHU observed in this study was Naga Jolokia (119,016 SHU). Also according to Lopes,[Citation23] the main determinants of pungency levels in peppers are species and cultivars. Other factors such as planting season, fruit ripening, and the environmental conditions in the region of cultivation are crucial to the pungency of the fruit, so these may be the reasons for the significant variation between the values of SHU. Additionally, the perception of pungency is variable between different individuals and seems to be also affected by the habit, since a decrease in sensitivity has been observed after continued exposure.[Citation24] Therefore, pungency is considered a subjective attribute, and a universal scale has been built by the American Spice Trade Association (ASTA), based on ppm of capsaicinoids instead of human perception, as it was done in the past by the Scoville Organoleptic Test.
Total Phenolic Determination
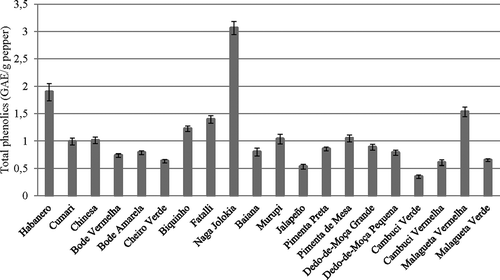
Phenolic compounds are interesting due to their biological activities, such as antioxidant, protection against free radicals, and action in the prevention of cancer, cardiovascular diseases, and neurodegenerative disorders.[Citation25] The phenolic contents of fresh Capsicum peppers were determined, and a large variability was observed from one specie to other. Phenolic contents ranged from 0.35 (±0.03) mg GAE/g for Cambuci Verde to 3.06 (±0.12) mg GAE/g for Naga Jolokia. In this study, Naga Jolokia showed phenolic content higher than all other evaluated peppers, as can be observed in .
The results of phenolic compounds found in this work are in agreement with those obtained for Capsicum peppers by other researchers: Zhuang et al.[Citation4] evaluated the total phenolic content of seven pepper cultivars from China and found values from 1.09 to 4.99 mg GAE/g fresh pepper. The red ripe cultivars of Malagueta and Cambuci showed phenolic contents significantly higher than those of green unripe fruits, and according to Ghasemnezhad et al.[Citation26] this is due to the phenolics accumulated along the developmental stages of pepper fruits. Plant maturity and color were the major determinants of variation in phenolic content. Additionally, there is no absolute correlation between the total capsaicinoid and total phenolic contents. As an example, the Biquinho pepper did not present capsaicinoids in its composition, although it ranks fourth in the total amount of phenolic compounds.
Conclusions
A wide variation was observed among the composition of capsaicinoids in 20 studied Brazilian Capsicum peppers. C and DHC were the most abundant capsaicinoids. Sweet Brazilian peppers (Cheiro Verde, Cambuci Verde, Cambuci Vermelha, and Biquinho) did not present capsaicinoids in their compositions. The spiciest pepper was Naga Jolokia followed by Murupi. Regarding the phenolic compounds, a large variability was observed, ranging from 0.35 mg GAE/g for Cambuci Verde to 3.06 mg GAE/g for Naga Jolokia. There was no direct relationship between the total contents of capsaicinoids and phenolic compounds, although there is certain relation in some peppers, such as Naga Jolokia. The current work may benefit consumers, the food, and pharmaceutical industries due to the increasing interest in pharmacological compounds present in hot and sweet Capsicum peppers.
Acknowledgment
The authors wish to thank CITA-ZARAGOZA (Agrifood and Technology Centre of Aragón) for the taxonomic identification of the pepper samples.
Funding
The authors wish to thank FAEPEX, CAPES, CNPq (Project 473342/2011-1), and FAPESP (Projects 2011/08656-7 and 2013/02203-6) for the financial support.
Additional information
Funding
References
- Giuffrida, D.; Dugo, P.; Torre, G.; Bignardi, C.; Cavazza, A.; Corradini, C.; Dugo, G. Characterization of 12 Capsicum Varieties by Evaluation of Their Carotenoid Profile and Pungency Determination. Food Chemistry 2013, 140, 794–802.
- Lutz, D.L.; Freitas, S.C. Valor Nutricional. Ribeiro, C.S.C.; Lopes, C.A.; Carvalho, S.I.C.; Henz, G.P.; Reifschneider, F.J.B.; Eds.; Pimentas Capsicum: Empresa Brasileira de Pesquisas Agropecuárias: Brasília, Brasil, 2008; 31–38 pp.
- Topuz, A.; Ozdemir, F. Assessment of Carotenoids, Capsaicinoids, and Ascorbic Acid Composition of Some Selected Pepper Cultivars (Capsicum Annuum L.) Grown in Turkey. Journal of Food Composition and Analysis 2007, 20, 596–602.
- Zhuang, Y.; Chen, L.; Sun, L.; Cao, J. Bioactive Characteristics and Antioxidant Activities of Nine Peppers. Journal of Functional Foods 2012, 4, 331–338.
- Korkutata, N.F.; Kavaz, A.A. Comparative Study of Ascorbic Acid and Capsaicinoid Contents in Red Hot Peppers (Capsicum Annum L.) Grown in Southeastern Anatolia Region. International Journal of Food Properties 2015, 18, 725–734.
- Laskaridou-Monnerville, A. Determination of Capsaicin and Dihydrocapsaicin by Micellar Electrokinetic Capillary Chromatography and Its Application to Various Species of Capsicum, Solanaceae. Journal of Chromatography A 1999, 838, 293–302.
- Barbero, G.F.; Liazid, A.; Palma, M.; Barroso, C.G. Fast Determination of Capsaicinoids from Peppers by High-Performance Liquid Chromatography Using a Reversed Phase Monolithic Column. Food Chemistry 2008, 107, 1276–1282.
- Aguiar, A.C.; Sales, L.P.; Coutinho, J.P.; Barbero, G.F.; Godoy, H.T.; Martínez, J. Supercritical Carbon Dioxide Extraction of Capsicum Peppers: Global Yield and Capsaicinoid Content. The Journal of Supercritical Fluids 2013, 81, 210–216.
- Luo, X.J.; Peng, J.; Li, Y.J. Recent Advances in the Study on Capsaicinoids and Capsinoids. European Journal of Pharmacology 2011, 650, 1–7.
- Boh, G.; Rocha, J.B.T. Distribution and Antioxidant Activity of Polyphenols in Ripe and Unripe Tree Pepper (Capsicum Pubescens). Journal of Food Biochemistry 2007, 31, 456–473.
- Saha, S.; Walia, S.; Kundu, A.; Kaur, C.; Singh, J.; Sisodia, R. Capsaicinoids, Tocopherol, and Sterols Content in Chili (Capsicum sp.) by Gas Chromatographic-Mass Spectrometric Determination. International Journal of Food Properties 2015, 18, 1535–1545.
- Gogus, F.; Ozel, M.Z.; Keskin, H.; Yanik, D.K.; Lewis, A.C. Volatiles of Fresh and Commercial Sweet Red Pepper Pastes: Processing Methods and Microwave Assisted Extraction. International Journal of Food Properties 2015, 18, 1625–1634.
- Eggink, P.M.; Maliepaard, C.; Tikunov, Y; Haanstra, J.P.W.; Bovy, A.G.; Visser, R.G.F. A Taste of Sweet Pepper: Volatile and Non-Volatile Chemical Composition of Fresh Sweet Pepper (Capsicum Annuum) in Relation to Sensory Evaluation of Taste. Food Chemistry 2012, 132, 301–310.
- Barbero, G.F.; Liazid, L.; Azaroual, L.; Palma, M.; Barroso, C.G. Capsaicinoid Contents in Peppers and Pepper-Related Spicy Foods. International Journal of Food Properties. 2016, 19(3), 485–493.
- Barbero, G.F.; Liazid, A.; Palma, M.; Barroso, C.G. Ultrasound-Assisted Extraction of Capsaicinoids from Peppers. Talanta 2008, 75, 1332–1337.
- Reilly, C.A.; Crouch, D.J.; Yost, G.S.; Fatah, A.A. Determination of Capsaicin, Nonivamide, and Dihydrocapsaicin in Blood and Tissue by Liquid Chromatography-Tandem Mass Spectrometry. Journal of Analytical Toxicology 2002, 26, 313–319.
- Thompson, R.Q.; Phinney, K.W.; Welch, M.J.; White, E.V. Quantitative Determination of Capsaicinoids by Liquid Chromatography–Electrospray Mass Spectrometry. Analytical and Bioanalytical Chemistry 2005, 381, 1441–1451.
- Schweiggert, U.; Carle, R.; Schieber, A. Characterization of Major and Minor Capsaicinoids and Related Compounds in Chili Pods (Capsicum Frutescens L.) by High-Performance Liquid Chromatography/Atmospheric Pressure Chemical Ionization Mass Spectrometry. Analytica Chimica Acta 2006, 557, 236–244.
- Dong, M.W. Food, Environmental, Chemical, and Life Sciences Applications. In Modern HPLC for Practicing Scientists; Dong, M.W.; Ed.; John Wiley & Sons, Inc.: Hoboken, NJ, 2006; 157–191 pp.
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of Total Phenols and Other Oxidation Substrates and Antioxidants by Means of Folin-Ciocalteu Reagent. Methods in Enzymology 1999, 299, 152–178.
- Reyes-Escogido, M.L.; Gonzalez-Mondragon, E.G.; Vazquez-Tzompantzi, E. Chemical and Pharmacological Aspects of Capsaicin. Molecules 2011, 16, 1253–1270.
- Zewdie, Y.; Bosland, P.W. Capsaicinoid Profiles Are Not Good Chemotaxonomic Indicators for Capsicum Species. Biochemical Systematics and Ecology 2001, 29, 161–169.
- Lopes, C.A. Ardume, Picância, Pungência. In: Ribeiro, C.S.C.; Lopes, C.A.; Carvalho, S.I.C.; Henz, G.P.; Reifschneider, F.J.B.; Eds.; Pimentas Capsicum: Empresa Brasileira de Pesquisas Agropecuárias: Brasília, Brasil, 2008; 31–38 pp.
- Rozin, P.; Mark, M.; Schiller, D. The Role of Desensitization to Capsaicin in Chili Pepper Ingestion and Preference. Chemical Senses 1981, 6, 23–31.
- Chandrasekara, A.; Shahidi, F. Determination of Antioxidant Activity in Free and Hydrolyzed Fractions of Millet Grains and Characterization of Their Phenolic Profiles by HPLC-DAD-ESI-MSn. Journal of Functional Foods 2011, 3, 144–158.
- Ghasemnezhad, M.; Sherafati, M.; Payvast, G.A. Variation in Phenolic Compounds, Ascorbic Acid, and Antioxidant Activity of Five Coloured Bell Pepper (Capsicum Annum) Fruits at Two Different Harvest Times. Journal of Functional Foods 2011, 3, 44–49.