Abstract
Shanxi aged vinegar (SAV) is the most famous one among the four typical famous vinegars in China, which is produced by spontaneous solid-state fermentation using sorghum, bran, and millet chaff and Daqu. In this study, free phenolic acids (FPAs) species and changes during SAV aging, and the synergistic antioxidant activities between FPAs was investigated. The results revealed that eight FPAs, including protocatechuic acid, p-hydroxybenzoic acid, salicylic acid, dihydro sinapic acid, p-coumaric acid, sinapic acid, dihydro ferulic acid, and ferulic acid, stably existed during SAV 0–5 year aging periods, whereas during the aging time evaporation of water and FPAs combining with melanoidins made prediction of precise FPAs contents difficult. With regard to FPAs synergistic antioxidant activities, it was observed that the FPAs antioxidative interrelations were antagonism in antiradical activity experiments. And, only a few groups of FPAs mixtures showed antagonism in reduction experiments.
Introduction
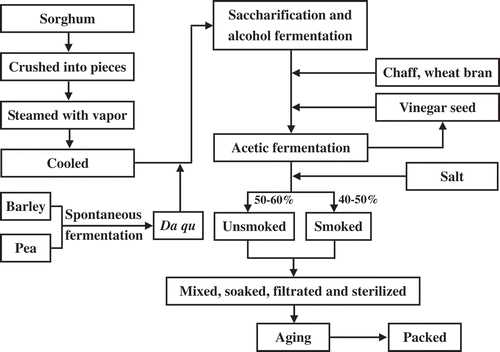
Vinegar is used as acid condiment in almost all countries of the world.[Citation1] In China, it has been produced and consumed for more than 3000 years and every region has its own local vinegar.[Citation2] Shanxi aged vinegar (SAV) is having the top position among four traditional famous vinegars of China including Zhenjiang aromatic vinegar, Sichuan bran vinegar, and Fujian Monascus vinegar and itself.[Citation3,Citation4] SAV is made from sorghum, bran, millet chaff, and Daqu (starter culture, made from barley and pea by spontaneous fermentation), leading it to contain abundant organic acids, amino acids, and free phenolic acids (FPAs).[Citation5–Citation7] During SAV brewing (), a long-term aging is always needed by the traditional technology named as “insolating in summer and taking out ice in winter,” to raise density, balance fragrance, and increase color and luster.[Citation4]
In the past two decades, phenolic acids widely present in plant products have been intensely investigated due to their antioxidant activities,[Citation8–Citation10] food preservation,[Citation11] and chronic disease prevention such as atherosclerosis, cancer, and degenerative diseases.[Citation12–Citation15] Normally phenolic acids exist in plant tissues combined with macromolecular compound like polysaccharose and protein,[Citation16] but they can be released by microbial catalysts during fermentation processes in the fermented products such as wine, vinegar, and others.[Citation17,Citation18] So the fermented products usually contain a certain amount of FPAs which can be directly extracted by organic solvents[Citation19,Citation20] and detected by liquid chromatography–photo-diode array (LC–PDA),[Citation21] LC–MS (mass spectrum),[Citation22] and gas chromatography (GC)–MS.[Citation23] According to previous studies the vinegars including sherry wine vinegars, traditional balsamic vinegar, and Kurosu (traditional Japanese vinegar) contained a mass of FPAs with varying species and contents of these FPAs were influenced by the type of vessels during aging.[Citation21,Citation23–Citation25] However, the species and contents of FPAs in SAV haven’t been reported. In the present work, FPAs species in SAV were identified and changes among them were determined during SAV aging process. Furthermore, the analyses regarding synergistic antioxidant activities were carried out.
Materials and methods
Materials and Chemicals
SAV (different aging time 0, 1, 2, 3, and 5 years) sampled from SAV Group Co. Ltd. (Shanxi, China). FPAs standards (protocatechuic acid, p-hydroxybenzoic acid, salicylic acid, p-coumaric acid, ferulic acid, sinapic acid, caffeic acid, vanillic acid, syringic acid, and gallic acid) were purchased from Aladdin Chemical Co. Ltd. (Shanghai, China). Dihydro ferulic acid was purchased from Tokyo Chemical Industry Co. Ltd. (Shanghai, China). 2, 2’-Azinobis-(3-ethylbenzthiazoline-6- sulphonate; ABTS) was purchased from Sigma-Aldrich Co. Ltd. (San Francisco, USA). All the chemicals were of analytical or chromatographic quality.
Extraction of Total Free Phenolic Acids (TFPAs) from Vinegar
The FPAs in SAV were extracted according to the previous methods with some modifications.[Citation22] First, 10 g vinegar was freeze-dried into powder and ultrasonically extracted by 50 mL 100% alcohol at 40°C five times (Removing melanoidins which is hardly soluble in alcohol). Then the extracts were combined and evaporated at 35°C to dry using a rotary evaporator (Rotary evaporator RE-52AA, Zhengzhou Great Wall Scientific Industry and Trade Co. Ltd.). Subsequently, the residue was dissolved in 5 mL distilled water and re-extracted by 15 mL ethyl acetate three times. After the ethyl acetate was completely volatilized at 35°C by rotary evaporator, the residue was dissolved in 2 mL d-H2O containing 20% alcohol for subsequent analysis.
TFPAs Determination in SAV during Aging Time
The TFPAs in SAV at different aging time (0, 1, 2, 3, and 5 years) were prepared by the method in 2.2. The contents of TFPAs were detected using the Folin–Ciocalteu method.[Citation26] Briefly, 1 mL of appropriately diluted TFPAs in distilled water (1 mL water as control) was mixed well with 0.8 mL of Folin–Ciocalten reagent and 10 mL of 10% Na2CO3, and kept at 25°C for 2 h. Then optical density (OD) values were measured by a spectrophotometer (UV-1750 UV-Vis spectrophotometer, Shimadzu Instruments Co. Ltd.) at 765 nm. Gallic acid was used as the standard and the content of TFPAs in SAV at various aging time were expressed as gallic acid equivalents in ppm.
Determination of FPAs in SAV during Aging Process
Method to identify FPAs in SAV
TFPAs from 5-year-old SAV were prepared by the method in 2.2. The accurate molecular weights of FPAs in SAV were detected by the high resolution mass spectrometry (Thermo Scientific Q Exactive, Thermo Fisher Scientific Co. Ltd.). HYPERSIL GOLD C18 column (150 × 2.1 mm, 1.9 μm) was selected to separate FPAs in a mobile phase [0.1% formic acid in pure water (mobile phase A) and pure acetonitrile (mobile phase B)] in following gradient elution: 0–3 min, 95–95% A; 3–12 min, 95–60% A; 12–13 min, 60–10% A; 13–15 min, 10–10% A; 15–16 min, 10–90% A; 16–20 min, 95–95% A. The injection volume was 5 μL, the flow rate was 0.2 mL/min. The ionization method was electrospray ionization (ESI) at negative ion mode and the scan range of mass spectra was from 100 to 1000 m/z. The FPAs species in SAV were confirmed by ultra performance liquid chromatography-mass spectrum (UPLC–MS; Waters Xevo TQ mass spectrometer, Waters Co. Ltd.) using FPAs standards. The FPAs separation was implemented with ACQUITY UPLC HSST column (100 × 2.1 mm, 1.8 μm). The mobile phase, injection volume, flow rate, and gradient elution were the same as described above. The ionization method was ESI at negative ion mode and the scan range of mass spectra was from 50 to 300 m/z. After TFPAs in SAV of different aging years (0, 1, 2, 3, and 5 years) was extracted, the FPAs species were identified and quantified following the method as described in 2.4.1.
Synergistic Antioxidant Activities between FPAs
Determination of the synergistic antiradical activity
The antiradical activity of FPAs were determined by previous methods with some modifications.[Citation27] Briefly, 0.1 mL of single FPAs standard (50 mg/L) or 0.1 mL of two kinds of mixed FPAs standards (each was 25 mg/L) was mixed well with 2.9 mL of ABTS, 7 mmol/L) and kept at 25°C for 5 min. The OD values were measured by a spectrophotometer (UV-1750 UV-Vis spectrophotometer, Shimadzu Instruments Co. Ltd.) at 735 nm. Gallic acid was used as standard and the antiradical activity was expressed as gallic acid equivalents in ppm.
Determination of the synergistic reducing power
The reducing power of FPAs were determined by Folin–Ciocalteu method.[Citation26] Briefly, 0.1 mL of single FPAs standard (50 mg/L) or 0.1 mL of two kinds of mixed FPAs standards (each was 25 mg/L) was mixed with 0.8 mL of Folin–Ciocalten reagent and 10 mL of 10% Na2CO3 at 25°C for 2 h. The OD values were measured by a spectrophotometer (UV-1750 UV-Vis spectrophotometer, Shimadzu Instruments Co. Ltd.) at 765 nm. Gallic acid was used as standard and the reducing power was expressed as gallic acid equivalents in ppm.
Statistical analyses and graph drawing
The data of samples was analyzed by SPSS 18.0 (Expressed as mean ± SD). One-sided t-test was analyzed by an Independent-Sample Test with SPSS 18.0. The graph was drawn by OriginPro 8.0.
Results
Changes of TFPAs Content in SAV during Aging Process
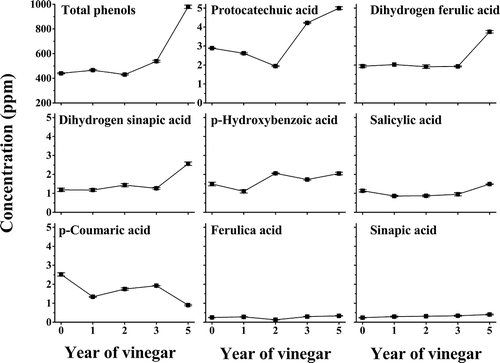
The contents of TFPAs in various aging-old SAV were analyzed by the Folin–Ciocalteu method. The results showed that the TFPAs contents in SAV increased year-by-year except 2-year-old vinegar and its contents in 5-year-old SAV reached up to 980 ± 11 μg gallic acid/g.
Determination of FPAs in SAV during Aging
The kinds of FPAs in 5-year SAV
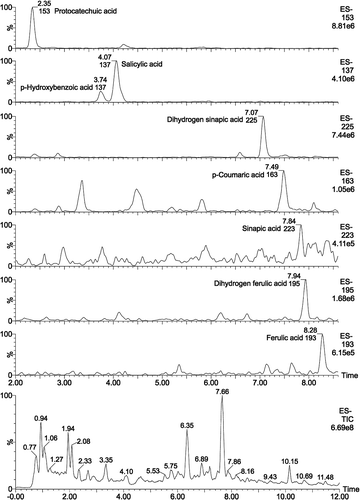
In preliminary work, we found that it was difficult to separate FPAs in SAV by HPLC–diode array detection (DAD) probably due to abundance of fermentation products and Maillard reaction products in it (data unpublished). So the accurate molecular weights of FPAs in SAV were first detected using high-resolution mass spectrometry. The FPAs species in SAV were confirmed by UPLC–MS using FPAs standards. First, 14 kinds of possible FPAs (Molecular formula; Molecular weight-1.00782: C7H6O3; 137.02387, C7H6O4; 153.01854, C9H8O3; 163.03957, C9H10O3; 165.05521, C9H10O4; 181.05016, C9H10O4; 181.05022, C10H8O4; 191.03474, C10H10O4; 193.05000, C10H12O4; 195.06587, C9H10O5; 197.04527, C9H10O5; 197.04523, C11H10O4; 205.05046, C11H12O5; 223.06131, and C11H14O5; 225.07702) were determined by the accurate molecular weight analysis. After confirmed by standards, eight kinds of FPAs were identified ( and ) including protocatechuic acid, p-hydroxybenzoic acid, salicylic acid, dihydro sinapic, p-coumaric acid, sinapic acid, dihydro ferulic acid, and ferulic acid. The error between detected molecular weight and theoretical molecular weight were all less than 5 ppm. And all the FPAs were confirmed by standard except dihydro ferulic acid which was done without standard. Therefore, dihydro ferulic acid was just confirmed by the accurate molecular weight, retention time (before sinapic acid) and mass fragments (181 = [M – COO]–, 151 = [M – COO – CH3 – CH3]–, 125 = [M – COO – CO – C2H4]–).
TABLE 1 The chromatogram of FPAs extracted from 5 year SAV
Identification and quantification of FPAs in SAV during aging
The kinds and contents of FPAs in SAV (0–5 year) were detected by UPLC–MS using external standard method. The standard included the phenolic acids identified in 5-year SAV (except dihydro sinapic acid) and the common phenolic acids in plant like caffeic acid, vanillic acid, syringic acid, and gallic acid. Result showed () that the kinds of FPAs were unchanged during the aging of SAV. Though, the content of TFPAs was increased with aging time mainly due to evaporation of water, the contents of some FPAs like p-hydroxybenzoic acid, salicylic acid, p-coumaric acid, ferulic acid, and sinapic acid were not increased with aging time. This is may be due to combination of these phenol acids with melanoidins during aging.[Citation28] The order of the contents of FPAs was: protocatechuic acid > dihydro ferulic acid > dihydro sinapic acid ≈ p-hydroxybenzoic acid > salicylic acid ≈ p-coumaric acid > ferulic acid > sinapic acid.
Synergy of Antioxidant Activity between FPAs
The antioxidant activity of FPAs (except dihydro sinapic acid) was reflected by antiradical activity () and reducing power (). The antiradical activity result showed that, in most cases, the total antiradical activity of the mixture of two FPAs was lower than the half of the total antiradical activity of two FPAs, except salicylic acid which had hardly scavenging effects on ABTS radicals. However, the reducing power result revealed that only four groups (p-hydroxybenzoic acid + protocatechuic acid, p-hydroxybenzoic acid + dihydro ferulic acid, salicylic acid + protocatechuic acid, salicylic acid + dihydro ferulic acid) showed antagonistic effect.
TABLE 2 The antiradical activity of FPAs (ppm)
TABLE 3 The reducing power of FPAs (ppm)
Discussion
Research demonstrated that the raw material of SAV like sorghum, bran, barley, and pea have a certain amount of bound phenolic acids and a small quantity of FPAs.[Citation29–Citation32] These bound phenolic acids could be released by microbial catalysis during fermentation processes.[Citation17,Citation18] The FPAs in SAV mainly come from raw material. Some FPAs were changed to other FPAs during fermentation, such as sinapic acid and ferulic acid reduced to dihydro sinapic acid and dihydro ferulic acid, respectively.[Citation33] Some FPAs might have been changed to other compounds, like gallic acid and caffeic acid, which were in the raw material of SAV but not detected in SAV.[Citation29–Citation32] The reason of keeping the kinds of FPAs unchanged in SAV during aging might be the vessel of SAV, terrine, unlike the wood made vessels having high influence on FPAs.[Citation21,Citation34] The evaporation of water and FPAs combining with melanoidins during aging result in the unpredictable change in FPAs contents in SAV. Study indicated that the FPAs antioxidant activities depends on the number and position of the hydroxyl groups bound to the aromatic ring and the type of substituent, which is positively correlated with the number of phenolic hydroxyl.[Citation35] The hydroxyl groups in the meta-positions of carboxyl groups can increase antioxidant activity more than that in the ortho- or para-positions due to the electron pushing effect of carboxyl group promoting H-donating ability of hydroxyl groups. Of course, ortho substitution of hydroxyl group with electron-donating groups like methoxy groups can also increase the antioxidant activity.[Citation36] The -CH=CH-COOH groups in cinnamic acid can produce greater H-donating ability and subsequent antioxidant activity than the -COOH groups in benzoic acids through stabilizing the radical by resonance of -C=C-.[Citation36,Citation37] The detected antioxidant activity of these FPAs was according with these rules. The antioxidant activity of dihydro ferulic acid which came from the reduction of ferulic acids was more than the antioxidant activity of ferulic acids possibly due to the electron pushing effect of -CH2-CH2- group promoting H-donating ability of phenolic hydroxyl group. The interrelation between most FPAs in scavenging ABTS radical was antagonistic unlike with the interrelation between different phenolic compounds;[Citation38] phenolic compounds, and non-phenolic antioxidants,[Citation39] and different phytochemical extracts.[Citation40] It could be because of the similar structure of these FPAs in SAV cannot assist each other in antioxidant activity. According to the poor synergy of antioxidant activity between different phenolic compounds and the low content of FPAs relative to TFPAs, there should be many other antioxidation ingredients in SAV which need been researched later. Moreover the types and contents of prime FPAs in SAV during ageing had been determined but their transformation during fermentation and the mechanism of synergy between them needs further studies.
Acknowledgment
We wish to thank Dr. Zeqiang Wu of Wuhan Institute of Biotechnology for providing the UPLC–MS.
Funding
This study was supported by Programs of International S & T Cooperation, Ministry of Science and Technology, P. R. China (No. 2014DFG32380), the Fundamental Research Funds for the Central Universities (No. 2014PY034, No. 2662015PY167, No. 2013PY006), and the project of Wuhan Science & Technology Bureau (No. 2015030809020368).
LJFP_S_1075216.pdf
Download PDF (174.8 KB)Additional information
Funding
References
- Solieri, L.; Giudici, P; Eds. Vinegars of the World. In Vinegars of the World Springer: Milan, Italy, 2009; 1–16.
- Chen, F.; Li, L.; Qu, J.; Chen, C. Cereal Vinegars Made by Solid-State Fermentation in China. In Vinegars of the World; Solieri, L.; Giudici, P.; Eds.; Springer: Milan, Italy, 2009; 243–259.
- Liu, D.; Zhu, Y.; Beeftink, R.; Ooijkaas, L.; Rinzema, A.; Chen, J.; Tramper, J. Chinese Vinegar and Its Solid-State Fermentation Process. Food Reviews International 2004, 20(4), 407–424.
- Wu, J.J.; Ma, Y.K.; Zhang, F.F.; Chen, F.S. Biodiversity of Yeasts, Lactic Acid Bacteria, and Acetic Acid Bacteria in the Fermentation of “Shanxi Aged Vinegar,” a Traditional Chinese Vinegar. Food Microbiology 2012, 30(1), 289–297.
- Chen, T.; Gui, Q.; Shi, J.J.; Zhang, X.Y.; Chen, F.S. Analysis of Variation of Main Components During Aging Process of Shanxi Aged Vinegar. Acetic Acid Bacteria 2013, 2(1s), e6.
- Chen, S.; Feng, B.; Liu, C.; Su, J.; Zhang, H.; Liu, Y.; Wu, X. Antioxidant Activity of Polyphenolic Extract from Shanxi Aged Vinegar. Food Science 2012, 1, 31–34.
- Wang, A.; Song, H.; Ren, C.; Li, Z. Key Aroma Compounds in Shanxi Aged Tartary Buckwheat Vinegar and Changes During Its Thermal Processing. Flavour and Fragrance Journal 2012, 27(1), 47–53.
- Schmidt, C.G.; Goncalves, L.M.; Prietto, L.; Hackbart, H.S.; Furlong, E.B. Antioxidant Activity and Enzyme Inhibition of Phenolic Acids from Fermented Rice Bran with Fungus Rizhopus Oryzae. Food Chemistry 2014, 146, 371–377.
- Shao, Y.; Xu, F.; Sun, X.; Bao, J.; Beta, T. Phenolic Acids, Anthocyanins, and Antioxidant Capacity in Rice (Oryza Sativa L.) Grains at Four Stages of Development After Flowering. Food Chemistry 2014, 143, 90–96.
- Chou, C.-H.; Liu, C.-W.; Yang, D.-J.; Wu, Y.-H.S.; Chen, Y.-C. Amino Acid, Mineral, and Polyphenolic Profiles of Black Vinegar, and Its Lipid Lowering and Antioxidant Effects in vivo. Food Chemistry 2015, 5, 168, 63–69.
- González-Rompinelli, E.M.; Rodríguez-Bencomo, J.J.; García-Ruiz, A.; Sánchez-Patán, F.; Martín-Álvarez, P.J.; Bartolomé, B.; Moreno-Arribas, M.V. A Winery-Scale Trial of the Use of Antimicrobial Plant Phenolic Extracts As Preservatives During Wine Ageing in Barrels. Food Control 2013, 33(2), 440–447.
- Zamora-Ros, R.; Rothwell, J.A.; Scalbert, A.; Knaze, V.; Romieu, I.; Slimani, N.; Fagherazzi, G.; Perquier, F.; Touillaud, M.; Molina-Montes, E. Dietary Intakes and Food Sources of Phenolic Acids in the European Prospective Investigation into Cancer and Nutrition (EPIC) Study. British Journal of Nutrition 2013, 110(08), 1500–1511.
- Ky, I.; Crozier, A.; Cros, G.; Teissedre, P.-L. Polyphenols Composition of Wine and Grape Sub-Products and Potential Effects on Chronic Diseases. Nutrition and Aging 2014, 2(2), 165–177.
- Henning, S.M.; Wang, P.; Abgaryan, N.; Vicinanza, R.; de Oliveira, D.M.; Zhang, Y.; Lee, R.P.; Carpenter, C.L.; Aronson, W.J.; Heber, D. Phenolic Acid Concentrations in Plasma and Urine from Men Consuming Green Or Black Tea and Potential Chemopreventive Properties for Colon Cancer. Molecular Nutrition & Food Research 2013, 57(3), 483–493.
- Boeing, H.; Bechthold, A.; Bub, A.; Ellinger, S.; Haller, D.; Kroke, A.; Leschik-Bonnet, E.; Muller, M.J.; Oberritter, H.; Schulze, M.; Stehle, P.; Watzl, B. Critical Review: Vegetables and Fruit in the Prevention of Chronic Diseases. European Journal of Nutrition 2012, 51(6), 637–663.
- Nicoletti, I.; Martini, D.; De Rossi, A.; Taddei, F.; D’Egidio, M.G.; Corradini, D. Identification and Quantification of Soluble Free, Soluble Conjugated, and Insoluble Bound Phenolic Acids in Durum Wheat (Triticum turgidum L. var. durum) and Derived Products by RP-HPLC on a Semimicro Separation Scale. Journal of Agricultural and Food Chemistry 2013, 61(48), 11800–11807.
- Svensson, L.; Sekwati-Monang, B.; Lutz, D.L.; Schieber, A.; Ganzle, M.G. Phenolic Acids and Flavonoids in Nonfermented and Fermented Red Sorghum (Sorghum Bicolor (L.) Moench). Journal of Agricultural and Food Chemistry 2010, 58(16), 9214–9220.
- Martins, S.; Mussatto, S.I.; Martinez-Avila, G.; Montanez-Saenz, J.; Aguilar, C.N.; Teixeira, J.A. Bioactive Phenolic Compounds: Production and Extraction by Solid-State Fermentation. A Review. Biotechnology Advances 2011, 29(3), 365–373.
- Naczk, M.; Shahidi, F. Extraction and Analysis of Phenolics in Food. Journal of Chromatography A 2004, 1054(1–2), 95–111.
- Robbins, R.J. Phenolic Acids in Foods: An Overview of Analytical Methodology. Journal of Agricultural and Food Chemistry 2003, 51(10), 2866–2887.
- Garcı́a Parrilla, M.C.; Heredia, F.J.; Troncoso, A.M. Sherry Wine Vinegars: Phenolic Composition Changes During Aging. Food Research International 1999, 32(6), 433–440.
- Kenny, O.; Smyth, T.J.; Hewage, C.M.; Brunton, N.P. Antioxidant Properties and Quantitative UPLC-MS Analysis of Phenolic Compounds from Extracts of Fenugreek (Trigonella Foenum-Graecum) Seeds and Bitter Melon (Momordica Charantia) Fruit. Food Chemistry 2013, 141(4), 4295–4302.
- Plessi, M.; Bertelli, D.; Miglietta, F. Extraction and Identification by GC-MS of Phenolic Acids in Traditional Balsamic Vinegar from Modena. Journal of Food Composition and Analysis 2006, 19(1), 49–54.
- Samanidou, V.F.; Antoniou, C.V.; Papadoyannis, I.N. Gradient RP-HPLC Determination Of Free Phenolic Acids In Wines And Wine Vinegar Samples after SPE, with Photodiode Array Identification. Journal of Liquid Chromatography & Related Technologies 2001, 24(14), 2161–2176.
- Qiu, J.; Ren, C.; Fan, J.; Li, Z. Antioxidant Activities of Aged Oat Vinegar in Vitro and in Mouse Serum and Liver. Journal of the Science of Food and Agriculture 2010, 90(11), 1951–1958.
- Greco, E.; Cervellati, R.; Litterio, M.L. Antioxidant Capacity and Total Reducing Power of Balsamic and Traditional Balsamic Vinegar from Modena and Reggio Emilia by Conventional Chemical Assays. International Journal of Food Science & Technology 2013, 48(1), 114–120.
- Szwajgier, D. Content of Individual Phenolic Acids in Worts and Beers and Their Possible Contribution to the Antiradical Activity of Beer. Journal of the Institute of Brewing 2009, 115(3), 243–252.
- Tagliazucchi, D.; Verzelloni, E.; Conte, A. Contribution of Melanoidins to the Antioxidant Activity of Traditional Balsamic Vinegar During Aging. Journal of Food Biochemistry 2010, 34(5), 1061–1078.
- Troszynska, A.; Ciska, E. Phenolic Compounds of Seed Coats of White and Coloured Varieties of Pea (Pisum Sativum L.) and Their Total Antioxidant Activity. Czech Journal of Food Sciences 2002, 20(1), 15–22.
- Dykes, L.; Rooney, L.W. Sorghum and Millet Phenols and Antioxidants. Journal of Cereal Science 2006, 44(3), 236–251.
- Dykes, L.; Rooney, L.W. Phenolic Compounds in Cereal Grains and Their Health Benefits. Cereal Foods World 2007, 52(3), 105–111.
- Dykes, L.; Seitz, L.M.; Rooney, W.L.; Rooney, L.W. Flavonoid Composition of Red Sorghum Genotypes. Food Chemistry 2009, 116(1), 313–317.
- Shimoji, Y.; Tamura, Y.; Nakamura, Y.; Nanda, K.; Nishidai, S.; Nishikawa, Y.; Ishihara, N.; Uenakai, K.; Ohigashi, H. Isolation and Identification of DPPH Radical Scavenging Compounds in Kurosu (Japanese Unpolished Rice Vinegar). Journal of Agricultural and Food Chemistry 2002, 50(22), 6501–6503.
- Cerezo, A.B.; Alvarez-Fernandez, M.A.; Hornedo-Ortega, R.; Troncoso, A.M.; Garcia-Parrilla, M.C. Phenolic Composition of Vinegars over An Accelerated Aging Process Using Different Wood Species (Acacia, Cherry, Chestnut, and Oak): Effect of Wood Toasting. Journal of Agricultural and Food Chemistry 2014, 62, 4369–4376.
- Gulcin, I. Antioxidant Activity of Food Constituents: An Overview. Archives of Toxicology 2012, 86(3), 345–391.
- Rice-Evans, C.A.; Miller, N.J.; Paganga, G. Structure-Antioxidant Activity Relationships of Flavonoids and Phenolic Acids. Free Radical Biology and Medicine 1996, 20(7), 933–956.
- Natella, F.; Nardini, M.; Di Felice, M.; Scaccini, C.; Benzoic and Cinnamic Acid Derivatives As Antioxidants: Structure−Activity Relation. Journal of Agricultural and Food Chemistry 1999, 47(4), 1453–1459.
- Rossetto, M.; Vanzani, P.; Mattivi, F.; Lunelli, M.; Scarpa, M.; Rigo, A. Synergistic Antioxidant Effect of Catechin and Malvidin 3-Glucoside on Free Radical-Initiated Peroxidation of Linoleic Acid in Micelles. Archives of Biochemistry and Biophysics 2002, 408(2), 239–245.
- Dai, F.; Chen, W.-F.; Zhou, B. Antioxidant Synergism of Green Tea Polyphenols with Α-Tocopherol and L-Ascorbic Acid in SDS Micelles. Biochimie 2008, 90(10), 1499–1505.
- Liu, R.H. Potential Synergy of Phytochemicals in Cancer Prevention: Mechanism of Action. The Journal of Nutrition 2004, 134(12), 3479S–3485S.