Abstract
Riboflavin or B2, isolated from a wide variety of animals and plant products is an important vitamin commonly found in the diets of various cultures. This vitamin has been generally known to impart antimicrobial properties when exposed to ultra-violet A irradiation. In the current work, we investigated the possibility of preventing or reducing pathogenic infections using both ultra-violet A assisted and stand-alone riboflavin solutions on selected strains of bacteria. The antimicrobial properties of riboflavin were determined by the effective inhibition of the growth of pathogens through the disc diffusion method. Zones of inhibition studies indicated that riboflavin on its own was found to be quite effective and was able to inhibit the pathogens when diffused at a concentration of 50.0 µL. Stand-alone riboflavin solution successfully inhibited Staphylococcus aureus, Enterococcus faecalis, Salmonella typhi, and Pseudomonas aeruginosa with an inhibition range of (18.7 ± 0.6) mm; (17.7 ± 0.6) mm; (17.3 ± 0.6) mm, and (15.7 ± 0.6) mm, respectively. Intermediate zones of inhibition were observed for Escherichia coli and Candida albicans with a range of (11.7 ± 0.6) mm and (11.7 ± 0.6) mm, respectively. Klebsiella pneumoniae was the only resistant pathogen at (7.7 ± 0.6) mm for the riboflavin concentration used in this work. These results may indicate the exciting prospects of the applications of riboflavin as a complementary approach toward inactivation of pathogenic infections.
INTRODUCTION
Riboflavin is a naturally available yellow-green fluorescent pigment also known as lactoflavin, vitamin G, or vitamin B2. This material commanded the attention of chemists in as early as 1879.[Citation1] In the year 1936 in Pittsburgh, the American Chemical Society suggested the term flavin to be used to describe the water soluble pigment, which has been demonstrated to be necessary for the normal nutrition of rat and for growth of chicks. The terms lactoflavin and vitamin G were also terminated and the new term riboflavin was accepted as referring to vitamin B2.[Citation1]
Riboflavin (7, 8-dimethyl-10-(2R, 3R, 4S)-2, 3, 4, 5–tetrahydroxypentyl) benzo [g] pteridine-2, 4 (3H, 10H)-dione) is a naturally occurring compound essential as a human nutrient. It is present in aerobic organisms and can be found in many types of food, such as cereals, meat, especially offal, fatty fish, and certain fruits and vegetables, especially dark-green vegetables, in reasonably high concentrations. It exists as an orange-yellow crystal that is stable to heat, acid, and oxidation. However, it is also light sensitive, especially to ultraviolet (UV) radiation present in sunlight. When washing or cooking the foods for long periods of time, some amount of riboflavin will be lost. Once eaten, vitamin B2 is easily absorbed from the small intestine into the blood, which transports it to the tissues. However, excess intake will be eliminated through the urine, as riboflavin in the form of other metabolites, such as 7-hydroxymethylriboflavin (7-hydroxyriboflavin) and lumiflavin (which gives it the yellow-green fluorescent glow) are water soluble.
Riboflavin is also non-toxic, as confirmed by a broad range of testing carried out prior to its designation as “generally recognized as safe” (GRAS) by the U.S. Food and Drug Administration (FDA).[Citation2] Riboflavin is not stored in the body, except for a small quantity in the liver and kidneys,[Citation3] so it is needed regularly in the diet. Current recommended nutrient intakes in the United Kingdom range from 0.4 mg/d in infancy to 1.3 mg/d in adult females. An increment has been set to 0.3 mg in pregnancy and 0.5 mg during lactation to recover increased tissue synthesis for foetus and maternal development with riboflavin secretion in milk being ~0.010–0.55 mg.[Citation4] These values are similar to recommendations made by the World Health Organization in 1974,[Citation2] the European population reference intake[Citation3] and the United States recommended dietary allowance.[Citation5]
Riboflavin has maximum absorption at 220, 265, 375, and 446 nm in water and appears yellow-orange in color. When aqueous solutions containing riboflavin are exposed to sunlight, riboflavin will be converted into lumichrome under neutral conditions, and into lumiflavin in alkaline solutions. Lumichrome is also a known metabolic breakdown product of riboflavin in the human body. Riboflavin also functions as the precursor to two coenzymes, flavin mononucleotide (FMN) and flavin adenine dinucleotide (FAD) that are important in energy production.[Citation6] The two coenzymes act as hydrogen carriers to help make energy in the form of adenosine triphosphate (ATP) through the metabolism of carbohydrates and fats.[Citation7] Furthermore, it is instrumental in cell respiration, helping each cell utilize oxygen efficiently.[Citation3] It is also helpful in maintaining good vision, healthy hair, skin, and nails, and is necessary for normal cell growth.[Citation8]
Riboflavin is commonly used to treat and help prevent visual problems, eye fatigue, and cataracts to maintain good vision.[Citation8] It seems to help alleviate with burning eyes, excess tearing, and decreased vision resulting from eye strain. Riboflavin is also used for treating many kinds of stress conditions, fatigue, and vitality or growth problems.[Citation7] For people with allergies and chemical sensitivities, riboflavin-5-phosphate may be more readily assimilated than riboflavin.[Citation3] Riboflavin is given for skin complications such as acne, dermatitis, eczema, and skin ulcers and to maintain healthy hair, skin, and nails.[Citation3] It is also used in the treatment for alcohol-related problems; ulcers, digestive difficulties, and leg cramps. Riboflavin supplements may be advantageous for prevention or during treatment of cancer.[Citation3,Citation8] However, there are not many published research articles to support these common uses.[Citation5]
Recent observations show lack of riboflavin intake in humans even though there are many types of available riboflavin-rich foods.[Citation3,Citation8] Riboflavin deficiency or ariboflavinosis, causes cheilosis (lip chapping and fissuring), sore tongue, and scaly rashes on scrotum or vulva.[Citation3,Citation8] Deficiency has also been associated with night blindness, photosensitivity, lack of growth, inability to stand, cataract, migraine, mild anaemia, and fatigue.[Citation3,Citation8] Although rarely performed, red blood cell glutathione reductive activity can be measured for evidence of deficiency, but usually clinical findings are sufficient. Riboflavin deficiency also presents a risk factor to cancer[Citation9] and cardiovascular diseases.[Citation6,Citation10] However, most diseases, including pathogenic infections, in humans can be prevented when normal diet and riboflavin related supplements are consumed.[Citation11]
In this work, we show for the first time the efficacy of stand-alone riboflavin without ultra-violet A (UVA) assisted photo-activation on inhibiting the growth of several types of bacteria and fungus.
MATERIALS AND METHODS
Preparation of Riboflavin Stock Solution
Twenty-five grams of riboflavin-5-Phosphate (Sigma-Aldrich) was prepared in a dark environment by diluting with 25.0 mL of Phosphate Buffered Saline (InvitrogenTM). The solution was further protected from light by storing in light sensitive bottles and used within 30 min of preparation.
Preparation of the Discs
From the prepared riboflavin stock solution, 50.0 µL was applied onto 6.0 mm sterile discs placed in multiwell culture plates (Sigma-Aldrich) using a pipette. The preparation was utilized within 30 min to reduce possible light contamination and other measurement errors.
Selection of Pathogens
Microbe strains were identified and obtained from Gribbles Pathology Laboratory (Petaling Jaya, Selangor, Malaysia). The bacterial strains studied were Staphylococcus aureus (SA), Enterococcus faecalis (EF), Escherichia coli (EC), Pseudomonas aeruginosa (PA), Salmonella typhi (ST), Klebsiella pneumoniae (KP), and Candida albicans (CA). Biochemical analysis was done as confirmation tests for all the microorganisms using Analytical Profile Index (API) kits.
Preparation of Inoculums
Selected strains were grown overnight on blood agar plates. Mueller-Hinton agar plates were used for antibiotic sensitive tests for bacteria while Sabouraud’s agar plates were used for fungus. Following optimum growth, the colonies on the agar plates were streaked and the plates incubated for 18 to 24 h until equal growth was observed. The inoculums were then prepared by diluting with saline and turbidity adjusted to 0.5 McFarland standard by using the broth. To avoid changes in inoculum densities, the plates were inoculated within 30 min of standardizing the inoculums.
Inoculation of Agar Plates
The inoculums were adjusted on the agar plate and excess inoculums removed within 15 min. Following which, the entire surface was swabbed on agar plates three times and the plates rotated approximately 60° between streaking to ensure even distribution. The discs were applied to the agar surface by using sterile forceps.[Citation12,Citation13] After which, the inoculums were allowed to dry at room temperature before inverting the plates for incubation at 35–37°C for 18 to 24 h.
Experimental Design
Riboflavin treatments were tested against three types of organisms; gram positive (SA, EF) and gram negative bacteria (EC, PA, ST, KP), and fungus (CA). The assay employed was a disc diffusion susceptibility testing based on the principal where standardized inoculums of organism was swabbed onto the surface of a Muller-Hinton agar plate. A sterile disc paper (Kirby-Bauer disc) was later impregnated with riboflavin of the selected concentration and the disc placed on the cultured agar plate. In this study, the discs were placed in the culture plates as reference points to locate the areas where riboflavin was previously diffused. Riboflavin drops (50.0 µL) were placed adjacent to the discs placed on each disc during the testing of drugs in the culture plates. These pathogens were tested by using Kirby-Bauer discs with negative control (Control-C), testing drug (50.0 µL of riboflavin) and standard drug on each disc from prepared stock solutions. Experiments were performed three times on each microorganism to obtain average results.[Citation14] Commercially available antibiotics and its interpretative criteria for standard drugs according to the Clinical and Laboratory Standards Institute (CLSI)[Citation15] guidelines were vancomysin (S ≥ 15 mm; I and R no zone) used on SA and EF (S ≥ 17 mm; I 15–16 mm and R ≤ 14 mm), imipenem (S ≥ 16 mm; I 14–15 mm, and R ≤ 13 mm) on ST, gentamicin (S ≥ 15 mm; I 13–14 mm, and R ≤ 12 mm) on PA, EC, and KP, and nystatin (S ≥ 15 mm; I 10–14 mm, and R no zone) used on CA.[Citation15] Then, the agar plates were inverted and incubated for 18–24 h at 35–37°C in an incubator.[Citation12]
Analyzing Plates
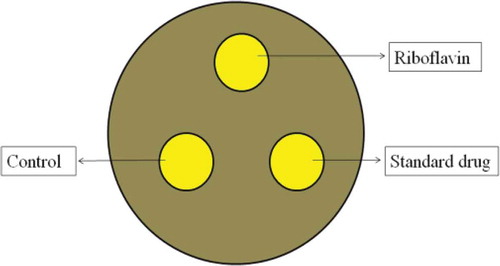
After incubation, each disc and its surrounding agar area was photographed using a 16 megapixel digital camera (Canon A3300IS). Images of each disc and its surrounding agar area were analyzed to measure the zones of inhibition (mm). Mean zone of inhibition of microorganisms were then calculated. The experimental design and the method of analysis are summarized in .
FIGURE 1 Experimental design and analysis of plates. Kirby-Bauer discs were used as a reference point with two sterile paper discs and one standard antibiotic disc. The zone of inhibition of each pathogens were measured by the diameter of the surrounding clear zone around the 6 mm disc in millimeters.
Statistical Analysis
Obtained results were analyzed using SPSS (Version 18.0) and Microsoft Excel softwares. Results were expressed as mean values with their standard errors (mean ± SD). Results from the tested drug were analyzed by using one-way analysis of variance (ANOVA) and assumed as a theoretically normal population. Post-hoc test was carried-out to observe the difference between the tested groups and to find the significant differences. Outcomes of the results were stated in terms of p-values, where p < 0.05 were considered statistically significant.
RESULTS
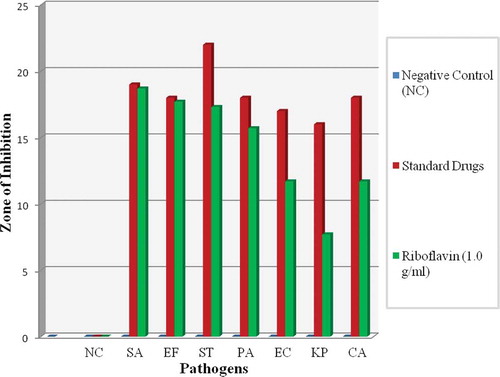
Zone of inhibition of Group 1 (gram positive bacteria) were significantly greater when compared with Group 2 (gram negative bacteria), Group 3 (fungus), and control. Riboflavin on its own was found to be very effective and able to inhibit the pathogens when diffused at a concentration of 1.0 g/mL as shown in (refer illustration). Meanwhile (refer illustration) shows the measurement of zone of inhibition of each pathogen (in mm) and its standard deviation (SD). Zones of inhibition were observed at (18.7 ± 0.6) mm for SA; (17.7 ± 0.6) mm for EF; (17.3 ± 0.6) mm for ST; and PA at (15.7 ± 0.6) mm. It was found to be moderately effective against EC at (11.7 ± 0.6) mm and CA at (11.7 ± 0.6) mm. The smallest zone of inhibition was observed for KP at (7.7 ± 0.6) mm.
TABLE 1 Average mean zone of inhibitions of selected pathogens
FIGURE 2 Testing the susceptibility of microorganisms to riboflavin solution by using Kirby-Bauer disc diffusion method. The labels refer as following: A-Staphylococcus aureus, B-Enterococcus faecalis, C-Salmonella thyphi, D-Pseudomonas aeruginosa, E-Escherichia coli, F-Klebsiella pneumoniae, and G-Candida albicans, R-Riboflavin, SD-Standard drug, and C-Control.
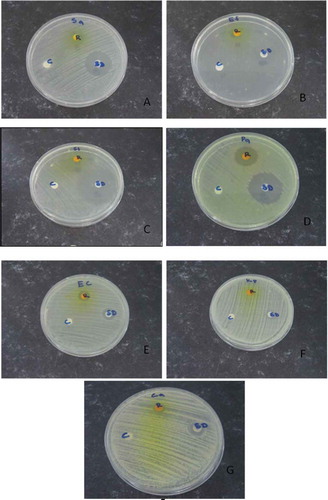
SA, EF, ST, and PA were susceptible toward riboflavin treatment while EC and CA showed intermediate reactions toward riboflavin. Meanwhile KP was found to be resistant to the riboflavin treatment. This might be due to its ability to produce extended-spectrum beta-lactamases (ESBL), which imparts these bacteria resistant to many classes of antibiotics.[Citation16,Citation17] Furthermore, presences of various peptides in mucosal surfaces might also protect the encapsulated bacteria.[Citation18] These features in general, cause the encapsulation layer to act as a shield against riboflavin activity.
In this work, the antimicrobial properties of riboflavin were determined by its effectiveness in inhibiting the growth of pathogens demonstrated by the disc diffusion method. Furthermore the concentration of riboflavin used, imparts antimicrobial properties against four out of seven pathogens tested in this study. The mean difference of riboflavin was also found to be significant against standard drugs at p = 0.001 (p < 0.05).
DISCUSSIONS
Riboflavin supplements have been used as part of the phototherapy treatment to treat neonatal jaundice, and migraine for over 30 years. Researchers such as Martins, Tsugita, Ruane, Goodrich, Corbin,[Citation14,Citation17–Citation21] and others have demonstrated that combination of riboflavin with UVA could be used to treat and inactivate pathogens in blood products. One study in the year 2000 was conducted without the application of UVA but, however, this only involved eradication of malarial infection in blood. Thomas Akompong[Citation22] used stand-alone riboflavin solution to treat malaria infection caused by Plasmodium falciparum in human blood. His studies highlighted the successful inhibition of the growth of the parasite besides demonstrating its antimicrobial properties. Coimbra and Junqueira[Citation23] meanwhile, in their studies on high doses of riboflavin and the elimination of dietary red meat observed the recovery of some motor functions in patients with Parkinson’s disease.
In this current study, we demonstrated that the selected common pathogens that cause infections to humans through blood and site of wounds could be treated with riboflavin alone. Widespread antibiotic resistance of pathogenic microorganisms urges the search for new antibiotics due to their toxic effects on the blood system and more importantly to the organs of the body. Coincidentally, this approach has also been researched for pathogens inactivation via the by-products of riboflavin after UVA.[Citation11] This mechanism affects a large list of pathogens, including bacteria, fungus, and virus.[Citation18,Citation20,Citation24,Citation25] The lack of penetration and a strong dependence on the distance from the source also causes limitation to the application of UVA, which may result in non-homogeneous microbial inactivation. This presents an obstacle for researches dealing with the treatment of blood borne infections deep within the biological tissues. In addition, the negative effects of UVA ionization on the biological tissues also deter advances in in vivo investigations. Therefore, the need for optimizing the efficacy of riboflavin without UVA combination has become quite necessary.
Riboflavin can also be acquired easily since it is a naturally occurring compound in both plants and animals. While being an essential nutrient, compounds containing antibacterial, antifungal, and anticancer could also be isolated from riboflavin.[Citation8,Citation26,Citation27] There is also a possibility that riboflavin naturally present in human may confer antimicrobial properties. This could be supported by the fact that riboflavin present in an infant’s blood may serve as a natural antimicrobial substance.[Citation28] Therefore, the antimicrobial capacity of biologically active substances from riboflavin proves to be an exciting field of research to be explored.
Furthermore, the efficacy of riboflavin was effective approximately at 50.0 µL toward inhibiting the growth of pathogens (refer ). In the non-resistant group, the efficacy of riboflavin treatment was greater against SA, EF, ST, and PA when compared with the treatment applied against EC, CA, and KP. Here, the treatment was more effective against gram positive compared to gram negative bacteria and fungus. Despite these findings, we cannot exclude these types of microorganisms from being treated with riboflavin as zones of inhibition were still observed in different levels of riboflavin solutions.
FIGURE 3 Comparison zone of inhibition between gram positive and gram negative bacteria and fungus. SA showed the highest susceptibility toward riboflavin solution compared to other pathogens. All the data were calculated through one way ANOVA and post-hoc multiple comparison tests. The results are expressed as mean ± SD for all pathogens.
CONCLUSIONS
In general, the results indicate the efficacy of stand-alone riboflavin against selected gram positive and negative bacteria and fungi. Riboflavin demonstrated comparable zones of inhibition against standard drugs for SA, EF, ST, and PA. However, KP exhibited resistance against riboflavin solution probably due to its encapsulation layer. Therefore, the current work reflects an effort to demonstrate a potentially exciting method for inactivation of human related pathogens using stand-alone riboflavin solution. We believe that this study may enable further in-depth investigations into the potentials of riboflavin as a natural alternative option for inhibiting the growth of pathogens.
FUNDING
The authors are grateful for the financial assistance sourced from various grants; High Impact Research (HIR) (UM.C/625/1/HIR/MOHE/SC/06), FRGS (FP004-2013A), UMRG (RG066/09AFR), and PPP (PG048-2012B). Facilities provided by Gribbles Pathology Laboratory Sdn Bhd, Petaling Jaya, Malaysia are also greatly acknowledged.
Additional information
Funding
REFERENCES
- Joel John, H. A stability and solubility study of riboflavin and some derivatives. Ph.D. dissertation, The University of Florida, 1954.
- Geneva: World Health Organization: WHO Handbook on Human Nutritional Requirements. Monograph series 61, 1974.
- Elson, M.H. Expected from Staying Healthy with Nutrition: The Complete Guide to Diet and Nutritional Medicine. 21th Century Ed. The False Fat Diet, the Staying Healthy Shopper’s Guide, the Detox Diet, and a Cookbook for All Seasons 2003, 114–115.
- Lindsay, H.A. B Vitamins in Breast Milk: Relative Importance of Maternal Status and Intake, and Effects on Infant Status and Function. Advances in Nutrition An International Review Journal 2012, 3, 362–369.
- National Research Council. Food and Nutrition Board, Commission on Life Sciences: Recommended Dietary Allowances; 10th Ed.; National Academy Press: Washington, DC, 1989.
- Jane, H. Riboflavin. Linus Pauling Institute, Oregon State University: Corvallis, OR, 2000–2015.
- Ball, F.M. Riboflavin in Vitamins in Foods, Analysis, Bioavailability, and Stability; Taylor and Francis Group: New York, NY, 2006; 168–175 pp.
- Powers, H.J. Riboflavin (Vitamin B-2) and Health. The American Journal of Clinical Nutrition 2003, 77, 1352–1360.
- Yuvaraj, S.; Premkumar, V.G.; Vijayasarathy, K.; Gangadaran, S.G.; Sachdanandam, P. Augmented Antioxidant Status in Tamoxifen Treated Postmenopausal Women with Breast Cancer on Co-Administration with Coenzyme Q10, Niacin, and Riboflavin. Cancer Chemotherapy Pharmacology 2008, 61(6), 933–941.
- Hassan, I.; Chibber, S.; Khan, A.A.; Naseem, I. Riboflavin Ameliorates Cisplatin Induced Toxicities Under Photo Illumination. PLOS One 2012, 7(5), e36273.
- Somer, E. The Essential Guide to Vitamins and Minerals; Harper Collins: New York, NY, 1995.
- Isenberg, H.D. ASM Clinical Microbiology Procedures Handbook 2004, 1, 5.1.1–5.1.12.
- Clinical and Laboratory Standards Institute (CLSI). Performance Standards for Antimicrobial Disk Susceptibility Tests; Approved Standard-Eleventh Edition. CLSI document M02-All. Clinical and Laboratory Standards Institute: Wayne, PA, 2012
- Martins, S.A.; Combs, J.C.; Noguera, G. Antimicrobial Efficacy of Riboflavin/UVA Combination (365 Nm) in Vitro for Bacterial and Fungal Isolates: A Potential New Treatment for Infectious Keratitis. Investigative Ophthalmology & Visual Science 2008, 49, 3402–3408.
- Clinical and Laboratory Standards Institute (CLSI). Performance Standards for Antimicrobial Susceptibility Testing; Seventeenth Informational Supplement. CLSI document M100-S17. Clinical and Laboratory Standards Institute: Wayne, PA, 2007.
- Cress, B.F.; Englaender, J.A.; He, W.; Kasper, D.; Linhardt, R.J.; Koffas, M.A. Masquerading Microbial Pathogens: Capsular Polysaccharides Mimic Host-Tissue Molecules. FEMS Microbiology Reviews 2014, 38(4), 660–697.
- Tsugita, A.; Okada, Y.; Uchara, K. Photosensitized Inactivation of Ribonucleic Acids in the Presence of Riboflavin. Biochimica et Biophysica Acta 1965, 103, 360–363.
- Llobet, E.; Tomas, J.M.; Bengoechea, J.A. Capsule Polysaccharide Is a Bacterial Decoy for Antimicrobial Peptides. Microbiology 2008, 154, 3877–3886.
- Ruane, P.H.; Edrich, R.; Gampp, D.; Keil, S.D.; Leonard, R.L.; Goodrich, R.P. Photochemical Inactivation of Selected Viruses and Bacteria in Platelet Concentrates Using Riboflavin and Light. Transfusion 2004, 44, 877–885.
- Goodrich, R.P. The Use of Riboflavin for Inactivation of Pathogens in Blood Products. Vox Sanguinis 2000, 78, 211–215.
- Corbin, F. Pathogen Inactivation of Blood Components: Current Status and Introduction of An Approach Using Riboflavin As a Photosensitizer. International Journal of Hematology 2002, 76(S2), 253–257.
- Akompong, T.; Ghori, N.; Haldar, K. In Vitro Activity of Riboflavin Against the Human Malaria Parasite Plasmodium Falciparum. Antimicrobial Agents and Chemotherapy 2000, 44, 88–96.
- Coimbra, C.; Junqueira, V. High Dose of Riboflavin and the Elimination of Dietary Red Meat Promote the Recovery of Some Motor Functions in Parkinson’s Disease Patients. Brazilian Journal of Medical and Biological Research 2003, 36, 1409–1417.
- Samar, B.M.; Goodrich, R.P. Viral Inactivation in Plasma Using Riboflavin-Based Technology. Transfusion 2001, 41, 88S.
- McAteer, M.J.; Tay-Goodrich, B.; Doane, S. Photoinactivation of Virus in Packed Red Blood Cell Units Using Riboflavin and Visible Light. Transfusion 2000, 3, 99S.
- Thakuri, P.S.; Joshi, R.; Basnet, S.; Pandey, S.; Taujale, S.D.; Mishra, N. Antibacterial Photodynamic Therapy on Staphylococcus Aureus and Pseudomonas Aeruginosa in Vitro. Nepal Medical College Journal 2011, 13(4), 281–284.
- Imrana, S. Importance, Polarographic Behaviour, and Determination of Riboflavin. Asian Journal of Biochemical and Pharmaceutical Research 2012, 2(2), 69–81.
- Sauer, A.; Letscher-Bru, V.; Speeg-Schatz, C.; Touboul, D.; Colin, J.; Candolfi, E.; Bourcier, T. in Vitro Efficacy of Antifungal Treatment Using Riboflavin/UV-A (365 nm) Combination and Amphotericin B. Investigate Ophthalmology & Visual Science 2010, 51(8), 3950–3953.
