?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Thymus capitatus has traditionally been considered as an anthelmintic, antispasmodic, carminative, emmenagogue, expectorant, rubefactient, sedative, stimulant, and tonic. This work was carried out to determine total polyphenol and total flavonoids chemical composition of phenolics and to evaluate the antioxidant activities of the methanolic extract. Total phenolic contents were assayed using the Folin–Ciocalteu reagent, total flavonoid content was measured spectrophotometrically and phenolics were analyzed by reverse phase–high performance liquid chromatography. The 1,1-diphenyl-2-picrylhydrazyl and reducing power were calculated. Total phenols and total flavonoids varied significantly among the studied regions. Chromatographic analysis by reverse phase–high performance liquid chromatography showed that phenolic acids: tannic, gallic, chlorogenic hemihydrate, caffeic, syringic, ferulic, p-coumaric acid, and trans-cinnamic rosmarinic were the main compounds. The anti-radical activity was region-dependent and could be summarized as follows in ascending order: Boukornine > Kef > Bizerte > Grombelia. Reducing power in the four studied regions was stronger than positive control (ascorbic acid). From these results we have conclude that thyme methanolic extract may have a role in pharmaceutical preparations and preservatives as an antioxidant.
Introduction
The genus Thymus which belongs to the family Lamiaceae (Labiatae) includes 350 species widespread all around the world. Recently, the essential oils of various species of the Thymus genus have been screened for their traditional indigenous uses and intensively investigated as promised sources of antibacterial, antifungal, antioxidant, and other natural products. Therefore, various species were studied for their antioxidant activities.[Citation1] In Tunisian flora,[Citation2] the genus Thymus is mainly represented by Thymus capitatus, a perennial, herbaceous shrub commonly used as a spicy herb and locally known under the common name “zaâtar.” On the other hand, antioxidants are compounds that neutralize chemically active products of metabolism, such as free radicals, which can damage the body. Plant phenols and polyphenols, with their potential to act as antioxidants; play a major role in the prevention of various pathological conditions, such as cancer, cardiovascular and neurodegenerative diseases, believed to be associated with oxidative stress.[Citation3] Plant phenols also exhibit significant antioxidant, antitumoral, antiviral, and antibiotic properties.[Citation4] Safety and efficacy of the synthetic antioxidants used in the food industry are frequently questioned because such antioxidants are unstable and highly volatile,[Citation5] therefore, interest in finding naturally occurring antioxidants that have the potential to protect human beings from damage induced by oxidative stress has intensified.[Citation6] To the best of our knowledge, there is no literature information available on the chemical compositions and biological activities of methanolic extract of the Tunisian T. capitatus as affected by the growing region. Therefore, this work is the first report about the chemical compositions of methanolic extract and antioxidant activities of the aerial parts of the plant.
Materials and methods
Chemicals and Reagents
All solvents used in the experiments were purchased from LAB-SCAN. Chlorhydric acid (HCl), trifluoroacetic acid (TFA), butylated hydroxytoluene (BHT), ethylenediaminetetraacetic acid (EDTA), 3-(2-pyridyl)-5,6-bis(4-phenyl-sulphonic acid)-1,2,4-triazine (ferrozine), iron (II) chloride tetrahydrate (FeCl2.4H2O), iron (II) chloride (FeCl2), iron (III) chloride (FeCl3), 1,1-diphenyl-2-picrylhydrazyl (DPPH), and (2,4,6 tripyridyl-s-triazine; [TPTZ]) were purchased from Sigma-Aldrich (Steinheim, Germany).
Plant Material
T. capitatus aerial parts were harvested randomly by hand (three times) from plants growing wild in different Tunisian regions (Kef, Bizerte, Boukornine, and Grombelia). The differences between studied regions were summarized in . The harvested plants were identified by professor Abderrzek Smaoui (Biotechnology Center in Borj-Cedria Technopole, Tunisia) and according to the Tunisian flora.[Citation2] The voucher specimen was deposited at the herbarium of the Laboratory of Bioactive Substances, Biotechnology Center in Borj-Cedria Technopole under the “TC2011-2405” number. After that, the aerial parts samples were air-dried and then ground to fine powder by an electric mill and conserved in a dessicator at room temperature (~25°C) in darkness for further extractions.
TABLE 1 Geographical and bioclimatic collection sites parameters
Polyphenol Extraction
The air-dried aerial parts were finely ground with a blade-carbide gringing (IKA-WERK Type: A: 10). Triplicate sub-samples of 1 g of each ground organ were separately extracted by stirring with 10 mL of pure methanol for 30 min. The extracts were then kept for 24 h at 4°C, filtered through a Whatman No. 4 filter paper, evaporated under vacuum to dryness and stored at 4°C until analyzed.[Citation7]
Total Phenolic Content
Total phenolic contents were assayed using the Folin–Ciocalteu reagent, following Singleton’s method slightly modified by Dewanto et al.[Citation8] An aliquot (0.125 mL) of a suitable diluted methanolic organ extract was added to 0.5 mL of deionized water and 0.125 mL of the Folin–Ciocalteu reagent. The mixture was shaken and allowed to stand for 6 min, before adding 1.25 mL of 7% Na2CO3 solution. The solution was then adjusted with deionized water to a final volume of 3 mL and mixed thoroughly. After incubation for 90 min at 23°C, the absorbance versus prepared blank was read at 760 nm. Total phenolic contents of aerial parts (three replicates per treatment) were expressed as mg gallic acid equivalents per gram (mg GAE/g) through the calibration curve with gallic acid. The calibration curve range was 50–400 mg/mL (R2 = 0.99). All samples were performed in triplicates.
Total Flavonoid Content
Total flavonoid content was measured according to Dewanto et al.[Citation8] Of the 250 µL methanolic aerial extract appropriately diluted was mixed with 75 µL NaNO2 (5%). After 6 min, 150 µL of 10% AlCl3 and 500 µL of NaOH (1 M) were added to the mixture. Finally, the mixture was adjusted to 2.5 mL with distilled water. The absorbance versus prepared blank was read at 510 nm. Total flavonoid contents of aerial parts (three replicates per treatment) were expressed as mg catechin equivalents per gram (mg CE/g) through the calibration curve with catechin. The calibration curve range was 50–500 mg/mL.
Analysis of Individual Phenolic Compounds by Analytical Reverse Phase–High-Performance Liquid Chromatography (RP–HPLC)
Air-dried samples from aerial parts of thyme were hydrolyzed according to the method of Proestos et al.[Citation9] which was slightly modified. The acidic hydrolysis was used to release the aglycones in order to simplify the identification process since the free forms of phenolic compounds are rarely present in plants and they occur as esters, glycosides or bound to the cell wall.[Citation10] Of 20 mL methanol containing BHT (1 g/L) were added to 0.5 g of a dried sample. Then 10 mL of 1 M HCl were added. The mixture was stirred carefully and sonicated for 15 min and refluxed in a water bath at 90°C for 2 h. The obtained mixture was injected to HPLC. The phenolic compound analysis was carried out using an Agilent Technologies 1100 series liquid chromatograph (RP–HPLC) coupled with an UV–vis multiwavelength detector. The separation was carried out on a 250 × 4.6-mm, 4 µm Hypersil ODS C18 reversed phase column at ambient temperature. The mobile phase consisted of acetonitrile (solvent A) and water with 0.2% sulphuric acid (solvent B). The flow rate was kept at 0.5 mL/min. The gradient program was as follows: 15%A/85%B 0–12 min, 40%A/60%B 12–14 min, 60%A/40%B 14–18 min, 80%A/20%B 18–20 min, 90%A/10%B 20–24 min, and 100%A 24–28 min.[Citation11] The injected volume was 20 µL, and peaks were monitored at 280 nm. Samples were filtered through a 0.45 µm membrane filter before injection. Phenolic compounds were identified according to their retention times and spectral characteristics of their peaks against those of standards, as well as by spiking the sample with standards. Analyses were performed in triplicate.
Antioxidant Activity
DPPH assay
The electron donation ability of the aerial parts extracts was measured by bleaching of the purple-colored solution of DPPH radical according to the method of Hanato et al.[Citation12] One half milliliter of 0.2 mM DPPH methanolic solution was added to aerial parts of T. capitatus (2 mL, 10–1000 μg/mL). After an incubation period of 30 min at room temperature, the absorbance was read against a blank at 517 nm. The inhibition percentage of free radical DPPH (IP%) was calculated as follows:
where Ablank is the absorbance of the control reaction and Asample is the absorbance in the presence of plant extract. Extract concentration providing 50% inhibition (IC50) was calculated from the regression equation prepared from the concentration of the extracts and the inhibition percentage. BHT was used as a positive control (R2 = 0.93).
Reducing power
The method of Oyaizu[Citation13] was used to assess the reducing power of T. capitatus aerial parts extracts. These extracts (1 mL) were mixed with 2.5 mL of a 0.2M sodium phosphate buffer (pH = 6.6) and 2.5 mL of 1% potassium ferricyanide (K3Fe (CN)6) and incubated in a water bath at 50°C for 20 min. Then, 2.5 mL of 10% trichloroacetic acid was added to the mixture that was centrifuged at 650 g for 10 min. The supernatant (2.5 mL) was then mixed with 2.5 mL of distilled water and 0.5 mL of 0.1% iron (III) chloride solution. The intensity of the blue green colour was measured at 700 nm. The extract concentration at which the absorbance was 0.5 for the reducing power (EC50) was obtained from the linear regression equation prepared from the concentrations of the extracts and the absorbance values. High absorbance indicates high reducing power. Ascorbic acid was used as a positive control (R2 = 0.97).
Data Analysis
All analyses were performed in triplicate, and the results are expressed as mean values ± standard deviations (SD). The data were subjected to statistical analysis using statistical program package.[Citation14] The one-way analysis of variance (ANOVA) followed by the Duncan multiple range test was employed and the differences between individual means and each means used were deemed to be significant at p < 0.05. A principal component analysis (PCA) was performed in order to discriminate between different regions of collection on the basis of their phenolic composition.
Results and Discussion
Total Phenols and Flavonoids Content
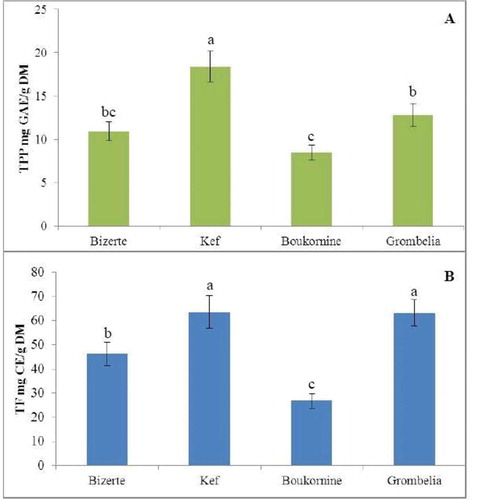
Contents of total phenols and total flavonoids are shown in and . The total phenol contents varied significantly (p < 0.05) with regards to the region of collection and reaches a maximum in the region of Kef (18.40 mg EAG/g DM) followed by the region of Grombelia, Bizerte, and Boukornine where levels were 18.40, 10.92, and 8.44 mg EAG/g DM, respectively. As for total polyphenols, total flavonoids differ significantly (p < 0.05) depending of the collection region, but with higher contents than those of total polyphenols with maximum amounts detected in the regions of Kef and Grombelia. The total flavonoid contents were 63.64, 63.26, 46.31, and 26.83 mg CE/g DM in the regions of Kef, Grombelia, Bizerte, and Bokornine, respectively. These differences could probably be due to the differences in the soil, climate, temperature, humidity, and solar lighting in each studied region that could have effects on the samples (). In addition, Safaei-Ghomi et al.[Citation15] reported that the total polyphenol content in the sub-polar fraction of Iranian Thymus caramanicus was 124.3 mg GAE/mg which was significantly higher when compared with our results. Previous work showed that total phenol were 15.06 ± 0.73 mg GAE/g DM and total flavonoid were 10.62 ± 0.24 mg CE/g DM in thyme dried aerial parts.[Citation16] Total polyphenol content which is higher than in our work was found by Aidi Wannes et al.[Citation17] in flowers of two varieties of Tunisian myrte: baetica and italica. These same authors have reported a higher content (0.5 mg CE/g DM) in flavonoids.
TABLE 2 ANOVA analysis and phenolic composition (expressed in %, w/w, and in mg/g DM) of methanolic extracts of thyme aerial parts
Identification and Quantification of Phenolic Compounds by HPLC
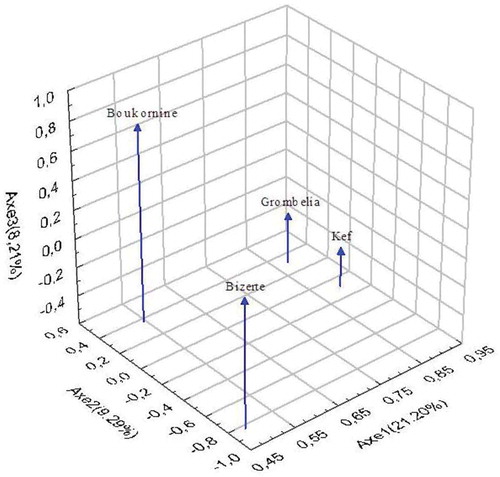
Chromatographic analysis by RP–HPLC of aerial parts of Thymus capitatus methanolic extracts collected from four different regions is summarized in . All the identified compounds are highly (p < 0.001) influenced by region factor. Phenolic acids: tannic, gallic, chlorogenic hemihydrate, caffeic, syringic, ferulic, and p-coumaric acid, trans-cinnamic rosmarinic represent the major compounds of polyphenols analyzed with proportions ranging from 64.52 ± 5.87% at the region of Grombelia to 44.59 ± 5.34% at the Bizerte one. In the latter region, trans-cinnamic acid was the major phenolic acid (13.41 ± 1.42%, 0.87 ± 0.09 mg/g DM), whereas in the region of Boukornine, chlorogenic acid hemihydrate was the predominant compound (14.29 ± 1.55%, 1.23 ± 0.14 mg/g DM). Regions of Kef and Grombelia were characterized by the dominance of rosmarinic acid with proportions of 33.26 ± 4.26% (1.45 ± 0.11 mg/g DM) and 32.79 ± 2.91% (4.35 ± 0.59 mg/g DM), respectively. Chromatographic analysis identified three flavonoids belonging to the class of flavonols (myristine, quercetine dihydrite and campherol), 2 flavanols (catechine and epicatechine), a flavanone (naringenine), two flavonoids (apigenin and flavone), and coumarin and resorcinol which were detected only in Bizerte. The compounds listed above vary significantly depending on the region (). Previous studies have found that phenolic compounds such as rosmarinic, caffeic, ferulic, and carnosic acids, and flavonoids such as quercetin, apigenin, and luteolin, were identified by Jordán et al.[Citation18] in some species of the genus Thymus. In addition, Kruma et al.[Citation19] have studied the phenolic compounds of basil and thyme and concluded that thyme is richer in phenolic compounds. According to these authors, basil is six times less rich in polyphenols than thyme. Recent studies have shown that natural antioxidants, such as polyphenols, are often incorporated into foods to ensure their stability and protect oxidation.[Citation20] Indeed, phenolic acids such as gallic and vanillic acids are involved in the organoleptic, nutritional, and antioxidant foods.[Citation21] Gallic acid is widely used as an additive to prevent food from spoilage, and is known for its activities, anti-carcinogenic, anti-inflammatory, and antimutagenic.[Citation22] In addition, PCA was undertaken to discriminate between the studied regions regarding their phenolic composition (). A better discrimination was revealed on the three-dimensional visualization of the plotted scores, where the three PC accounted for 38.7% of total variance. As shown in () Kef and Grombelia constitute the first group and the second was formed by Bizerte and Boukornine regions suggesting similar phenolic composition. These results confirmed that phenolic compounds distribution was region-dependant.
Antioxidant Activity
Anti-radical power of the thyme methanol extract
The radical scavenging power was determined by following the decrease in the absorbance at 517 nm which is induced by antioxidants that act as hydrogen donors.[Citation23] This extract is highly influenced by the region of collect (anti-radical activity of methanolic extracts showed activity region-dependent). Boukornine extract shows the highest activity (10 ± 0.98 µg/mL) and this activity is stronger than that positive control; BHT (16 ± 1.89 µg/mL). The lowest activity was detected in the region of Grombelia (50 ± 4.32 µg/mL). The anti-radical activity of the four regions could be summarized as follows in ascending order: Boukornine > Kef > Bizerte > Grombelia. Bounatirou et al.[Citation1] reported a lower antiradical activity with an IC50 exceeding 250 µg/mL of methanol extract and essential oil of thyme flowers. According to these authors, concentrations of 750 and 1000 mg/mL of the extract lead to the most effective antioxidant capacity. Statistical analysis revealed on positive and not significant correlation coefficient (R = 0.09) between the antiradical activity and total polyphenol content. In this case, the anti-radical activity is due to the quality of the extract, not to the quantity. Different results were found by Wojdyło et al.[Citation24] who reported a highly significant positive correlation (R = 0.83; p < 0.05) between the anti-radical activity of Lamiaceae and total polyphenols demonstrating the importance of these antioxidant compounds in spices and their significant contribution to the total antioxidant activity.
Reducing power
The obtained results demonstrated that the methanol extracts of thyme aerial parts was characterized by EC50 values lower than that of the positive control; ascorbic acid (EC50 = 32 µL). This can be explained by the presence of natural antioxidants such as phenolic compounds.[Citation1] According Hazzit et al.[Citation25] the reducing power of Thymus algeriensis essential oil with minor quantities of thymol and carvacrol was significantly lower than that of an essential oil rich in these two phenols. Bounatirou et al.[Citation1] noted higher EC50 values (0.4 to 1000 µg/mL) indicating a lower antioxidant activity of the Thymus capitatus essential oil. Contrary to the results presented by Wojdyło et al.[Citation24] on the antioxidant activity of Lamiaceae, we found a negative correlation and not significant (r = –0.17; p < 0.05) between total polyphenol content and the reducing power of the methanolic extract of the aerial parts. This means that the activity is mainly due to the quality of polyphenols in the methanol extract of thyme aerial parts and not to their individual quantities.
Conclusion
The obtained results of the present study showed that polyphenols and flavonoids of thyme methanolic extract have a high potential antioxidant activities. Anti-radical activity of the four regions could be summarized as follows in ascending order: Boukornine > Kef > Bizerte > Grombelia. However, these metabolites could be used as natural additives in the food industry, pharmaceutical industries, and in medical preparations. Further chromatographic purification of extracts and phenolic compound isolation were necessary to understand their mechanism of action.
References
- Bounatirou, S.; Smiti, S.; Miguel, M.G.; Faleiro, L.; Rejeb, M.N.; Neffati, M.; Costa, M.M.; Figueiredo, A.; Barroso, J.G.; Pedro, L.G. Chemical Composition Antioxidant and Antibacterial Activities of the Essential Oils Isolated from Tunisian Thymus capitatus Hoff et Link. Food Chemistry 2007, 105, 146–155.
- Pottier-Alapetite, G. Tunisian Flora. Angiosperms-Dicotyledons Gamopetalaes. Official Printing of the Republic of Tunisia. Tunis, Tunisia. 1979; 1074 pp.
- Losso, J.N.; Shahidi, F.; Bagchi, D. Anti-Angiogenic Functional and Medicinal Foods; Taylor & Francis: Boca Raton, FL, 2007.
- Apak, R.; Guclu, K.; Demirata, B.; Ozyurek, M.; Celik, S.E.; Bektasolu, B.; Berker, K.I.; Özyurt, D. Comparative Evaluation of Various Total Antioxidant Capacity Assays Applied to Phenolic Compounds with the CUPRAC Assay. Molecules 2007, 12, 1496–1547.
- Sokmen, A.; Gulluce, M.; Akpulat, H.A.; Daferera, D.; Tepe, B.; Polissiou, M.; Sokmen, M.; Sahin, F. The in Vitro Antimicrobial and Antioxidant Activities of the Essential Oils and Methanol Extracts of Endemic Thymus Spathulifolius. Food Control 2004, 15, 627–634.
- Scalbert, A.; Manach, C.; Morand, C.; Remesy, C. Dietary of Polyphenols and the Prevention of Diseases. Critical Reviews in Food Science and Nutrition 2005, 45, 287–306.
- Mau, J.L.; Chao, G.R.; Wu, K.T. Antioxidant Properties of Methanolic Extracts from Several Ear Mushrooms. Journal of Agriculture and Food Chemistry 2001, 49, 5461–5467.
- Dewanto, V.; Wu, X.; Adom, K.; Liu, R.H. Thermal Processing Enhances the Nutritional Value of Tomatoes by Increasing Total Antioxidant Activity. Journal of Agriculture and Food Chemistry 2002, 50, 3010–3014.
- Proestos, C.; Boziaris, I.S.; Nychas, G.J.E.; Komaitis, M. Analysis of Flavonoids and Phenolic Acids in Greek Aromatic Plants: Investigation of Their Antioxidant Capacity and Antimicrobial Activity. Food Chemistry 2006, 95, 664–671.
- Nuutila, A.M.; Kammiovirta, K.; Oksman-Caldentey, K.M. Comparison of Methods for the Hydrolysis of Flavonoids and Phenolic Acids from Onion and Spinach for HPLC Analysis. Food Chemistry 2002, 76, 519–525.
- Bourgou, S.; Ksouri, R.; Bellila, A.; Skandarani, I.; Falleh, H.; Marzouk, B. Phenolic Composition and Biological Activities of Tunisian Nigella Sativa L. Shoots and Roots. Comptes Rendus Biologies 2008, 331, 48–55.
- Hanato, T.; Kagawa, H.; Yasuhara, T.; Okuda, T. Two New Flavonoids and Other Constituents in Licorice Root: Their Relative Astringency and Radical Scavenging Effect. Chemical and Pharmaceutical Bulletin 1988, 36, 1090–1097.
- Oyaizu, M. Studies on Products of Browning Reaction: Antioxidative Activity of Products of Browning Reaction. Japanese Journal of Nutrition 1986, 44, 307–315.
- Statsoft. STATISTICA for Windows (Computer Program Electronic Manual); Statsoft Inc.: Tulsa, OK, 1998.
- Safaei-Ghomi, J.; Ebrahimabadi, A.H.; Djafari-Bidgoli, Z.; Batooli, H. GC/MS Analysis and in vitro Antioxidant Activity of Essential Oil and Methanol Extracts of Thymus caramanicus Jalas and Its Main Constituent Carvacrol. Food Chemistry 2009, 115, 1524–1528.
- Jabri Karoui, I.; Bettaieb, I.; Msaada, K.; Hammami, M.; Marzouk, B. Research on the Phenolic Compounds and Antioxidant Activities of Tunisian Thymus capitatus. Journal of Functional Foods 2012, 4, 661–669.
- Aidi Wannes, W.; Mhamdi, B.; Sriti, J.; Ben Jemia, M.; Ouchikh, O.; Hamdaoui, G.; Kchouk, M.E.; Marzouk, B. Antioxidant Activities of the Essential Oils and Methanol Extracts from Myrtle (Myrtus communis var. italica L.) Leaf Stem and Flower. Food and Chemical Toxicology 2010, 48, 1362–1370.
- Jordán, M.J.; Martínez, R.M.; Martínez, C.; Moñino, I.; Sotomayor, J.A. Polyphenolic Extract and Essential Oil Quality of Thymus Zygis ssp. Gracilis Shrubs Cultivated Under Different Watering Levels. Industrial Crops and Products 2009, 29, 145–153.
- Kruma, Z.; Kreicbergs, V.; Adams, A. Volatile Compounds in Oils Aromatized with Spices Grown in Latvia. Chemina Technologia 2006, 4, 74–79.
- Espin, J.C.; Garcia-Conesa, M.T.; Tomas-Barberan, F.A. Nutraceuricals: Facts and Fiction. Phytochemistry 2007, 68, 2986–3008.
- Rodriguez Vaquero, M.J.; Alberto, M.R.; Manca De Nadra, M.C. Antibacterial Effect of Phenolic Compounds from Different Wines. Food Control 2007, 18, 93–101.
- Soong, Y.Y.; Barlow, P.J. Quantification of Gallic Acid and Ellagic Acid from Longan (Dimocarpus longan Lour.) Seed and Mango (Mangifera indica L.) Kernel and Their Effects on Antioxidant Activity. Food Chemistry 2006, 97, 524–530.
- Soares, J.R.; Dinis, T.C.P.; Cunha, A.P.; Almeida, L.M. Antioxidant Activities of Some Extracts of Thymus zygis. Free Radical Research 1997, 26, 469–478.
- Wojdyło, A.; Oszmianski, J.; Czemerys, R. Antioxidant Activity and Phenolic Compounds in 32 Selected Herbs. Food Chemistry 2007, 105, 940–949.
- Hazzit, M.; Baaliouamer, A.; Verissimo, A.R.; Faleiro, M.L.; Miguel, M.G. Chemical Composition and Biological Activities of Algerian Thymus Oils. Food Chemistry 2009, 116, 714–721.