Abstract
The profiles of tocopherol (T) and tocotrienol (T3) homologues in 37 samples of seven different types of bran (rye, wheat, oat, spelt, buckwheat, rice, and corn), available on the Polish market, were studied. Tocochromanols were identified and quantified by reverse phase-high-performance liquid chromatograph/fluorescence detector and reverse phase-ultra performance liquid chromatography-electrospray ionization/mass spectrometry. Only rice bran contained all eight tocochromanol types. Corn bran lacked β-T3; rye, wheat, oat, and spelt bran lacked γ-T3 and δ-T3; and buckwheat bran lacked β-T3, γ-T3, and δ-T3. In buckwheat and corn bran tocopherols predominated (98 and 78%, respectively); whereas rye, wheat, oat, spelt, and rice bran were rich in tocotrienols (78, 76, 66, 87, and 66%, respectively). The average total tocochromanol contents in the oat, corn, spelt, buckwheat, wheat, rye, and rice bran were 5.5, 16.2, 15.8, 14.7, 12.8, 10.7, and 9.1 mg/100 g of dry weight, respectively. Tocochromanol concentrations in samples of the same type bran from different sources varied considerably. Better labeling of bran products to reflect this variation would assist with control of vitamin E daily dietary requirements.
INTRODUCTION
The term “bran” describes the by-product of different cereal grains obtained during white flour processing; however, is a term that still remains without a standardized chemical definition. Bran composition is associated with several different factors such as grain species, variety, maturity, shape, kernel size, thickness of the outermost layer, size of germ, duration and condition of grain storage, as well as the system of grain conditioning before and during the milling process.[Citation1] All of these factors contribute to a different phytochemical composition of the same type of bran, produced by various manufacturers.
The utilization of, for instance, wheat bran in the feed and food industry has increased significantly over the last decade. Between 2001 and 2011 the number of developed products in the food sector containing wheat bran as one of the ingredients increased from 52 to 798. Over 58% of those products on the market consisted of baked and cereal foodstuffs, which were consumed daily.[Citation2] Improved management of the harvested plant material and high nutritional value of the bran may have contributed to this trend. The most studied types of bran are rye and wheat, which are a valuable source of dietary fiber, proteins, minerals, fats, sterols, stanols, alkylresorcinols, phenolic acids, lignans, and tocochromanols.[Citation3] A significant amount of data on the phytochemical composition of rice bran also exists.[Citation4] Unfortunately, there is either a lack of or a very limited amount of information about buckwheat, corn (maize), oat, and spelt bran tocochromanol composition.
Tocochromanols are fat-soluble molecules with unique biological activity[Citation5] and significant antioxidant activity, which can be increased or decreased during synergistic or antagonistic interactions, respectively, with phenolic compounds.[Citation6] Of all eight bioactive forms of vitamin E—namely four tocopherol (T) and four tocotrienol (T3) homologues (α, β, γ, and δ)—the α-T seems to be the most extensively studied in both, model (liposomal) and biological systems.[Citation5,Citation7] Much less is known about T3s and their health benefits, but they are considered as a “potential ally” against cancer and other chronic diseases of the 21st century.[Citation8]
Plant oils and margarines are among the richest natural sources of Ts and T3s,[Citation9–Citation12] and these products are major sources of vitamin E in the daily diet of a significant part of the human population. Bran, rich in tocochromanols, may also be considered as an important and valuable source of vitamin E in the daily diet. Moreover, the number of commercially available food sector products that contain bran is increasing each year. Nonetheless, most bran products lack detailed information on the label about their vitamin E content. Hence, the present study was performed. This investigation included the comprehensive analysis of 37 samples of different type of bran—rye (6), wheat (6), oat (7), spelt (7), buckwheat (4), rice (5), and corn (2)—from various manufacturers, available in the Polish market.
MATERIALS AND METHODS
Reagents
Ethyl acetate, n-hexane, ethanol and methanol (high-performance liquid chromatography [HPLC] grade), potassium hydroxide, sodium chloride, and pyrogallol were obtained from Sigma-Aldrich (Steinheim, Germany). T3 and T homologues (α, β, γ, and δ) standards (>95% of purity) were received from LGC Standards (Teddington, Middlesex, UK) and Merck (Darmstadt, Germany), respectively.
Bran Samples
Thirty-seven commercially available samples of different types of bran: rye (6), wheat (6), oat (7), spelt (7), buckwheat (4), rice (5), and corn (2), of 18 different producers were provided directly by manufacturers or distributors located in Poland. The samples of bran (15 ± 2 g) were milled with a KnifetecTM 1095 (Foss, Höganäs, Sweden) universal laboratory mill to pass through a sieve of 0.75 mm mesh size to finally obtain a powder, which was directly used in micro-saponification and extraction of tocochromanols. Dry weight basis (dw) in the studied samples were measured gravimetrically.
Micro-Saponification and Extraction of Ts and T3s
Micro-saponification and extraction of T and T3 homologues were performed according to Górnaś, et al.[Citation13] Powdered bran (0.1 g) was placed in a 15 mL tube, followed by 0.05 g of pyrogallol, 2.5 mL of ethanol, and 0.25 mL of aqueous potassium hydroxide (600 g/L). The tube was immediately closed, mixed for 10 s. on the Vortex REAX top (Heidolph, Schwabach, Germany) and incubated in a water bath at 80°C for a total of 25 min, during which, after 10 min, samples were mixed (10 s). After incubation, samples were immediately cooled in an ice-water bath and 2.5 mL of sodium chloride (10 g/L) was added; the samples were mixed (5 s), 2.5 mL of n-hexane:ethyl acetate (9:1; v/v) was added, and the samples were mixed (15 s) and centrifuged (5 min, 1000 × g, at 4°C). The organic layer was removed to a round bottom flask and the residue was re-extracted with 2.5 mL of n-hexane:ethyl acetate (9:1; v/v) as before (three times), and the combined organic layer fractions were evaporated in a vacuum rotary evaporator at 40°C and re-dissolved in 2 mL ethanol.
Determination of Tocochromanols by Reverse Phase-High-Performance Liquid Chromatograph/Fluorescence Detector (RP-HPLC/FLD)
Tocochromanols were determined as described by Górnaś et al.,[Citation14] using a Shimadzu HPLC system (Shimadzu, Kyoto, Japan) consisting of a fluorescence detector (RF-10AXL)—set an excitation of 295 nm and emission of 330 nm—and a Luna PFP column (3 μm, 150 × 4.6 mm) with a guard column (4 × 3 mm; Phenomenex, Torrance, CA, USA). The analysis was performed under the following conditions: mobile phase methanol:water (93:7; v/v); flow (1.0 mL/min); column oven temperature (40°C); room temperature (22 ± 1°C); and runtime (13 min).
Identification of Tocochromanols by Reverse Phase-Ultra Performance Liquid Chromatography-Electrospray Ionization/Mass Spectrometry (RP-UPLC-ESI/MSn)
The mass spectrometry analyses were conducted as previously described for T[Citation15] and T3[Citation16] homologues. The chromatographic separation was carried out on an Acquity Ultra Performance LC system (Waters, Milford, MA, USA) and a KinetexTM PFP (2.6 µm, 150 × 4.6 mm), Phenomenex, Torrance, CA, USA) column. The methanol:water mixture (9:1, v/v) was used as the mobile phase at the flow rate of 0.8 mL/min and at 20°C temperature of the column oven. The QTRAP5500 mass spectrometer (AB SCIEX, Framingham, MA, USA) equipped with an electrospray ionization (ESI) source in the positive ion mode was under the following operating conditions: 30 psi curtain gas, 5500 V ion spray voltage, 550°C temperature, 10 V entrance potential, and 50 V declustering potential.
Statistical Analysis
Each bran sample was analyzed in three replicates and the results are presented as means of n samples of the same type of bran ± standard deviation. The P-value < 0.05 was used to denote significant differences between mean values of different type of bran determined by Scheffe’s test with the assistance of Statistica 10.0 software (StatSoft, Tulsa, OK, USA).
RESULTS AND DISCUSSION
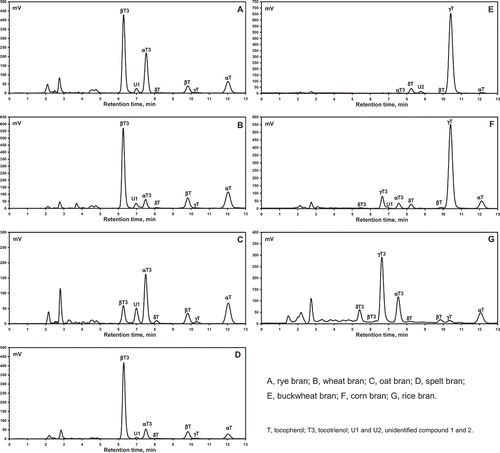
All four T homologues were detected in all the bran samples examined. However, the distribution of T3s was more varied. Only in the rice bran were all four T3 homologues detected (). In corn bran only three T3s (α-T3, γ-T3, and δ-T3) were identified (). γ-T3 and δ-T3 were absent from the rye, wheat, oat, and spelt brans (, , , and ), and in buckwheat bran only α-T3, in trace amounts, was recorded (). The RP-HPLC/FLD identification of all tocochromanols was confirmed by ESI/MSn (data not shown). In the majority of samples studied by RP-HPLC/FLD two unknown peaks U1 and U2 were detected (, , , , , and ) which could not be identified. The failure of ESI/MSn in this case may be due to poor ionization of these compounds.
Rye and oat bran had similar tocochromanol compositions. In both types α-T3 (4.7 and 3.0 mg/100 g dw, respectively) predominated; and both had identical average levels of α-T, γ-T, and δ-T (1.4, 0.1, and 0.1 mg/100 g dw, respectively). However their concentrations of β-T and β-T3 were significantly different (P < 0.05). Rye bran contained higher average amounts of β-T and β-T3 (0.7 and 3.7 mg/100 g dw, respectively) than oat bran (0.3 and 0.6 mg/100 g dw, respectively; ). A wider concentration range of α-T3, β-T3, α-T, and β-T was found in rye—2.8–6.6, 2.6–4.7, 0.7–2.9, and 0.4–1.3 mg/100 g dw, respectively—than in oat bran (1.7–4.1, 0.4–1.0, 1.1–2.1, and 0.1–0.5 mg/100 g dw, respectively; ). The four predominant tocochromanols in the rye brans we studied were also measured in six rye bran samples from Nordic countries.[Citation3] The levels of α-T, β-T, α-T3, and β-T3 in the oat brans we examined are comparable to those previously reported in fresh oat products and bran.[Citation17] The lower concentration of tocochromanols in oat, compared to rye bran, may be the result of high amounts adhering to the inner core starchy endosperm. A clean and efficient separation of oat grain’s inner endosperm from the outer layer (bran part) was stated to be a very problematic procedure.[Citation1] Moreover, it was also reported that the cultivar of the grains had a significant impact on the tocochromanol levels in the morphological fractions of the rye.[Citation18]
TABLE 1 Content of tocopherol and tocotrienol homologues in various types of bran determined by RP-HPLC/FLD
T3s were also the main type of tocochromanols found in the wheat and spelt brans, as found in the rye and oat brans, although the wheat and spelt brans mainly contained β-T3, not α-T3. Significant differences (P < 0.05) in the average concentrations of β-T and β-T3 were noted between the wheat and spelt brans. In general, wheat bran had higher average concentrations of Ts (1.9 and 1.1 versus 1.4 and 0.6 mg/100 g dw of α-T and β-T, respectively) and lower levels of T3s (2.2 and 7.4 versus 2.8 and 10.9 mg/100 g dw of α-T3 and β-T3, respectively) than spelt bran (). The concentration range of α-T, β-T, α-T3, and β-T3 in wheat and spelt brans were 0.6–3.1, 0.5–1.6, 1.5–2.8, and 5.0–10.3 versus 0.8–1.9, 0.2–0.9, 2.5–3.1, and 7.2–13.8 mg/100 g dw, respectively (). Near identical levels were reported in two wheat bran samples from Nordic countries.[Citation3] The sample of spelt bran investigated by Nielsen and Hansen[Citation19] had comparable levels of Ts, but approximately four times lower concentrations of T3s, when compared with our findings. Previously, the HEALTHGRAIN study of 175 genotypically different wheat types, including five spelt cultivars, and different types of wheat (winter, spring, and durum) reported a large variation in tocochromanol levels.[Citation20] Variability in tocochromanol levels in different spelt bran samples in the present study was noted. In wheat and spelt bran, as well in rye and oat bran, only minor levels of γ-T and δ-T in the range 0.0–0.2 mg/100 g dw were recorded ().
The buckwheat and corn bran, in contrast to the rye, wheat, oat and spelt bran samples, contained γ-T (13.3 and 10.1 mg/100 g dw, respectively) as the major tocochromanol component. They also had significantly higher levels of δ-T (0.6 and 0.4 mg/100 g dw, respectively). The average concentration of α-T in buckwheat brans was lower than in corn brans (0.8 and 2.1 mg/100 g dw, respectively). Significantly lower levels of β-T in buckwheat and corn brans (trace and 0.1 mg/100 g dw, respectively) compared with the rye, wheat, and spelt brans were noted (). Of the T3 homologues, only trace amounts α-T3 were detected in the buckwheat bran, but in the corn bran, three homologues (α, γ, and δ) with the levels 1.5, 1.9, and 0.1 mg/100 g dw, respectively, were found. We wish to highlight the concentration range of α-T, δ-T, and especially γ-T in the buckwheat brans (0.4–1.3, 0.3–1.0, and 5.8–24.4 mg/100 g dw, respectively), indicating a wide variation of T levels in the buckwheat bran available in the market. In contrast, in corn bran, similar concentrations for each of the individual tocochromanols were measured; however, only two samples of corn bran were studied. To the best of our knowledge, this study is the first to report the tocochromanol composition of buckwheat and corn bran; thus, a direct comparison with previous data was not possible. Nevertheless, oil extracted from corn bran had a similar tocochromanol profile, with γ-T as the major component, despite the use of a different extraction protocol.[Citation21] Moreover, in relation to the data in the present study, the lack of β-T and δ-T3 in corn bran oil was also stated.[Citation21] However, β-T and δ-T3 were found in corn germ, endosperm, and hull oil samples.[Citation22] Zielinski et al.[Citation23] detected only three Ts (α-T, γ-T, and δ-T) in two morphological fractions—whole grain and endosperm with the germ—of buckwheat. γ-T was constituted over 90% of the identified tocochromanols. Similar observations were made in the presented study. γ-T and α-T, are the most abundant T homologues in the plant world, particularly characteristic of the seeds and kernels of different cultivars of fruits.[Citation24–Citation26] In contrast, levels of γ-T (average 20–40%) higher than α-T and δ-T have been reported in buckwheat grains of different species.[Citation27] The different T concentrations in whole buckwheat grains reported by Zielinski et al.[Citation23] and Kim et al.[Citation27] could be the result of their different extraction procedures. Zielinski et al.[Citation23] extracted with 80% methanol, while Kim et al.[Citation27] used hexane. According to Ryynänen et al.,[Citation28] the saponification assay, used in our study, is the best available method to ensure a high recovery rates of extracted Ts. Moreover, the n-hexane:ethyl acetate (9:1, v/v) solvent ratio used in our study, according to Eitenmiller et al.,[Citation29] significantly improved the recovery of Ts, especially the β, γ, and δ homologues.
The rice bran samples contained mainly T3s, similar to rye, wheat, oat, and spelt bran. However, they contained significantly (P < 0.05) higher amounts of γ-T3 and δ-T3 (2.8 and 1.0 mg/100 g dw, respectively) and lower levels of α-T3 and β-T3 (1.9 and 0.3 mg/100 g dw, respectively). Compared to corn bran, rice bran had a similar T3 composition (a predominance of α-T3 and γ-T3), and a significantly (P < 0.05) lower concentration of γ-T. The average concentration α-T, β-T, γ-T, and δ-T in rice bran were 1.8, 0.3, 0.8, and 0.2 mg/100 g dw, respectively. The concentration range of the tocochromanols (α-T, β-T, γ-T, δ-T, α-T3, β-T3, γ-T3, and δ-T3) in rice bran samples were 0.7–5.5, 0.1–0.8, 0.4–2.3, 0.1–0.3, 0.2–7.4, 0.1–0.6, 0.8–10.1, and 0.1–3.9 mg/100 g dw, respectively. Similar tocochromanol ranges were reported in bran of eight rice varieties from the United States.[Citation4] Nevertheless, the total vitamin E content of our rice brans, was lower than reported in a previous study.[Citation4] The observed large variations of tocochromanols in the same type of bran produced by different manufacturers, is not surprising, since the concentration of Ts and T3s in the bran is effected by numerous factors such as, grain and bran storage times, storage conditions, the cultivar of grain, and the milling process. Milling time is an important factor, as longer milling times result in lowered concentrations of tocochromanols.[Citation30]
A significantly higher (P < 0.05) average amount of tocochromanols was noted in the corn, spelt, buckwheat, and wheat bran (16.2, 15.8, 14.7, and 12.8 mg/100 g dw, respectively) compared to the oat bran (5.5 mg/100 g dw, respectively). The tocochromanol concentrations in the rice and rye bran (9.1 and 10.7 mg/100 g dw, respectively) were in between these extremes. However, taking into account the maximum range values, the highest total amount of tocochromanols can be found in the buckwheat and rice bran (27.3 and 27.7 mg/100 g dw, respectively).
CONCLUSION
This study has shown that bran type significantly influenced the composition and concentration of individual Ts and T3s. The buckwheat and corn bran mainly consisted of Ts (98 and 78%, respectively), more specifically γ-T; whereas rye, wheat, oat, spelt, and rice bran were rich in T3s (78, 76, 66, 87, and 66%, respectively). The concentration range of individual tocochromanols, also varied considerably between different samples of the same type of bran. This observation highlighted the importance of labelling the content of T and T3s in commercial bran, since bran products are becoming more and more popular in the market, and are a potentially significant source of vitamin E in the daily diet.
REFERENCES
- Zitterman, A. In Encyclopedia of Food Sciences and Nutrition: Volumes 1– 10; Caballero, B.; Trugo, L.; Finglas, P.; Eds.; Elsevier Science BV: Amsterdam, 2003; 1844–1850.
- Prückler, M.; Siebenhandl-Ehn, S.; Apprich, S.; Höltinger, S.; Haas, C.; Schmid, E.; Kneifel, W. Wheat Bran-Based Biorefinery 1: Composition of Wheat Bran and Strategies of Functionalization. LWT–Food Science and Technology 2014, 56, 211–221.
- Kamal-Eldin, A.; Lærke, H.N.; Knudsen, K.-E.B.; Lampi, A.-M.; Piironen, V.; Adlercreutz, H.; Katina, K.; Poutanen, K.; Ɨman, P. Physical, Microscopic, and Chemical Characterisation of Industrial Rye and Wheat Brans from the Nordic Countries. Food & Nutrition Research 2009, 53, 1–11.
- Min, B.; McClung, A.M.; Chen, M.H. Phytochemicals and Antioxidant Capacities in Rice Brans of Different Color. Journal of Food Science 2011, 76, C117–C126.
- Eitenmiller, R.; Lee, J. Vitamin E: Food Chemistry, Composition, and Analysis; Marcel Dekker Inc.: New York, NY, 2004.
- Nogala-Kałucka, M.; Dwiecki, K.; Siger, A.; Górnaś, P.; Polewski, K.; Ciosek, S. Antioxidant Synergism and Antagonism Between Tocotrienols, Quercetin, and Rutin in Model System. Acta Alimentaria 2013, 42, 360–370.
- Dwiecki, K.; Górnaś, P.; Jackowiak, H.; Nogala-Kałucka, M.; Polewski, K. The Effect of D‐Alpha‐Tocopherol on the Solubilization of Dipalmitoylphosphatidylcholine Membrane by Anionic Detergent Sodium Dodecyl Sulfate. Journal of Food Lipids 2007, 14, 50–61.
- Aggarwal, B.B.; Sundaram, C.; Prasad, S.; Kannappan, R. Tocotrienols, the Vitamin E of the 21st Century: Its Potential Against Cancer and Other Chronic Diseases. Biochemical Pharmacology 2010, 80, 1613–1631.
- Górnaś, P.; Siger, A. Simplified Sample Preparation and Rapid Detection by RP-HPLC/FLD of Tocopherols and Tocotrienols in Margarines: Preliminary Screening of Plant Fats—Potential Quality Markers. European Journal of Lipid Science and Technology 2015, 117, 1589–1597.
- Górnaś, P.; Siger, A.; Juhņeviča, K.; Lācis, G.; Šnē, E.; Segliņa, D. Cold-Pressed Japanese Quince (Chaenomeles Japonica (Thunb.) Lindl. ex Spach) Seed Oil As a Rich Source of α-Tocopherol, Carotenoids, and Phenolics: A Comparison of the Composition and Antioxidant Activity with Nine Other Plant Oils. European Journal of Lipid Science and Technology 2014, 116, 563–570.
- Górnaś, P.; Soliven, A.; Segliņa, D. Seed Oils Recovered from Industrial Fruit By-Products are a Rich Source of Tocopherols and Tocotrienols: Rapid Separation of α/β/γ/δ Homologues by RP-HPLC/FLD. European Journal of Lipid Science and Technology 2015, 117, 773–777.
- Schwartz, H.; Ollilainen, V.; Piironen, V.; Lampi, A.-M. Tocopherol, Tocotrienol, and Plant Sterol Contents of Vegetable Oils and Industrial Fats. Journal of Food Composition and Analysis 2008, 21, 152–161.
- Górnaś, P.; Pugajeva, I.; Segliņa, D. Seeds Recovered from by-Products of Selected Fruit Processing As a Rich Source of Tocochromanols: RP-HPLC/FLD and RP-UPLC-ESI/Msn Study. European Food Research and Technology 2014, 239, 519–524.
- Górnaś, P.; Siger, A.; Czubinski, J.; Dwiecki, K.; Segliņa, D.; Nogala-Kalucka, M. An Alternative RP-HPLC Method for the Separation and Determination of Tocopherol and Tocotrienol Homologues As Butter Authenticity Markers: A Comparative Study Between Two European Countries. European Journal of Lipid Science and Technology 2014, 116, 895–903.
- Górnaś, P.; Siger, A.; Polewski, K.; Pugajeva, I.; Waśkiewicz, A. Factors Affecting Tocopherol Contents in Coffee Brews: NP-HPLC/FLD, RP-UPLC-ESI/MSn and Spectroscopic Study. European Food Research and Technology 2014, 238, 259–264.
- Górnaś, P.; Segliņa, D.; Lācis, G.; Pugajeva, I. Dessert and Crab Apple Seeds As a Promising and Rich Source of All Four Homologues of Tocopherol (a, b, g, and d). LWT–Food Science and Technology 2014, 59, 211–214.
- Peterson, D.M. Oat Tocols: Concentration and Stability in Oat Products and Distribution Within the Kernel. Cereal Chemistry 1995, 72, 21–24.
- Zielinski, H.; Michalska, A.; Szawara-Nowak, D.; Wiczkowski, W.; Piskuta, M.K. Tocotrienols in Three Rye Varietes: From the Grain to the Bread. Polish Journal of Food and Nutrition Sciences 2007, 57, 441–446.
- Nielsen, M.M.; Hansen, Å. Rapid High-Performance Liquid Chromatography Determination of Tocopherols and Tocotrienols in Cereals. Cereal Chemistry 2008, 85, 248–251.
- Lampi, A.-M.; Nurmi, T.; Ollilainen, V.; Piironen, V. Tocopherols and Tocotrienols in Wheat Genotypes in the HEALTHGRAIN Diversity Screen. Journal of Agricultural and Food Chemistry 2008, 56, 9716–9721.
- Moreau, R.A.; Hicks, K.B. The Composition of Corn Oil Obtained by the Alcohol Extraction of Ground Corn. Journal of the American Oil Chemists’ Society 2005, 82, 809–815.
- Ko, S.-N.; Kim, C.-J.; Kim, H.; Kim, C.-T.; Chung, S.-H.; Tae, B.-S.; Kim, I.-H. Tocol Levels in Milling Fractions of Some Cereal Grains and Soybean. Journal of the American Oil Chemists’ Society 2003, 80, 585–589.
- Zielinski, H.; Ciska, E.; Kozlowska, H. The Cereal Grains: Focus on Vitamin E. Czech Journal of Food Science 2001, 19, 182–188.
- Górnaś, P.; Mišina, I.; Grāvīte, I.; Soliven, A.; Kaufmane, E.; Segliņa, D. Tocochromanols Composition in Kernels Recovered from Different Apricot Varieties: RP-HPLC/FLD and RP-UPLC-ESI/MSn Study. Natural Product Research 2015, 29, 1222–1227.
- Górnaś, P.; Mišina, I.; Lāce, B.; Lācis, G.; Segliņa, D. Tocochromanols Composition in Seeds Recovered from Different Pear Cultivars: RP-HPLC/FLD and RP-UPLC-ESI/MSn Study. LWT–Food Science and Technology 2015, 62, 104–107.
- Górnaś, P.; Mišina, I.; Ruisa, S.; Rubauskis, E.; Lācis, G.; Segliņa, D. Composition of Tocochromanols in Kernels Recovered from Different Sweet Cherry (Prunus Avium L.) Cultivars: RP-HPLC/FLD and RP-UPLC-ESI/MSn Study. European Food Research and Technology 2015, 240, 663–667.
- Kim, S.L.; Kim, S.K.; Park, C.H. Comparisons of Lipid, Fatty Acids, and Tocopherols of Different Buckwheat Species. Food Science and Biotechnology 2002, 11, 332–336.
- Ryynänen, M.; Lampi, A.-M.; Salo-Väänänen, P.; Ollilainen, V.; Piironen, V. A Small-Scale Sample Preparation Method with HPLC Analysis for Determination of Tocopherols and Tocotrienols in Cereals. Journal of Food Composition and Analysis 2004, 17, 749–765.
- Eitenmiller, R.R.; Landen Jr, W.; Ye, L. Vitamin Analysis for the Health and Food Sciences, 2nd Ed; CRC Press: New York, NY, 2007.
- Rohrer, C.A.; Siebenmorgen, T.J. Nutraceutical Concentrations Within the Bran of Various Rice Kernel Thickness Fractions. Biosystems Engineering 2004, 88, 453–460.