Abstract
Bok choy, Brassica rapa subsp. Chinensis, is a type of Chinese cabbage. Stir frying could affect antioxidant activity and aroma composition of bok choy. Antioxidant activity such as diphenyl picryl hydrazinyl radical and ferric-reducing antioxidant power of the leave and stem samples were determined. The highest ferric-reducing antioxidant power activity was obtained at 1.5 min stir-frying under 160ºC for bok choy leaves (49.7 ± 2.1 µmol FeSO4/g dry weight). In ferric-reducing antioxidant power assay, antioxidant capacities of bok choy stem samples increased significantly after stir-frying. Dimethyl sulfide (DMS), a typical volatile organic sulfur compound with a characteristic cabbage-like odor, was only observed in leave groups. After cooking treatment, antioxidant activities of bok choy stems were higher than those of leaves in diphenyl picryl hydrazinyl radical assay. Odor-active compounds were identified using gas chromatography-olfactometry-mass spectrometry technique. Compared to stem sample data, more aroma components were quantified in leaves sample in 160, 180, and 210ºC, respectively. It was first time to detect and analysis the aroma components and antioxidant activity in different edible part of bok choy after Chinese domestic cooking.
INTRODUCTION
Epidemiological researches proved that the increasing vegetables consumption could play important part in protecting human from various diseases, because vegetables contain high levels of beneficial materials such as vitamins, antioxidants, and anticancer compounds.[Citation1] Bok choy, a dark green Chinese cabbage, is a kind of popular and favorite Brassicaceae vegetables in China. Brassicaceae vegetables are salutary for human and can reduce the risk of cancers in several studies.[Citation2] Bok choy can be harvested more than once during the year, so they can provide plenty of flesh (leaf and stem) for human. What is more, bok choy plays a role in health by improving nutritional value quality through different cooking methods.[Citation3] Compared with culinary cultures of other countries, there are several features in Chinese traditional diet. In Chinese cuisine, most kind of vegetables are cooked by boiling, steaming, baking, stir frying, and deep frying. Some researches pointed out that these cooking methods would result in several changes in the physical characteristics and chemical composition of vegetables.[Citation4,Citation5] Wen et al.[Citation6] reported the comparison of bioactive substance contents and antioxidant activities of blanched and raw vegetables.
In China, stir frying is a fast and fresh cook pattern and is used to prepare most homemade dishes.[Citation7] Stir frying is a Chinese cooking technique in which edible ingredients are mixed and fried in a small amount of very hot vegetable oil. Some studies have indicated that nutrients from raw vegetable were less available than those from cooked vegetables.[Citation8,Citation9] Grosshauser’s study indicated that different cooking methods could influence mushrooms odors. During heat processing, key odorants or chemical construction of nutrient in vegetables could be formed or changed.[Citation10] Compared to some reports on odor analysis and sensory characteristics of thermal processed food of European cuisine style, studies on the odorants on china cuisine are rare, and the antioxidant capacity change has hardly been studied.
The objective of the present work was to investigate the aroma components and antioxidant capacity of bok choy during different period of stir frying. It is a challenge to carry out the identification and characterization of the delicious aroma in traditional Chinese food. Base on gas chromatography-olfactometry-mass spectrometry (GC-O-MS) method, aroma-active compounds in bok choy after stir frying were also studied. The findings of this study may contribute to enhance nutrition field knowledge of bok choy and Chinese cuisine.
MATERIAL AND METHODS
Materials
Bok choy (Brassica chinensis L.; Shanghai Qing) and maize oil (COFCO, PRC) were purchased from several local markets in Beijing used as research material.
Sample selection and preparation
Raw bok choy was kept for 1 h at 4ºC temperature to allow retreatment, for bok choy tissue tends to wither. Before washing, the withered bok choy with yellow leaf was picked out, only fresh bok choy was selected to prepare for cooking. The leaf and stem of bok choy were cut separately, in order to cook them respectively.
Processing treatments stir frying cooking
Tailor-made stainless steel container (12 × 9 × 7.5 cm) was placed in oil bath, and the temperature of oil bath was pre-set at 160, 180, or 210ºC. A thermal couple was used to detect temperature of container where the actual frying temperature was measured. Edible corn oil (8.5 g) was added into the container. When the temperature of edible oil reached the pre-set temperature, four pieces of stem slices in container were put inside. Each piece was fried for 30 s per side, then turn-over was done by using chopsticks. Stem slices were fried at different time intervals (i.e., 1, 2, 3, and 4 min) for subsequent analysis. For the leaf part, the leaf pieces were fried with the same procedure as that of stem part mentioned above. Leaf pieces were fried for 1, 1.5, 2, and 2.5 min, also for subsequent analysis. The sample was cooled rapidly on ice.
Instrumental method for volatile determination
The solid phase micro-extraction (SPME) devices (Supelco, Poole, UK) contained a 1 cm Stable-flex fiber coated with 50/30 μm DVB-Carboxen on PDMS. Gas chromatography 7890A coupled to a triple quadrupole mass spectrometry 7000B (Agilent, Palo Alto, CA), and equipped with a Sniffer 9000 Olfactometer (Brechbühler, Switzerland). Separation of odorant compounds in GC was performed on DB-WAX column (30 m × 0.25 mm × 0.25 μm, J & W Scientific, Folsom, CA, and USA). Mass spectrum was based on NIST 08 mass spectra libraries.
Diphenyl picryl hydrazinyl radical (DPPH) radical scavenging activity
The free radical scavenging activity of the samples was measured in accordance with the method of Zhang and Hamauzu[Citation5] with some modifications. The sample extract (0.1 mL) was added to a 3.0 mL 0.1 mM DPPH∙ methanolic solution. The mixture was vortexed (30 s) and left to stand at room temperature in the dark for 30 min. The absorbance was measured at 517 nm using a TU-1810 spectrophotometer (Beijing Purkinje General Instruments Co. Ltd., Beijing, China). The results were expressed as percentage of reduction of the initial DPPH∙ absorption by test samples as follows: DPPH∙ scavenging effect (%) = [(A0 – At) /A0] × 100. where: A0: absorbance of the control at t = 0 min; At: absorbance of the antioxidant at t = 30 min.
Ferric reducing antioxidant power (FRAP) assay
The FRAP assay is a method of measuring the ability of reductants to reduce Fe3+ to Fe2+. The formation of blue colored Fe2+-TPTZ complex increases at the absorbance of 593 nm. The reducing power of the extracts was evaluated by the modified method of Kubola and Siriamornpun.[Citation11] The FRAP reagent was freshly prepared by mixing 100 mL of acetate buffer (300 mM, pH 3.6), 10 mL 2,4,6-Tris(2-pyridyl)-s-triazine (tripyridyltriazine; TPTZ) solution (10 mM TPTZ in 40 mM/HCl), 10 mL FeCl3·6H2O (20 mM) in a ratio of 10:1:1, and 12 mL distilled water, at 37ºC. This working solution was allowed for 6 min of incubation and after that the absorbance of reaction mixture at 593 nm was measured using the FRAP working solution as a blank. The reading of relative absorbance should be within the range 0–2.0; otherwise, the sample should be diluted. The final result was expressed as the concentration of antioxidants having a ferric reducing ability equivalent to that of 1 mM FeSO4. Adequate dilution was needed if the FRAP value measured was over the linear range of standard curve.
Analysis of Aroma-Active Compounds in Chinese Cabbage
Volatiles extraction procedure
Aroma-active compounds in bok choy (Chinese cabbage) were extracted by headspace solid phase microextraction (HS-SPME). Five grams of the samples with 2-methyl-3-heptanone (1.632 μg/μL) added as the internal standard were placed in the sealed vial, and equilibrated for 30 min in the thermostatic bath at 50ºC. Then the stainless steel needle of SPME device, housing the fiber, was inserted into the vial and the fiber was exposed in headspace state above the sample for 40 min (50ºC). Afterward, the HS-SPME fiber was inserted into the injection port of gas chromatography-mass spectrometry, as described by Choi and Min.[Citation12,Citation13]
GC-O-MS instrumentation and operating conditions
Gas chromatography 7890A coupled to a triple quadrupole mass spectrometry 7000B (Agilent, Palo Alto, CA, USA), and equipped with a Sniffer 9000 Olfactometer (Brechbühler, Switzerland). Separation of odorant compounds in GC was performed on DB-WAX column (30 m × 0.25 mm × 0.25 μm, J & W Scientific, Folsom, CA, USA). The carrier gas was ultra-high purity helium (>99.99%) with a flow rate of 1.2 mL/min. The injector port was heated to 250ºC, an initial temperature was set at 40ºC for 3 min, then raised at 5ºC min−1 to 200ºC, held for 0 min, and elevated to 230ºC at 10ºC min−1. Mass spectra were acquired in an electron impact mode. MS was taken at 70 eV and the ion source temperature was 230ºC. The m/z scanned range from 40 to 600 amu. The data were identified and confirmed by comparison of their retention times and mass spectra with a Mass Spectra Library, Wiley 7N edition (Agilent Part No. G1035B).
Identification and quantification of aroma-active compounds
The chemical identification was based on the comparison of the mass spectrum, retention index (RI), and odor description with reference compound. Mass spectrum was based on NIST 2.0 mass spectra libraries. The RI values and odor descriptions on DB-WAX column should match with those of linear retention indices (LRIs) having the same/similar odor quality, previously reported in www.odour.org.uk and literatures.[Citation14,Citation15] n-Alkanes (C7-C22) were analyzed under the same conditions to calculate LRIs which was described by Dool and Krazt:[Citation16]
The internal standard, 2-methyl-3-heptanone at a concentration of 326.4 ng/g (326.4 ng of the internal standard/1 g of the sample), was added into the sample with the injection of 1 µL. The concentration for each odorant was calculated as the following:
The abbreviated letter of CX, CA, SX, and SA represented the concentration of an odorant, concentration of internal standard, peak area of an odorant, and peak area of internal standard on GC chromatogram respectively.
Statistical analysis
Data were expressed as mean ± standard error of mean (SEM). The dependent variables were tested by analysis of variance (ANOVA) and Tukey’s test was applied in order to determine statistical differences between group means. Significant differences were considered at p < 0.05.
RESULTS AND DISCUSSION
Determination of Antioxidant Activity
The effect of stir frying methods on antioxidant activities of bok choy stems and leaves was shown in and . Two antioxidant activity assays such as DPPH and FRAP were applied to assess the antioxidant capacities of both bok choy leaves and stems after stir frying. Because of the limitations of method for antioxidant determination, it was recommended to employ various assays, instead of relying on a single assay to assess antioxidant capacity.[Citation17]
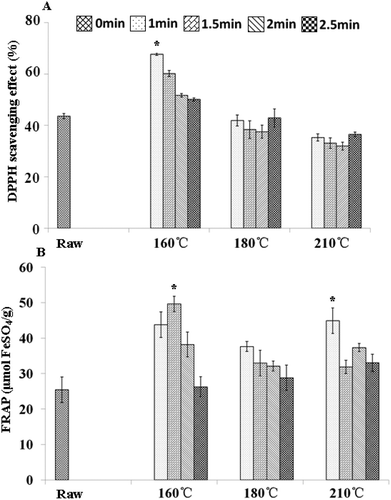
FIGURE 1 Effect of cooking methods on antioxidant activity of bok choy stems.
A: Bok choy stems DPPH scavenging effect; B: FRAP value of bok choy stems.Values are means ± standard deviation (n = 3). Statistically significant difference in comparison with the raw sample is indicated as * (*p < 0.05).
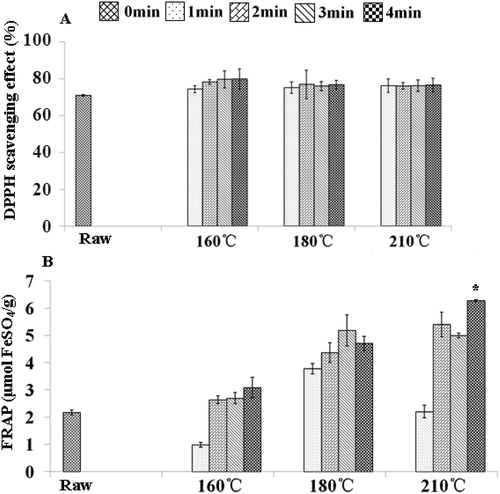
FIGURE 2 Effect of cooking methods on antioxidant activity of bok choy leaves.
A: Bok choy leaves DPPH scavenging effect; B: FRAP value of bok choy leaves.Values are means ± standard deviation (n = 3). Statistically significant difference in comparison with the raw sample is indicated as * (*p < 0.05).
DPPH is a common abbreviation for an organic chemical compound 2,2-diphenyl-1-picrylhydrazyl. DPPH assay is the activity of quenching free radicals or H-donor capability of the antioxidant.[Citation18] The DPPH electron paramagnetic resonance (EPR) reacted with a hydrogen donated from the antioxidant and form their corresponding hydrazine.[Citation19] FRAP, ferric ion reducing antioxidant power, is an antioxidant capacity assay that uses Trolox as a standard which is based on the reduction capability of ferrous ion (Fe3+) to ferricion (Fe2+) of a test sample.[Citation20]
Compared to raw bok choy samples, the antioxidant activities of bok choy leaves and stems after stir-frying were clearly influenced. The DPPH values of stem samples increased slightly during processing at 160, 180, and 210ºC, respectively. In stem samples the highest DPPH radical scavenging activity of 79.9% was obtained at 160ºC 4 min. DPPH radical scavenging activity of stem samples increased slightly along with processing time at 160ºC. There was no significant difference between the radical scavenging activities of raw bok choy samples and stem samples under 180 and 210ºC processing. The scavenging effects of 180ºC stem samples were nearly equal to those of 210ºC stem samples ().
In leaves samples the highest DPPH radical scavenging activity of 67.7% was obtained in leaves samples at 160ºC 1 min significantly (p < 0.05) in , whereas DPPH radical scavenging activities of leaves samples decreased clearly along with processing time at 160ºC. There were also changes in DPPH radical scavenging levels between 180ºC samples and 210ºC samples, which had decreases of 40 and 38%, respectively.
In order to fully evaluate the antioxidant capacity, FRAP assay was employed which has been widely used to quantify antioxidant activity.[Citation21] As shown in in all FRAP among samples, the leaves extracts showed the best antioxidant activity. The dynamic tendency of antioxidant capacity differences of stems and leaves for FRAP was noted ( and ). The FRAP value of stem samples increased significantly along with processing time and temperature, respectively, whereas leaves values of FRAP assay decreased along with processing. The FRAP data of leaves were higher than those of stems at same temperature. The higher FRAP capacities of 43.8 ± 3.6 µmol FeSO4/g dry weight (DW), 49.7 ± 2.1 µmol FeSO4/g DW, and 44.9 ± 3.5 µmol FeSO4/g DW were obtained in leaves samples at 160ºC 1 min, 160ºC 1.5 min, and 210ºC 1 min, respectively (p < 0.05).
The difference in antioxidant activity of leaves and stem after cooking processing could be attributed to concentration, composition, and structure of antioxidant substance.[Citation22] Although there were no reports on the composition of antioxidant substances in bok choy, but similar research results showed that antioxidant capacities of leaves were higher than those of stems, for leaves had more antioxidant substances.[Citation23] What is more, different parts of plant contain different compounds. Some of them are thermally more labile than others, therefore after the same cooking processing, notable effects could be observed on different types of plants.[Citation24] Cooking is complex progress which may cause many physico-chemical changes. Many researches showed that cooking methods could impact the antioxidant properties of vegetables.[Citation25] Turkmen found that the influence of cooking method on antioxidant property of vegetable was depended on the species.[Citation26]
Volatile Compounds
Volatile compounds identified and quantified in stems and leaves from raw and cooked bok choy samples were listed in and . Six and night volatile odorants were identified from the stems and leaves of fresh bok choy, respectively, but interestingly, heptanal, 2-pentanal, 2-hexenal, 2-ethylfuran, 2-ethylthiophene, and 1-hexanol were not identified from stir fried bok choy samples. Three cooking temperatures (160, 180, and 210ºC) and four cooking time points were chosen in this study. Amount of aroma compounds increased with cooking time and temperature in stems and leaves samples. The amount of volatile compounds in leaves was more than those of stems. Volatile compounds (20, 25, and 26) were identified in leaves at 160, 180, and 210ºC, respectively. Volatile compounds (17, 20, and 19) were detected in stems at 160, 180, and 210ºC, respectively. The numbers and concentrations of volatile odorants identified from leaves were higher than those from stems. It might due to the fatty acids in vegetable tissues. The fatty acids were oxidized to hydrogen peroxides by lipoxygenase under aerobic conditions, and then by actions of a serial of other enzymes, the hydrogen peroxides were broken down into volatile odorants such as aldehydes and alcohols eventually. Hydroperoxide lyase was one of the key enzymes involved in the progress. As hydroperoxide lyases differed from each other in different vegetable tissues, so the resulting aroma components were different.[Citation27,Citation28]
TABLE 1 Effect of stir frying on aroma compounds of bok choy stems
TABLE 2 Effect of stir frying on aroma compounds of bok choy leaves
The total 23 aroma components coming from stem part were identified and can be classified into five groups: aldehyde, alcohol, ether, furan, and pyridine, while 33 compounds from the leaf part, belonging to six classes: alcohol, ether, aldehyde, pyrazine, pyridine, and furan. Twenty aroma compounds were detected in both stem and leaf samples after stir-frying. Three and 12 aroma compounds were only present in the stem and leaf groups, respectively. Aldehydes were suggested as the largest class of aroma-active components in both stem and leaf groups, which accounted for 62.5 to 78% of the total aroma amount in stem samples and 43.5 to 90% in leaf samples. A total of five pyrazine compounds were found to be the second largest class of aroma-active compounds in leaf samples. Pyridines were only detected in leaf samples.
In this study, three sulfur-containing compounds were first identified from cooked bok choy. Sulfur compounds are known to play an important role in the formation of vegetable aroma. After stir frying, dimethyl trisulfide and DMS were detected in leaf group, while dimethyl disulfide was present in both parts of bok choy. DMS is a typical volatile organic sulfur compound with a characteristic seaweed-like and boiled cabbage-like odor. DMS is considered as a key flavor constituent in cooked vegetables such as tomato, cabbage, and asparagus.[Citation29,Citation30]
The concentrations of most volatile compounds including three sulfur-containing compounds were low in 160ºC and then these concentrations increased significantly after stir frying at 180ºC; however, they decreased at 210ºC. The concentration of most volatile compounds tended to decrease depending on the heating temperature (210ºC) and heating time. It was suggested that temperature- and time-related treatments could cause changes in dissociation of biological structures of some compounds and the alteration in their chemical structure and chemical reaction rate, which could make possible conversion of some volatile substances. Moreover it was assumed that heating treatment could influence endogenous formation of volatile substances and volatilization.[Citation31,Citation32]
Dynamic Aroma Profiles
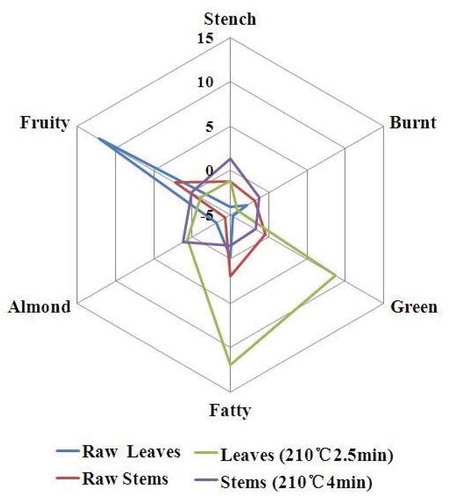
The aroma-active volatiles in and with similar sensory descriptors were grouped into six general aroma categories based on their primary aroma characters: stench, burnt, green, fatty, almond, and fruity, which were demonstrated in and .
The concentration ranges of aroma volatiles were different in several cooking condition, data normalization was employed for comparison purposes. The fruity note had the highest aroma intensity for both raw leaves and stems ( and ); however, the fruity aroma in raw leaves were clearly richer than that in raw stems. In 160ºC leaves sample, all aroma classes were similar (), however fruity aroma clearly decreased. What is more, the highest intensity of fruity aroma was detected from raw leaves in all samples (). Burnt and green smelling were major aroma categories in leaves after processing under 180ºC (). Green and fatty notes were predominant in leaves samples after cooking under 210ºC ().
FIGURE 3 Dynamic comparative aroma profiles of bok choy leaves sample with similar aroma characteristics.
A: Raw leaves samples; B: 160°C leaves samples; C: 180°C leaves samples; D: 210°C leaves samples.
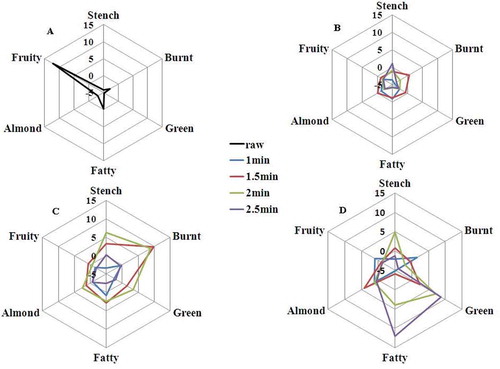
FIGURE 4 Dynamic comparative aroma profiles of bok choy leaves sample with similar aroma characteristics
A: Raw stems samples; B: 160°C stems samples; C: 180°C stems samples; D: 210°C stems samples.
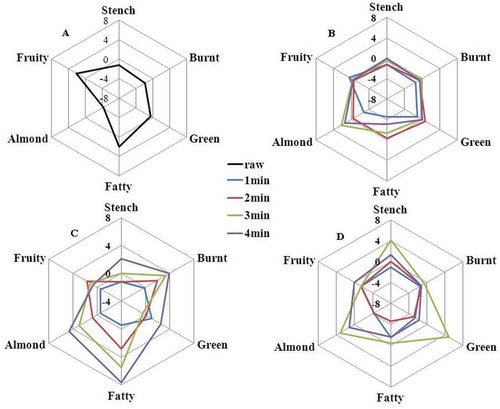
The most intense odor was almond smelling in stem samples (), which was obvious increasing with cooking time at the same temperature. At 180ºC 3 min, the stem sample presented harmonious aroma smell (). Compared to the stem samples, the leaf samples showed stronger and richer aroma intensities in final processing (). The volatile compounds of leaves and stems showed qualitative and quantitative differences. It might be due to the raw materials being frequently lost [Citation33] and several novel aroma compound were formed by degradation reaction or enzymatically hydrolyzed during the thermal treatment.[Citation34]
CONCLUSION
To the best of our knowledge, few studies have been carried out on antioxidant capacities and aroma components of different parts of bok choy. It was the first report in which an overall picture of the effects of common domestic cooking conditions on antioxidant activity and aroma-active compounds of bok choy as cooked was presented. Bok choy stems treated with stir frying showed higher DPPH scavenging, but the FRAP values of bok choy stems were lower than those of leaves. Thirty-five volatile compounds were identified. Most volatile compounds occurred in bok choy leaves. The results showed that the aldehydes and sulfur-containing compound increased upon stir frying treatment. Furthermore, more sulfur-containing compounds were identified in bok choy leaf parts. Compared to volatile compounds of bok choy stems, the total aroma-active of leaves increased significantly after cooking.
REFERENCES
- Bhupathiraju, S.N.; Wedick, N.M.; Pan, A.; Manson, J.E.; Rexrode, K.M.; Willett,W. C.; Rimm, E.B.; Hu, F.B. Quantity and Variety in Fruit and Vegetable Intake and Risk of Coronary Heart Disease. American. Journal of Clinic Nutrition 2013, 98, 1514–1523.
- Wei, J.; Miao, H.; Wang, Q. Effect of Glucose on Glucosinolates, Antioxidants, and Metabolic Enzymes in Brassica Sprouts. Scientia Horticulturae 2011, 129, 535–540.
- Sun, Y.P.; Chou, C.C.; Yu, R.C. Antioxidant Activity of Lactic-Fermented Chinese Cabbage. Food Chemistry 2009, 115, 912–917.
- Rehman, Z.U.; Islam, M.; Shah, W.H. Effect of Microwave and Conventional Cooking on Insoluble Dietary Fibre Components of Vegetables. Food Chemistry 2003, 80, 237–240.
- Zhang, D.; Hamauzu, Y. Phenolics, Ascorbic Acid, Carotenoids, and Antioxidant Activity of Broccoli and Their Changes During Conventional and Microwave Cooking. Food Chemistry 2004, 88, 503–509.
- Wen, T.N.; Prasad, K.N.; Yang, B.; Ismail, A. Bioactive Substance Contents and Antioxidant Activity of Blanched and Raw Vegetables. Innovative Food Science and Emerging Technologies 2010, 11, 464–469.
- Liu, X.Q.; Li, Y.H. Epidemiological and Nutritional Research on Prevention of Cardiovascular Disease in China. British Journal of Nutrition 2000, 84, S199–S203.
- Törrönen, R.; Lehmusacho, M.; Harkinen, S.; Hanninen, O.; Mykkanen, H. Serum β-Carotene Response to Supplementation with Raw Carrots, Carrot Juice or Purified β-Carotene in Healthy Non-Smoking Women. Nutrition Research 1996, 16, 565–575.
- Zhang, J.J.; Ji, R.; Hu, Y.Q.; Chen, J.C.; Ye, X.Q. Effect of Three Cooking Methods on Nutrient Components and Antioxidant Capacities of Bamboo Shoot. Journal of Zhejiang University Science B 2011, 12, 752–759.
- Grosshauser, S.; Schieberle, P. Characterization of the Key Odorants in Pan-Fried White Mushrooms (Agaricus Bisporus L.) by Means of Molecular Sensory Science, Comparison with the Raw Mushroom Tissue. Journal of Agricultural and Food Chemistry 2013, 24, 3804–3813.
- Kubola, J.; Siriamornpun, S. Phenolic Contents and Antioxidant Activities of Bitter Gourd (Momordica Charantia L.) Leaf, Stem, and Fruit Fraction Extracts in Vitro. Food Chemistry 2008, 110, 881–890.
- Choi, H.S.; Min, K.C. Aroma-Active Compounds of Elsholtzia Splendens Using AEDA and HS-SPME-GC-O Dilution Analysis. Flavour and Fragrance Journal 2008, 23, 58–64.
- Xiao, Z.B.; Zhang, N.; Niu, Y.W.; Feng, T.; Huai-Xiang Tian, H.X.; Zhu, J.C.; Yu, H.Y. Multivariate Classification of Cherry Wines Based on Headspace Solid Phase Microextraction and Gas Chromatography-Mass Spectrometry of Volatile Compounds. International Journal of Food Properties 2015, 18, 1272–1287.
- Rychlik, M.; Schieberle, P.; Grosch, W. Compilation of Odor Thresholds, Odor Qualities, and Retention Indices of Key Food Odorants. In: Deutsche Forschungsanstalt für Lebensmittelchemie und Institut für Lebensmittelchemie der TU München; Garching, Germany, 1998.
- Song, H.L.; Cadwallader, K.R. Aroma Components of American Country Ham. Journal of Food Science 2008, 73, C29–C35.
- van den Dool, H.; Kratz, D.P. Generalization of the Retention Index System Including Linear Temperature Programmed Gas-Liquid Partition Chromatography. Journal of Chromatography 1963, 2, 463–470.
- Pérez-Jiménez, J.; Arranz, S.; Tabernero, M.; Diaz-Rubio, M.E.; Serrano, J.; Goni, I.; Saura-Calixto, F. Updated Methodology to Determine Antioxidant Capacity in Plant Foods, Oils and Beverages: Extraction, Measurement, and Expression of Results. Food Research International 2008, 41, 274–285.
- Sasikumar, J.M.; Patharaj, J.; Adithya, E.S.; Christabel, P.H.; Shamna, R. Antioxidant Capacity and Phenolic Content of Elaeagnus Kologa Schlecht. An Underexploited Fruit from India. Free Radicals and Antioxidants 2012, 2, 28–35.
- Sanchez-Moreno, C. Review: Methods Used to Evaluate the Free Radical Scavenging Activity in Foods and Biological Systems. Food Science and Technology International 2002, 8, 121–137.
- Benzie, I.F.F.; Strain, J.J. The Ferric Reducing Ability of Plasma (FRAP) As a Measure of “Antioxidant Power,” The FRAP Assay. Analytical Biochemistry 1996, 239, 70–76.
- Müller, L.; Fröhlich, K.; Böhm, V. Comparative Antioxidant Activities of Carotenoids Measured by Ferric Reducing Antioxidant Power (FRAP), ABTS Bleaching Assay (αTEAC), DPPH Assay and Peroxyl Radical Scavenging Assay. Food Chemistry 2011, 129, 139–148.
- Guo, C.J.; Wei, J.Y.; Yan, G.J.; Li, Y.F.; Xu, J.; Jiang, Y.G. The Antioxidant Capacity of 66 Vegetables and Fruits: A Comparative Study. Acta Nutrimenta Sinica 2003, 25, 203–206.
- Wang, X.; Zhao, M.M.; Su, G.W.; Cai, M.S.; Zhou, C.M.; Huang, J.Y.; Lin, L.Z. The Antioxidant Activities and the Xanthine Oxidase Inhibition Effects of Walnut (Juglans Regia L.) Fruit, Stem, and Leaf. International Journal of Food Science and Technology 2015, 50, 233–239.
- Bernhardt, S.; Schlich, E. Impact of Different Cooking Methods on Food Quality: Retention of Lipophilic Vitamins in Fresh and Frozen Vegetables. Journal of Food Engineering 2006, 77, 327–333.
- Ilyasoğlu, H.; Burnaz, N.A. Effect of Domestic Cooking Methods on Antioxidant Capacity of Fresh and Frozen Kale. International Journal of Food Properties 2015, 18, 1298–1305.
- Turkmen, N.; Sari, F.; Velioglu, Y.S. The Effect of Cooking Methods on Total Phenolics and Antioxidant Activity of Selected Green Vegetables. Food Chemistry 2005, 93, 713–718.
- Sanz, C.; Olias, J.M.; Perez, A.G. Aroma Biochemistry of Fruits and Vegetables. In: Phytochemistry of Fruit and Vegetables; Tomas-Barberan, F.A.; Ed.; UK: Clarendon Press, 1997; 125–155.
- Beaulieu, J. C., & Baldwin, E. A. Flavor and aroma of fresh-cut fruits and vegetables. In: Fresh-Cut Fruits and Vegetables; Lamikanra O.; Ed; CRC Press: New York, NY, 2002; 391–425.
- Du, X.F.; Song, M.; Baldwin, E.; Rouseff, R. Identification of Sulphur Volatiles and GC-Olfactometry Aroma Profiling in Two Fresh Tomato Cultivars. Food Chemistry 2015, 171, 306–314.
- Ney, K.J.; Freytag, W. Dimethyl Sulfide As An Essential Component of Asparagus Flavor. Volatile Components of Boiled Asparagus. Zeitschrift fuer Lebensmittel-Untersuchung und Forschung 1972, 149, 154–155.
- Scherb, J.; Kreissl, J.; Haupt, S.; Schieberle, P. Quantitation of S-Methylmethionine in Raw Vegetables and Green Malt by a Stable Isotope Dilution Assay Using LC-MS/MS, Comparison with Dimethyl Sulfide Formation After Heat Treatment. Journal of Agricultural and Food Chemistry 2009, 57, 9091–9096.
- Annelie, J.; Tim, N.; Ingegerd, S. Influence of Temperature, Modified Atmosphere Packaging, and Heat Treatment on Aroma Compounds in Broccoli. Journal of Agricultural and Food Chemistry 2004, 52, 1607–1614.
- Yilmaztekin, M. Characterization of Potent Aroma Compounds of Cape Gooseberry (Physalis Peruviana L.) Fruits Grown in Antalya Through the Determination of Odor Activity Values. International Journal of Food Properties 2014, 17, 469–480.
- Sampaio, K.L.; Biasoto, A.C.T.; Marques, E.J.N.; Batista, E.A.C.; Da Silva, M.A.A.P. Dynamics of the Recovery of Aroma Volatile Compounds During the Concentration of Cashew Apple Juice (Anacardium Occidentale L.). Food Research International 2013, 51, 335–343.