Abstract
Sodium caseinate was modified by transglutaminase-catalyzed reaction in the presence of oligochitosan of 5 kDa at two different levels, aiming to generate two glycated and crosslinked caseinate products (GC-caseinate I and II) and to clarify their respective structure and property changes. Electrophoretic analysis with aid of protein and saccharide staining confirmed that both GC-caseinate I and II were glycated and crosslinked protein products, while high-performance liquid chromatrography analysis demonstrated that both GC-caseinate I and II contained glucosamine (12.8 and 30.8 g/kg protein, respectively). In comparison with sodium caseinate, GC-caseinate I and II also contained less reactable –NH2 groups (0.50 and 0.52 versus 0.62 mol/kg protein), more –OH groups in their molecules, but they had more ordered secondary structure than sodium caseinate. In addition, GC-caseinate I and II showed higher water-binding capacity, larger hydrodynamic radius (173.9 and 168.2 versus 145.3 nm), and larger negative zeta-potential (–32.9 and –30.9 versus –27.6 mV) in aqueous dispersions, but they exhibited lower thermal stability (namely, greater mass loss) at temperature higher than 286ºC. Oligochitosan-glycated sodium caseinate at lower and higher extents was observed to be unfavorable and favorable to the in vitro digestibility of GC-caseinate I and II, respectively. It is concluded that application of transglutaminase and the oligochitosan can modify structure and property of sodium caseinate to generate new protein ingredients with good hydration and colloidal stability.
INTRODUCTION
Food scientists have focused their attention to the practical methods to improve the properties of food proteins in recent decades, as these properties usually exert great impacts on the quality of processed foods.[Citation1] The structure of food proteins governs their properties. Structural modification of proteins via physical, chemical, and enzymatic treatment, thereof, is a direct way to improve protein’s properties. Both chemical and enzymatic modifications of food proteins are powerful and practical,[Citation2] such as saccharide attachment by the Maillard reaction, enzymatic crosslinking, and enzymatic hydrolysis.[Citation3] Covalent attachment of saccharides into protein molecules (i.e., protein glycation) via the Maillard reaction is usually carried out in dry-state condition and endows proteins with improved functional properties, for example emulsifying properties.[Citation4,Citation5] However, some disadvantages for this type of protein glycation exist, including difficult reaction control, the extended reaction time, and formation of mutagenic or colored products.[Citation6] Covalent attachment of saccharides into proteins by enzymatic catalysis can overcome some of these disadvantages. Two potential enzymes for protein glycation are glycosyltransferases and glycosidases.[Citation7] However, substrate specificity and low yield of trans-glycation are considered two limits.[Citation8] Fortunately, transglutaminase (TGase; EC 2.3.2.13) might be an alternative selection to catalyze enzymatic protein glycation based on some recent reported investigations.[Citation9–Citation12]
TGase can catalyze acyl transfer reaction, in which γ-carboxyamide groups of the glutamine residues act as acyl donors, while the most common acyl acceptors are ε-amino groups of lysine residues and primary amino groups (–NH2) of some naturally occurring polyamines.[Citation13] Thus, TGase is able to induce protein crosslinking, and has been used widely to modify the properties of soy protein, myosin, gluten, globulin, and milk proteins.[Citation14] Recently, TGase has been applied to generate glycated proteins via conjugating –NH2-containing saccharides (glucosamine and an oligochitosan of 1 kDa) into sodium caseinate,[Citation10,Citation11] soy protein,[Citation9,Citation15] and fish protein.[Citation12] Glucosamine has –NH2 groups, while oligochitosan comprises of glucosamine units. Both of them are acyl acceptors. TGase is capable of inducing protein crosslinking and saccharide attachment simultaneously. The generated products by this way are glycated and crosslinked proteins (GC-proteins) with modified properties. For example, both GC-soy protein and GC-caseinate have been observed to have improved water-binding capacity and rheological properties.[Citation9,Citation11] However, whether an oligochitosan with much larger molecular weight than that of the previous studies used can also be conjugated into food proteins by TGase, and more important, how this modification will bring about structure and property changes still remain unknown in the present time.
Caseinate (e.g., sodium caseinate) is one of the most used protein ingredients with good properties such as water-binding, gelation, emulsification, and others. Oligochitosan has been widely used in the fields like food and agriculture due to its applicable properties such as biocompatibility, biodegradability, and no toxicity.[Citation16] In this study, TGase and an oligochitosan with molecular weight of 5 kDa were used to modify sodium caseinate. Two glycated and crosslinked caseinate products (namely, GC-caseinates) generated were assessed for their structure and property (i.e., levels of –OH groups, secondary structure, thermal stability, hydration, and aggregation). The aim of this study was to assess the structure and property changes of sodium caseinate induced by this modification, and the potential of GC-caseinates as protein ingredients.
MATERIAL AND METHODS
Materials and Chemicals
Caseinate (protein content of 89.1% on dry weight basis) was purchased from Beijing Aoboxing Bio-Tech Co., Ltd. (Beijing, China). Oligochitosan with declared deacetylation degree about 90% and average molecular weight of 5 kDa was purchased from Zhejiang Golden-Shell Pharmaceutical Co., Ltd. (Hangzhou, China). TGase was donated by Jiangsu Yiming Fine Chemical Industry Co., Ltd. (Qinxing, Jiangsu, China) with a measured activity of 110 units (U) per gram. All reagents used in high-performance liquid chromatography (HPLC) analysis were of HPLC grade. The buffers and solutions were prepared by using highly purified water generated from Milli-Q Plus system (Millipore, New York, NY, USA) and filtered through a 0.45 μm Millipore HA membrane. Other chemicals used were of analytical grade.
Glycation and Crosslinking of Sodium Caseinate
Sodium caseinate and oligochitosan were dispersed in water, followed by adjusting pH value to 7.5 using 2 mol/L NaOH to obtain the fixed concentrations of 50 and 100 g/L, respectively. The solutions of caseinate and oligochitosan were mixed at two volume ratios of 8:1 and 4:1, to achieve two respective caseinate-oligochitosan ratios of 4:1 and 2:1 (w/w), and then diluted to the fixed total concentration of 50 g/L. TGase was added at 10 U/g protein to the mixed solutions. Two reaction systems were held at 37ºC for 3 h with continuous agitation, followed by prompt heating to 85ºC for 5 min in a water bath to inactivate TGase and then cooling rapidly to an ambient temperature of 20ºC. Forthcoming treatment was the same as our previous study[Citation17] to remove free oligochitosan via isoelectric precipitation and washing. The respective precipitates collected were dispersed in water, and neutralized to pH 7.0 using 2 mol/L NaOH. Two GC-caseinates were obtained, freeze-dried, heated at 50ºC overnight for complete drying, and named as GC-caseinate I (prepared with a caseinate-oligochitosan mass ratio of 4:1) and GC-caseinate II (prepared with a caseinate-oligochitosan mass ratio of 2:1), respectively. Sodium caseinate was also subjected to the same treatment as the GC-caseinates without the addition of TGase and oligochitosan, and used as a control for further study. All samples were sealed in plastic bags, and stored at –20ºC before using.
Chemical Analyses
Nitrogen content was assayed by the Kjeldahl method at a Kjeltec 2300 Protein Analyzer (Foss Tecator AB, Hoganas, Sweden), with a conversion factor of 6.38 to obtain protein content.[Citation18] Sodium dodecyl sulphate-polyacrylamide gel electrophoresis (SDS-PAGE) analysis of the samples as well as protein and saccharide staining were performed as per the study.[Citation11] Oligochitosan amount conjugated into the two GC-caseinates was evaluated using the reversed phase-HPLC method,[Citation19] and expressed as the amount of glucosamine (g) in protein fraction of 1 kg. The amount of reactable –NH2 groups of the samples was assayed using the o-phthaldialdehyde (OPA) method,[Citation15] and reported as the amount of –NH2 groups (in moles) in protein fraction of 1 kg. For the two GC-caseinates, the amount of –NH2 and nitrogen from the conjugated oligochitosan were obtained by HPLC analysis, and subtracted from the respective results to make correction for the measured reactable –NH2 groups and protein contents of the samples.
Fourier Transform Infrared (FT-IR) and Circular Dichroism (CD) Analyses
The samples were measured at a Spectrum One FT-IR spectrometer (Perkin Elmer Inc., Norwalk, CT, USA) to obtain their FT-IR spectra. The spectra were recorded from 3600 to 400 1/cm by using 1 1/cm resolution and an accumulation of 64 scans, according to the reported method.[Citation13] The samples used in FT-IR analysis were prepared using the pre-dried samples of 1 mg and KBr of 100 mg, respectively, to generate the compressed pellets.
Far-ultraviolet (UV) CD was used to examine secondary structure of proteins according to a reported method.[Citation20] The samples of 0.1 g/L (in 10 mmol/L phosphate buffer, pH 7.0) were assayed at 25ºC using a Jasco J-810 spectropolarimeter (Jasco Corporation, Tokyo, Japan). The spectra were recorded using a 0.1 cm path length cuvette at a speed of 100 nm/min, a response time of 0.125 s, and a bandwidth of 1 nm. Mean residue ellipticity (θ) was expressed in deg cmCitation2/dmol.
Thermogravimetric (TG) Analysis
TG analysis was carried out by using a SDT Q600 TG analyzer (TA Instruments Inc., New Castle, DE, USA). The samples were held at an ambient temperature of 20ºC for 24 h with a saturated NaCl atmosphere (relative humidity about 75.5%) prior to the analysis. During the TG analysis, the samples of 2 mg were weighed, and then heated from ambient temperature to 450ºC at 10ºC/min under air atmosphere (100 mL/min). The temperatures responsible for the maximum degradation rates (Tmax) of the samples at two stages (region I, ambient temperature to 105ºC; and region II, 105 to 450ºC), as well as the mass loss of the samples, were determined from the derivative TG (DTG) and TG curves, respectively.
Property Evaluation
Hydrodynamic radius and zeta-potential of the samples in dispersions (1.0 g/L in 10 mmol/L phosphate buffer, pH 7.0) were measured at 25ºC by dynamic light scattering, using the Zetasizer Nano-ZS90 (Malvern Instruments, Westborough, MA, USA). Sample dispersions of 3 mL were placed in a disposable sizing cuvette (1 cm path length) within the sample holder. The two indices were estimated by the respective Stokes-Einstein and Henry’s equations.[Citation21]
Water-binding capacities (WBC) of the samples was assayed as per the method,[Citation19] and expressed as the amount (grams) of water held in the samples on the basis of protein fraction of one gram. In vitro digestibility of the samples was assessed as per the study.[Citation9] Trichloroacetic acid-soluble nitrogen released into the final supernatants from the pepsin and pepsin-trypsin digestion was diluted by water of four-fold volumes; after that, the diluted solutions were detected for their absorbencies at 280 nm by a spectrophotometer (UV-2401 PC, Shimadzu Co., Kyoto, Japan). The detected absorbencies were used to reflect in vitro digestibility of the samples.
Statistical Analysis
All experiments and analyses were carried at three times. All data were expressed as mean values or mean values ± standard deviations. The differences between the mean values of multiple groups were analyzed by one-way analysis of variance (ANOVA) with Duncan’s multiple range tests by using SPSS 16.0 software (SPSS Inc., Chicago, IL, USA). MS Excel 2003 (Microsoft Corporation, Redmond, WA, USA) was used to report the data in the figures.
RESULTS AND DISCUSSION
Chemical Characteristics of GC-Caseinate I and II
SDS-PAGE analysis () shows that both GC-caseinate I and II contain protein polymers (, lanes and ) and saccharide moieties (, lanes and ). It was thus proved that both GC-caseinate I and II were oligochitosan-glycated and crosslinked protein products, namely, TGase induced glycation and crosslinking of sodium caseinate. HPLC analysis further indicated that both GC-caseinate I and II were glycated, as they were detected to have respective glucosamine amount of 12.8 and 30.8 g/kg protein. At the same time, no glucosamine was detected in sodium caseinate. GC-caseinate II was prepared with lower caseinate-oligochitosan mass ratio of 2:1. Higher level of oligochitosan ensured itself much opportunity for being conjugated into sodium caseinate. GC-caseinate II thus had higher glucosamine content than GC-caseinate I prepared with higher caseinate-oligochitosan mass ratio of 4:1. Meanwhile, OPA analysis results demonstrated that GC-caseinate I and II had reactable –NH2 groups of 0.50 and 0.52 mol/kg protein, respectively, which were lower than that of sodium caseinate (0.62 mol/kg protein). During sample preparation, some ε–NH2 groups of the lysine residues in sodium caseinate were blocked by the glutamine residues; therefore, the generated products GC-caseinate I and II should have decreased amount of –NH2 groups. OPA analysis results supported that both GC-caseinate I and II were crosslinked proteins, and GC-caseinate I was much crosslinked than GC-caseinate II.
FIGURE 1 SDS-PAGE profiles of three protein samples stained for (a) protein and (b) saccharide fractions. Lane M, standard protein markers; lane 1, horseradish peroxidase; lane 2, sodium caseinate; lane 3, GC-caseinate I; lane 4, GC-caseinate II. The arrows indicate protein polymers.
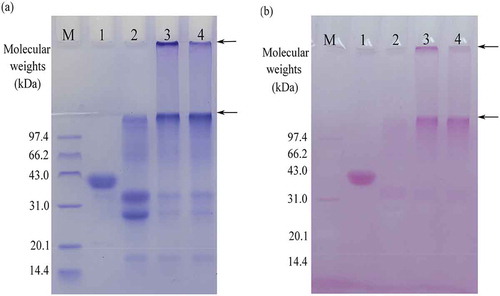
Two past studies reported that TGase could be used to conjugate glucosamine and oligochitosan of 1 kDa into sodium caseinate, resulting in glucosamine conjugation of 10.2 and 4.74 g/kg, respectively.[Citation10,Citation17] The oligochitosan used in this study had larger molecular weight than glucosamine and the oligochitosan of 1 kDa; that is, the oligochitosan of 5 kDa contained more glucosamine units in the molecules. Both GC-caseinate I and II thus showed higher glucosamine amount than the two reported GC-caseinates. OPA analysis for reactable –NH2 groups was applied in our previous study to reflect the crosslinking of soy protein,[Citation15] while SDS-PAGE analysis were used to confirm TGase-induced glycation and crosslinking of two proteins.[Citation9,Citation11] This study drew the same conclusion as these reported studies; sodium caseinate could be modified by TGase in the presence of the oligochitosan of 5 kDa to produce oligochitosan-glycated and crosslinked caseinates.
Structural Characteristics of GC-Caseinate I and II
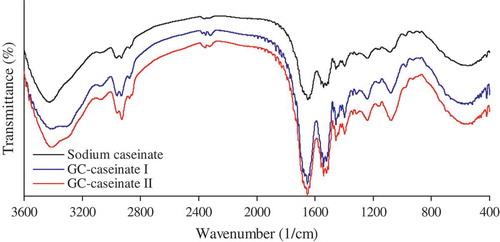
FT-IR analysis was used to reveal chemical characteristics of GC-caseinate I and II. IR spectra of GC-caseinate I and II shows some differences, compared with sodium caseinate (). Both GC-caseinate I and II had greater absorption around 3300–3600 and 1050–1150 1/cm. The first absorption peak was assigned to the N–H stretching vibration and O–H stretching vibration, while the second one was responsible for the O–H deforming vibration and C–O stretching.[Citation22] These results demonstrated that both GC-caseinate I and II contained more –OH groups in the molecules because of glycation of sodium caseinate by the oligochitosan of 5 kDa. Based on HPLC analysis, GC-caseinate I had lower oligochitosan conjugation than GC-caseinate II; therefore, it showed weaker absorption in the two regions.
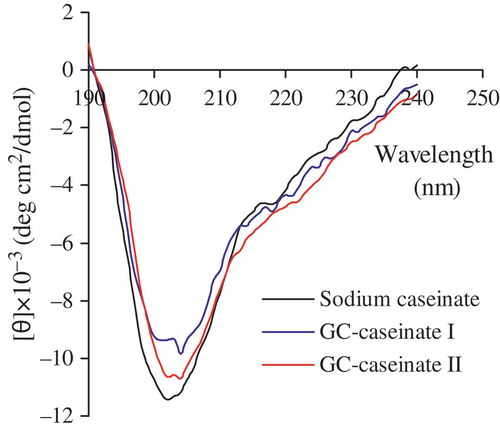
Secondary structural features of sodium caseinate, GC-caseinate I and II are described by the obtained CD spectra (). In comparison with sodium caseinate, GC-caseinate I and II displayed different profiles in secondary structure. First, they showed weaker negative absorption around 200 nm (an indicator of disordered structure), suggesting that they had a less disordered secondary structure. Second, they exhibited stronger negative absorption around 215 and 220 nm, which indicated they had more β-sheet and α-helix structure. Third, they also had stronger negative absorption in the region of 220–230 nm, indicating that they possessed more β-turn structure. Overall, both GC-caseinate I and II had a more ordered secondary structure than sodium caseinate. Darewicz and Dziuba[Citation23] found that glycation of β-casein by glucose was helpful to β-turn formation, leading to an ordered secondary structure. Niu et al.[Citation24] observed that glycation of wheat germ protein with dextran favored α-helix formation but decreased random coil. Similarly, TGase-treated oat globulin showed increased β-sheet structure.[Citation25] These studies indicated that protein glycation and crosslinking could result in an ordered secondary structure. Oligochitosan-glycation and crosslinking thereof conferred GC-caseinate I and II with a more ordered secondary structure. Structure and properties of food proteins are highly correlated.[Citation26] It is expected that both GC-caseinate I and II had modified structure, as results of introduction of hydrophilic –OH groups into molecules, protein crosslinking, and alteration of secondary structure; therefore, GC-caseinate I and II would have properties different from sodium caseinate.
Thermal Properties of GC-Caseinate I and II
The measured TG and DTG curves for the three samples are shown in and , respectively. Based on these curves, three samples had different thermal properties (). All the samples exhibited similar behavior during TG analysis with two stages of mass loss. In region I (ambient temperature to 105ºC), three samples were heated without any breakage of the covalent bonds in the sample molecules. Mass loss in region I was mostly attributed to the lost water physically retained by the samples. In comparison with sodium caseinate, both GC-caseinate I and II absorbed more water (12.6–12.7% versus 8.3%), but they lost water at lower temperature (Tmax1, 38.2–38.7ºC versus 47.5ºC). In region II (105 to 450ºC), the samples were heated at higher temperature, which finally resulted in partial decomposition of the samples. The measured Tmax2 of sodium caseinate, GC-caseinate I and II were 291.5, 286.2, and 286.3ºC, respectively, demonstrating that both GC-caseinate I and II were less stable than sodium caseinate. At the same time, the measured mass loss of sodium caseinate, GC-caseinate I and II in region II were 52.1, 54.2, and 58.3%, respectively, proving different thermal stability of the samples again. Sodium caseinate and GC-caseinate II exhibited the highest and least thermal stability, respectively. Therefore, TG analysis demonstrated that the carried out modification conferred the products with lower thermal stability.
FIGURE 4 (a) Original thermogravimetric (TG) curves for sodium caseinate, GC-caseinate I and II, and (b) the respective derivative function (DTG) versus temperatures ranging from ambient temperature to 450°C.
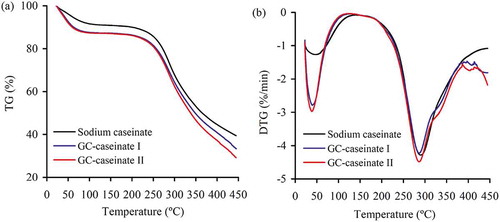
TABLE 1 Some thermogravimetric parameters of sodium caseinate, GC-caseinate I and II
Saccharides at high temperature show lower thermal stability than proteins, as Grega et al.[Citation27] had observed that potato starch gave higher mass loss than casein (94.3% versus 66.3%) in temperature ranging from 23 to 500ºC. Chitosan has a Tmax value of 277ºC;[Citation16] this value was lower than the measured value of sodium caseinate (291.5ºC). Both GC-caseinate I and II were oligochitosan-glycated caseinates. The attached oligochitosan fraction thus conferred GC-caseinate I and II with higher sensitivity toward heating. It is reasonable that both GC-caseinate I and II showed lower thermal stability with lower Tmax2 and greater mass loss than sodium caseinate. A support came from the study of Cardoso et al.,[Citation28] who reported that a casein-maltodextrin conjugate via the Maillard reaction also showed higher mass loss than casein in a temperature range of 300–800ºC.
Other Properties of GC-Caseinate I and II
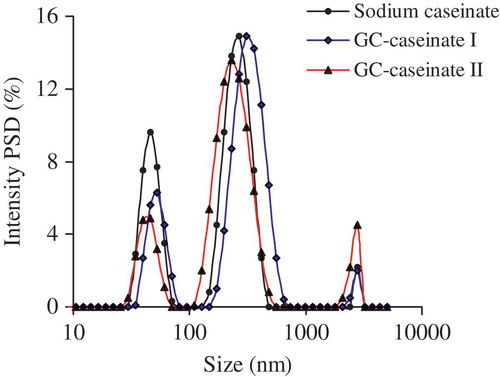
Hydrodynamic radius, zeta-potential, WBC, and in vitro digestibility of sodium caseinate, GC-caseinate I and II are exhibited in and , respectively. Based on these data, it is seen again that the modification caused property changes of sodium caseinate. The results in Fig. 5 show the three samples had different particle size distribution (PSD). The measured average hydrodynamic radius of sodium caseinate, GC-caseinate I and II in dispersions were 145.3, 173.9, and 168.2 nm, respectively (). Clearly, oligochitosan glycation and crosslinking of sodium caseinate led to that both GC-caseinate I and II more able to form greater aggregates in dispersions; that is, larger hydrodynamic radius was detected in the measurement. Caseins can aggregate into micelles of 50–500 nm (average 150 nm).[Citation29] TGase induces inter-micellar crosslinking of proteins, which therefore, brings about increased sizes of protein aggregates.[Citation30] When TGase was used to treat the ultra-high temperature-treated milk, larger hydrodynamic radius and enhanced micellar stability were observed, and formation of intra-micellar iso-peptide bonds in milk proteins was responsible for the enlarged radius.[Citation31] Similarly, both GC-caseinate I and II were crosslinked protein products by TGase with larger hydrodynamic radius and enhanced aggregation than sodium caseinate. GC-caseinate I had slightly larger hydrodynamic radius than GC-caseinate II, because it was much crosslinked during preparation.
TABLE 2 Some properties of sodium caseinate, GC-caseinate I and II
Zeta-potential provides information about the outer layer of the micelles and the role of electrostatics in the changed stability of the micelles.[Citation32] The measured respective zeta-potentials of sodium caseinate, GC-caseinate I and II dispersions were about −27.6, −32.9, and −30.9 mV (). It is thus seen that the occurred modification conferred both GC-caseinate I and II with good colloidal stability. For TGase-induced protein crosslinking, acyl acceptors (ε–NH2 groups of lysine residues) are blocked, resulting in reduced numbers of positive charges. Both GC-caseinate I and II thus had more negative values in their zeta-potentials than sodium caseinate. Zeta-potential of whey protein isolate in dispersion of pH 7.0 was −27.0 mV, whilst that of a crosslinked counterpart by TGase was −30.4 mV.[Citation33] The two values provided a direct support to the present measurement results.
Both GC-caseinate I and II showed better WBC than sodium caseinate (14.32 and 10.14 versus 5.62 g/g protein; ), indicating that they could bind much water. At the same time, TG analysis (, region I) also evidenced this result. Hydrophilic saccharides attached into proteins could enhance WBC of the conjugates,[Citation34] while TGase-induced protein crosslinking was also beneficial to WBC of caseinate.[Citation17] The present results were consistent with the two reported ones.
GC-caseinate I and II were different when they were subjected to in vitro proteolytic digestion (). In comparison with sodium caseinate, GC-caseinate I showed lower digestibility (0.385 versus 0.406 in pepsin digestion, or 0.956 versus 1.013 in pepsin-trypsin digestion), whereas GC-caseinate II exhibited higher one (0.438 versus 0.406 in pepsin digestion, or 1.042 versus 1.013 in pepsin-trypsin digestion). Crosslinked food proteins had lower digestibility. For example, the crosslinked soy protein isolate had lower in vitro digestibility due to its compact structure.[Citation35] However, conjugation of saccharides into proteins is beneficial to their digestion;[Citation36] for example, β-lactoglobulin-dextran conjugates via the Maillard reaction had enhanced digestibility.[Citation37] GC-caseinate I was crosslinked protein with less glycation (glucosamine amount 12.8 g/kg protein), indicating lower digestibility than sodium caseinate. However, GC-caseinate II was also crosslinked protein but had greater glycation (glucosamine amount 30.8 g/kg protein), suggesting better digestibility than sodium caseinate.
CONCLUSIONS
Sodium caseinate could be modified by TGase in the presence of the oligochitosan of 5 kDa at two levels, to generate two oligochitosan-glycated and crosslinked caseinates with different glucosamine contents. The modification was observed to induce structure and property changes in the two products, reflected by several indices in comparison with sodium caseinate. The two products contained less reactable –NH2 but more –OH groups, and they had more ordered secondary structure, much enhanced water-binding property, especially enlarged hydrodynamic radius as well as negative zeta-potential in dispersions. The two products also exhibited decreased thermal stability at temperatures higher than 286ºC. Increased glycation of sodium caseinate contributed to in vitro digestion. The two products might be served as potential protein ingredients with good hydration and colloidal stability.
ACKNOWLEDGMENTS
The authors thank Mr. Fu for his help in the article preparation, and the anonymous reviewers and editors for their valuable advice.
FUNDING
This study was funded by the National High Technology Research and Development Program (“863” Program) of China (Project No. 2013AA102205), the Specialized Research Fund for the Doctoral Program of Higher Education (Project No. 20132325130001), and the Innovation Foundation for Postgraduate of Northeast Agricultural University (Project No. YJSCX14062).
Additional information
Funding
REFERENCES
- Dube, M.; Schäfer, C.; Neidhart, S.; Carle, R. Texturisation and Modification of Vegetable Proteins for Food Applications Using Microbial Transglutaminase. European Food Research and Technology 2007, 225, 287–299.
- Hattori, M. Functional Improvements in Food Proteins in Multiple Aspects by Conjugation with Saccharides: Case Studies of β-Lactoglobulin-Acidic Polysaccharides Conjugates. Food Science and Technology Research 2002, 8, 291–299.
- Broyard, C.; Gaucheron, F. Modifications of Structures and Functions of Caseins: A Scientific and Technological Challenge. Dairy Science and Technology 2015, 95, 831–862.
- Golkara, A.; Nasirpoura, A.; Keramata J.; Desobryb, S. Emulsifying Properties of Angum Gum (Amygdalus Scoparia Spach) Conjugated to β-Lactoglobulin Through Maillard-Type Reaction. International Journal of Food Properties 2015, 18, 2042–2055.
- Hernández-García, S.; Salazar-Montoya, J.A.; Totosaus, A. Emulsifying Properties of Food Proteins Conjugated with Glucose Or Lactose by Two Methods (Spray-Drying Or Freeze-Drying). International Journal of Food Properties 2016, 19, 526–536.
- Zhu, D.; Damodaran, S.; Lucey, J.A. Formation of Whey Protein Isolate (WPI)-Dextran Conjugates in Aqueous Solutions. Journal of Agricultural and Food Chemistry 2008, 56, 7113–7118.
- Colas, B.; Caer, D.; Fournier, E. Transglutaminase-Catalyzed Glycosylation of Vegetable Proteins. Effect on Solubility of Pea Legumin and Wheat Gliadins. Journal of Agricultural and Food Chemistry 1993, 41, 1811–1815.
- Hrynets, Y.; Ndagijimana, M.; Betti, M. Transglutaminase-Catalyzed Glycosylation of Natural Actomyosin (NAM) Using Glucosamine As Amine Donor: Functionality and Gel Microstructure. Food Hydrocolloids 2014, 36, 26–36.
- Jiang, S.J.; Zhao, X.H. Transglutaminase-Induced Cross-Linking and Glucosamine Conjugation in Soybean Protein Isolates and Its Impacts on Some Functional Properties of the Products. European Food Research and Technology 2010, 231, 679–689.
- Jiang, S.J.; Zhao, X.H. Transglutaminase-Induced Cross-Linking and Glucosamine Conjugation of Casein and Some Functional Properties of the Modified Product. International Dairy Journal 2011, 21, 198–205.
- Song, C.L.; Zhao, X.H. Rheological, Gelling, and Emulsifying Properties of a Glycosylated and Cross-Linked Caseinate Generated by Transglutaminase. International Journal of Food Science and Technology 2013, 48, 2595–2602.
- Hong, P.K.; Gottardi, D.; Ndagijimana, M.; Betti, M. Glycation and Transglutaminase Mediated Glycosylation of Fish Gelatin Peptides with Glucosamine Enhance Bioactivity. Food Chemistry 2014, 142, 285–293.
- Sang, L.Y.; Zhou, X.H.; Yun, F.; Zhang, G.L. Enzymatic Synthesis of Chitosan-Gelatin Antimicrobial Copolymer and Its Characterisation. Journal of the Science of Food and Agriculture 2010, 90, 58–64.
- Yokoyama, K.; Nio, N.; Kikuchi, Y. Properties and Applications of Microbial Transglutaminase. Applied Microbiology and Biotechnology 2004, 64, 447–454.
- Song, C.L.; Zhao, X.H. Structure and Property Modification of An Oligochitosan- Glycosylated and Crosslinked Soybean Protein Generated by Microbial Transglutaminase. Food Chemistry 2014, 163, 114–119.
- Pereira, F.S.; da Silva Agostini, D.L.; Job, A.E.; González, E.R.P. Thermal Studies of Chitin-Chitosan Derivatives. Journal of Thermal Analysis and Calorimetry 2013, 114, 321–327.
- Song, C.L.; Zhao, X.H. The Preparation of An Oligochitosan-Glycosylated and Cross-Linked Caseinate Obtained by a Microbial Transglutaminase and Its Functional Properties. International Journal of Dairy Technology 2014, 67, 110–116.
- AOAC. Official Methods of Analysis of Association of Official Analytical Chemists International, 18th Ed; AOAC International: Gaithersburg, FL, 2005.
- Zhu, C.Y.; Wang, X.P.; Zhao, X.H. Property Modification of Caseinate Responsible to Transglutaminase-Induced Glycosylation and Crosslinking in the Presence of a Degraded Chitosan. Food Science and Biotechnology 2015, 24, 843–850.
- Wooster, T.J.; Augustin, M.A. Rheology of Whey Protein-Dextran Conjugate Films at The Air/Water Interface. Food Hydrocolloids 2007, 21, 1072–1080.
- Lam, R.S.H.; Nickerson, M.T. The Effect of pH and Heat Pre-Treatments on the Physicochemical and Emulsifying Properties of β-Lactoglobulin. Food Biophysics 2014, 9, 20–28.
- Guan, J.J.; Qiu, A.Y.; Liu, X.Y.; Hua, Y.F.; Ma, Y.H. Microwave Improvement of Soy Protein Isolate-Saccharide Graft Reactions. Food Chemistry 2006, 97, 577–585.
- Darewicz, M.; Dziuba, J. The Effect of Glycosylation on Emulsifying and Structural Properties of Bovine Beta-Casein. Nahrung-Food 2001, 45, 15–20.
- Niu, L.Y.; Jiang, S.T.; Pan, L.J.; Zhai, Y.S. Characteristics and Functional Properties of Wheat Germ Protein Glycated with Saccharides Through Maillard Reaction. International Journal of Food Science and Technology 2011, 46, 2197–2203.
- Siu, N.C.; Ma, C.Y.; Mine, Y. Physicochemical and Structural Properties of Oat Globulin Polymers Formed by a Microbial Transglutaminase. Journal of Agricultural and Food Chemistry 2002, 50, 2660–2665.
- Martínez, K.D.; Sánchez, C.C.; Ruíz-Henestrosa, V.P.; Patino, J.M.R.; Pilosof, A.M.R. Effect of Limited Hydrolysis of Soy Protein on the Interactions with Polysaccharides at the Air-Water Interface. Food Hydrocolloids 2007, 21, 813–822.
- Grega, T.; Najgebauer, D.; Sady, M.; Baczkowicz, M.; Tomasik, P.; Faryna, M. Biodegradable Complex Polymers from Casein and Potato Starch. Journal of Polymers and the Environment 2003, 11, 75–83.
- Cardoso, J.C.; Albuquerque Jr., R.L.C.; Padilha, F.F.; Bittencourt, F.O.; de Freitas, O.; Nunes, P.S.; Pereira, N.L.; Fonseca, M.J.V.; Araújo, A.A.S. Effect of the Maillard Reaction on Properties of Casein and Casein Films. Journal of Thermal Analysis and Calorimetry 2011, 104, 249–254.
- Fox, P.F. Milk Proteins: General and Historical Aspects. In Fox, P.F.; McSweeney, P.L.H.; Eds.; Advanced Dairy Chemistry–1 Proteins, 3rd Ed; Kluwer Academic/Plenum Publishers: New York, NY, 2003; 1–48.
- Mellema, M.; de Groot, P.W.N.; Golding, M. Impaired or Accelerated Aggregation of Proteins in Sterilised Milk by Adding Surfactants. International Dairy Journal 2009, 19, 728–736.
- Partschefeld, C.; Schwarzenbolz, U.; Richter, S.; Henle, T. Crosslinking of Casein by Microbial Transglutaminase and Its Resulting Influence on the Stability of Micelle Structure. Biotechnology Journal 2007, 2, 456–461.
- Anema, S.G.; Klostermeyer, H. ζ-Potentials of Casein Micelles from Reconstituted Skim Milk Heated at 120ºC. International Dairy Journal 1996, 6, 673–687.
- Wang, W.; Zhong, Q.X.; Hu, Z.X. Nanoscale Understanding of Thermal Aggregation of Whey Protein Pretreated by Transglutaminase. Journal of Agricultural and Food Chemistry 2013, 61, 435–446.
- Matemu, A.O.; Kayahara, H.; Murasawa, H.; Nakamura, S. Importance of Size and Charge of Carbohydrate Chains in the Preparation of Functional Glycoproteins with Excellent Emulsifying Properties from Tofu Whey. Food Chemistry 2009, 114, 1328–1334.
- Jiang, P.; Zhao, X.H. Gelation and in Vitro Digestibility of Soybean Protein Isolate Treated by a Ternary System Containing Horseradish Peroxidase, Glucose Oxidase, and Glucose. International Journal of Food Properties 2014, 17, 2284–2297.
- Thomas, M.E.C.; Scher, J.; Desobry-Banon, S.; Desobry, S. Milk Powders Ageing: Effect on Physical and Functional Properties. Critical Reviews in Food Science and Nutrition 2004, 44, 297–322.
- Wooster, T.J.; Augustin, M.A. β-Lactoglobulin-Dextran Maillard Conjugates: Their Effect on Interfacial Thickness and Emulsion Stability. Journal of Colloid and Interface Science 2006, 303, 564–572.