ABSTRACT
The antioxidant peptide was prepared from hydrolyzed egg of Apostichopus japonicus. Ultra-filtration, high-speed counter-current chromatography and gel filtration were used in peptide purification. In each step of purification, meanwhile, peptide was tested with its antioxidant ability. The purity and molecular weight was tested by using Tricine-sodium dodecyl sulphate-polyacrylamide gel electrophoresis. Results showed that the purified peptide obtained a sharply enhancing ability to scavenge hydroxyl radical from 37 to 89.82 U/mL. The purity of peptide was conformed and molecular weight was estimated about 30 KDa. It would be promising for purified peptide to be a good functional food resource.
Introduction
Sea cucumber Apostichopus japonicus was a traditional nutritional food in China and other East Asian countries. In recent years, many studies reported that sea cucumber had good effects of immunomodulatory properties,[Citation1] angiotensin I converting enzyme (ACE) inhibitory activity,[Citation2] hypolipidemic effects,[Citation3] antifungal activity,[Citation4] antitumor activity,[Citation5] antioxidative activity, etc.[Citation6] According to its multifunctional healthcare performance, sea cucumber was usually called “sea ginseng.”[Citation7,Citation8] The gonads of the sea cucumber were by-products in dried items processing, and yet their utilization was at a very low level in many countries.
Sea cucumbers have high protein and low fat contents.[Citation9] The body walls of sea cucumber are rich in nutrients such as proteins, essential amino acids (EAA), polysaccharides, and mineral components.[Citation10,Citation11] Studies showed that the gonads also had plenty of nutrients with the same and even higher levels than that in the usually edible part of the body wall.[Citation12] Among all specific nutrients in the sea cucumber, peptides were believed to have abilities in scavenging radicals, hydrogen peroxide, and other peroxides.[Citation13] These activities resulted in the protection of the cell membrane and delay of senescence. Currently, peptides from the sea cucumber were utilized in the area of foods, pharmaceuticals, cosmetics, etc. Some functional peptides with therapeutic effects had been applied for a patent.[Citation14]
In recent years, many biochemical properties extracted from the sea cucumber were reported.[Citation15] Fucosylated chondroitin sulfates were isolated from sea cucumbers which had anticoagulant activities.[Citation10] The total phenols and flavonoids were extracted from the digestive tract, gonads, muscles, and the respiratory apparatus of sea cucumbers, and their antioxidant activities were determined.[Citation6] Currently, direct extraction, the synthesis method, and proteolysis are the main methods in preparing peptides.[Citation2,Citation16–Citation18] In contrast to the former two methods using organic solvents or toxic chemicals, the method using enzymes to hydrolyze protein are moderate, safe, and more efficient. By controlling the parameters such as time, temperature, and other conditions, different bioactive peptides can be produced. The purification methods of the peptides include gel filtration, ultra-filtration, ion exchange separation, high-speed counter-current chromatography (HSCCC), capillary electrophoresis (CE), high-performance liquid chromatography (HPLC), etc. Based on the difference of molecular weight, charge, polarity, binding force, and other factors, the separation can be realized.
Most research papers published today focused on the material of the body wall. Few reports were written concerning the peptides found in the gonads of sea cucumbers. Many researchers payed more attention to purification and biological activity than extraction of gonad peptides.[Citation12,Citation17,Citation19] Facts such as short collection time, low yield, and no identity as a food resource may account for the lack of studies. Even in the research reported about gonads in the sea cucumber, the egg and sperm were not distinctly divided into egg and sperm. By using a microscope, the gonad was easily divided into two parts. According to our previous research, there were different nutritional compositions in egg and sperm. The egg had balanced nutrition compositions and more than 50% of protein on a dried basis, which made it a good resource for peptide preparation. It would be feasible to prepare peptides from the eggs in a sea cucumber and to manufacture functional foods according to their activities. In this study, we investigated the purification process of peptides, and in the process, tested its antioxidant activity in each step. The results would be helpful to exploit new functional foods with peptides from sea cucumber by-products.
Material and Methods
Sampling
Fresh Apostichopus japonicus was harvested from Yangma Island (Latitude~37° 47′ N, Longitude~121° 66′ E, Yantai, Shandong, China), during early June in 2012. The body wall of the sea cucumber was cut lengthwise, and the gonads were picked out from viscera. Then, the egg and sperm were separated from each other through observation by microscope (Eclipse E600w, Nikon, Tokyo, Japan). Egg emulsion was prepared for hydrolysis after the smash and homogenizing.
Enzyme Hydrolysis
The former optimized technology was used here,[Citation20] and the parameters were as follows: hydrolysis at 75°C for 4 h with a 1:1 (m/m) mixture of papain (8 × 105U/g, Pangbo Biological Engineering Co., Ltd., Nanning, Guangxi, China) and protamex (500 LAPU/g, Novozymes, Bagsvaerd, Denmark) at a total enzyme dosage of 2.0% and after enzyme inactivation, further hydrolysis with 1.5% flavourzyme (1.5AU/g, Novozymes, Bagsvaerd, Denmark) at 45°C for one h. When hydrolysis was completed, a five min boiling water bath was used to inactivate the enzyme.
Ultra-Filtration
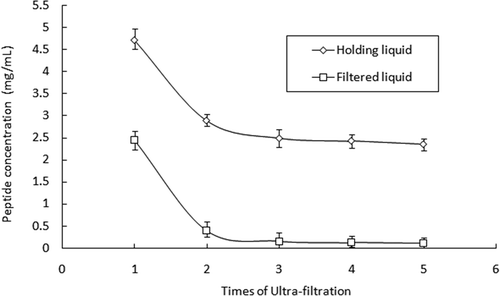
The hydrolysis liquid was centrifuged for 20 min using 8000 rpm centrifugal machine (5430R, Eppendorf AG, Hamburg, Germany). Then the supernatant liquid was filtrated by using a 0.45 and 0.22 μm pore size membrane (Pall, New York, NY, USA). Ultra-filtration was carried on by using one lab scale apparatus MinimateTM with cut-off molecular 10 KDa, 1 KDa, 650 Da cross-flow membrane module (Pall, Easthills, NY, USA). Under pressure of 10–30 psig, peptides with big molecular weights can be held back, while the smaller ones can filtrated out. After step-by-step filtration, the hydrolysis peptide liquid can be divided into four parts with molecular weights more than 10 KDa, 10-1 KDa, 1 K–650 Da, and smaller than 650 Da. The concentrations of peptide in different parts were detected using Biuret method.
The gradient dilution method was put into use during the ultra-filtration process. When the residual volume of the holding liquid was about 1/5, we added water to reach the initial volume.The next step was to start the next filtration process and repeat several times. Concentrations of the peptide were detected in two parts each time after filtration. When the concentration in the holding liquid was stable and was about zero in the filtered solution, the end of ultra-filtration was made.
HSCCC
The HSCCC method was selected as one procedure in the purification of the egg peptide by using the TBE200V (Shanghai Tauto Biotech Co., Ltd., Shanghai, China) apparatus.[Citation21] The mixed solution was composed by methyl tertiary butyl ether (MTBE):n-butanol:acetonitrile:1% trifluoroacetic acid (TFA) water solution with volume ratio 2:1:1:4. After being degassed by ultrasonic, the solution was layered into the up stationary phase and down mobile phase. The detected samples were dissolved into 20 mL mobile phase with the dose of 0.2 g. The samples were separated and detected with 214 nm wavelength under the main engine rotational speed of 850 rpm and 2.5 mLm–1 flow rate of mobile phase.
Gel Filtration
We consulted reported packing methods and selected Sephadexgel G-100 (GE Healthcare, Piscataway, NJ, USA) to separate peptides with different molecular weights.[Citation22,Citation23] All packing material was compacted into a glass column (Shanghai Jingke Industrial Co. Ltd., Shanghai, China) with an inner diameter of 2.6 cm and length of 70 cm. Peptides in the mobile phase were tested using an ultraviolet (UV)-detector (Shanghai Jingke industrial Co. Ltd., China) under a wavelength of 214 nm. After the first gel filtration, Sephadex gel G-50 (GE Healthcare, Piscataway, NJ, USA) was chosen for further filtration by packing it into a glass column with an inner diameter of 1.6 cm and length of 80 cm. Through these two steps of filtration, the peptide was purified.
Antioxidant Activity Tests
Antioxidant activity of peptides from sea cucumber eggs were investigated with the assay kit (Nanjing Jiancheng Bioengineering Institute, Nanjing, Jiangsu, China) of scavenging hydroxyl and superoxide anion free radicals. The Fenton reaction can produce hydroxyl radicals (OH) whose amount is in proportion to the concentration of H2O2 in the system. It receives electron acceptors and reacts with the Griess reagent to produce a red compound whose absorbance appears in direct proportion with the OH amount. Following the sequence provided by the assay kit, absorbance values were detected under the wavelength of 550 nm. The scavenging ability of hydroxyl radicals was calculated by Eq. (1).
The superoxide anion radical assay kit imitates the xanthine–xanthine oxidase reaction system to produce superoxide anion radical (O2), then the electron transfer sample and Gress’s chromogenic reagent are added in sequence to make the reaction system present a prunosus color. The scavenging ability of the superoxide anion radical can be measured with optical density values at a wavelength of 550 nm and then calculated by using vitamin C as the standard through Eq. (2).
Purity and Molecular Weight Tests
Tricine-sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE)
Separating and stacking gels were prepared with compositions shown in . The peptide standard marker (Shanghai Sangon Biological Engineering Co., Ltd., Shanghai, China) with small molecular weights was used during the sample test. Electrophoresis was conducted first in the stacking gel under a voltage of 50 V in 1 h, and then in the separating gel under a voltage of 100 V in 1.5 h. After being immobilized for 20 min in the mixed solution of methanol, acetic acid, and water with a ratio of 5:1:4, the separating gel was dyed under 45°C for 15–30 min in 10% acetic acid solution which contained Coomassie brilliant blue (CBB) with 0.5% and got rid of the color of the background in 10% acetic acid water solution.
Table 1. Compositions of separating and stacking gels.
Statistical Analysis
All analysis results were repeated three times. Analysis of variance and standard deviation of the means were obtained by using Statistical Product and Service Solutions software v.20.0 (IBM Inc., Chicago, IL, USA). When significant differences were found, comparisons among means were carried out by using Tukey’s honest significant difference test (p < 0.05). All charts in the article were created by EXCEL software (Microsoft, Redmond, WA, USA).
Results and Discussion
Ultra-Filtration
The first ultra-filtration was conducted by using a cross-flow membrane module with a cut-off molecular weight of 10 KDa, under 12 psi of pressure, and a temperature of 25°C. As can be seen in , by increasing the times of filtration, the concentration of the peptide in holding liquid was decreasing to a stable level about 2.5 mg/mL, and in filtered liquid it is about zero. This indicated that after three times of filtration, the peptide with cut-off molecular weight lower than 10 KDa was filtrated out almost completely. Using 1K membrane module, filtration could be completed by three times, while it was needed four times by using 650 Da membrane module. Three kinds of peptides with a cut-off molecular weight of 10-1KDa, 1 K-650 Da, and lower than 650 Da were given the name F1, F2, and F3, respectively. The contents of F1, F2, and F3 in the fresh sea cucumber eggs were 0.74, 0.30, and 0.52%, respectively, and totally occupied 1.56%. The amount of F1 was nearly half of the total peptide.
Antioxidant Activity Test of Ultra-Filtered Peptide
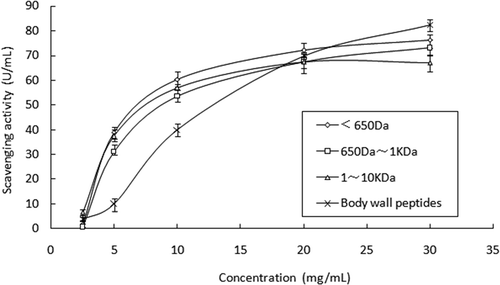
showed that three kinds of peptides from the eggs had an increasing ability to scavenge radicals with an increasing concentration of samples. Among the three egg peptides, there was also no significant difference in antioxidant activities. When the concentration of peptides was less than 10 mg/mL, scavenging ability improved sharply by increasing concentration. But when the concentration was greater than 10 mg/mL, the ability rose smoothly. Although they also had a similar trend as peptides from the eggs, peptides from the body wall exhibited a lower activity than the egg peptides with a low concentration. At a concentration of 5 mg/mL, three kinds of peptides from the eggs had the scavenging ability of F1: 37.41 U/mL, F2: 31.17 U/mL, and F3: 38.96 U/mL which were 3-fold more than that from the body wall. But there was no significant difference between the peptides from the two sources with a high concentration of about 20–30 mg/mL.
Among all active oxygen radicals, OH is one of the most aggressive radicals, and it is more harmful to human health than others.[Citation24] Peptides from the sea cucumber eggs presented good scavenging ability on OH radicals, and they would be expected to be new antioxidants.
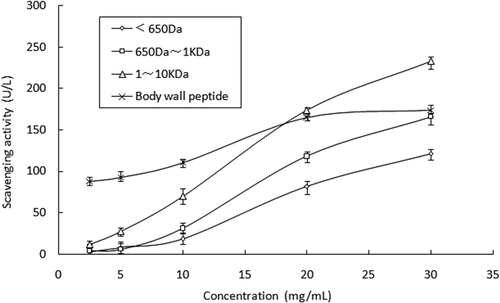
Radical O2−• was the one very important oxidant that can react with other molecules to produce new oxidant radicals.[Citation25] showed the results of abilities of peptides to scavenge O2−• radicals. Peptides presented an increasing scavenging ability with added concentration. Though they had the same trend in ability changing, three kinds of egg peptides had a distinct difference between each other. Peptide F1 with cut-off molecular weight 1-10 KDa was superior to the other two peptides in scavenging ability. With the low concentration of 5 mg/mL, peptides F1, F2, and F3 showed ability with F1: 27.79 U/L, F: 25.64 U/L, and F3: 8.04 U/L, and with a high concentration of 30 mg/mL it was F1: 233.56 U/L, F2: 165.95 U/L, and F3: 121.27 U/L, respectively. Compared with peptides from the body wall, the egg peptides had a lower ability with low concentration while they all had a similar ability with high concentration and even F1 had a higher ability with a concentration of 30 mg/mL. Then, F1 was chosen to make further purification according to its higher yield and scavenging ability on both radicals.
HSCCC
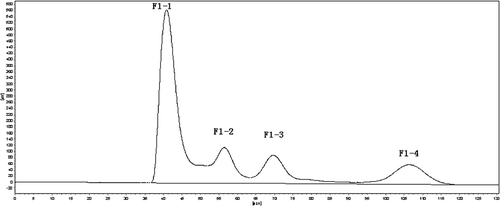
HSCCC is one of the liquid–liquid partition chromatographic techniques developed during the 1980s. It uses centrifugal force to retain one of the liquid phases in an multi-layered coil column, while the second liquid phase is pumped through the column.[Citation26,Citation27] Currently, HSCCC has been applied in many areas, such as the separation of biologically active substances, natural and synthetic peptides, and various plant hormones.[Citation28,Citation29] Some peptides, like the circle peptide, the antioxidant peptide, and some hydrophobic peptides, were successfully separated by using HSCCC.[Citation30–Citation32] As can be seen in , through HSCCC separation, peptide F1 was divided into four peaks F1-1, F1-2, F1-3, and F1-4 with a percentage content of 58.5, 14.1, 14.5, and 12.6%. According to the separating principle of HSCCC, the hydrophobic properties of four peptide peaks from strong to weak is F1-4 > F1-3 > F1-2 > F1-1.
Antioxidant Activity Test of Peptide from HSCCC
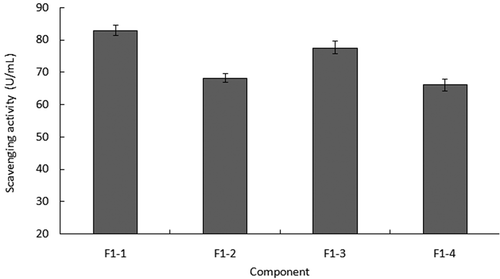
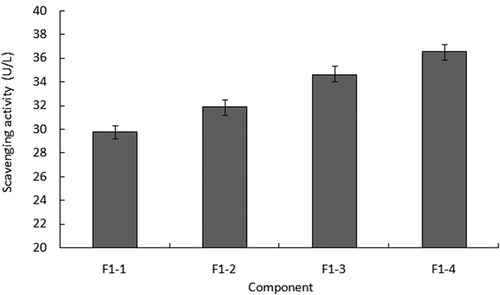
and showed the results of the radical scavenging ability of peptides separated by HSCCC. showed that hydroxyl radicals’ scavenging abilities of the four peptides separated from peptide F1 were all higher than that exhibited by three ultra-filtered peptides. The peptide F1-1 had the best ability among the four peptides by reaching 82.95 U/mL, which was two-fold more than peptide F1. This ability enhancement agreed with the result reported by Wang.[Citation33] When it comes to the ability to scavenge superoxide radicals, showed the increasing evidence with the increasing hydrophobic properties. Contrary to their hydrophobic properties situation, peptide F1-1 displayed the weakest ability in superoxide radical scavenging.
Some studies believed that hydrophobic peptides contributed more to the antioxidant activities of the peptide mixture than the hydrophilic peptides.[Citation32,Citation34] The result of superoxide radical scavenging seemed to be identified with the above viewpoint, but the result of hydroxyl radical scavenging was not in line with it. Peptide F1-1 provided the strongest ability in scavenging hydroxyl radicals which had weaker hydrophobic properties than the other three peptides. The hydroxyl radical was the most active in all oxygen radicals and it can be easily reacted with molecules like amino acid, protein, DNA, and so on. Scavenging hydroxyl radicals can strengthen the body’s defense ability of various diseases.[Citation35] Although it exhibited a weaker ability as a superoxide radical scavenger, peptide F1-1 had stronger ability in scavenging hydroxyl radical than the other three peptides. Meanwhile, in consideration of high yield, peptide F1-1 was selected as the material with which to do further purification.
Results of Gel Filtration
Gel filtration with another name, size-exclusion chromatography (SEC), is a chromatographic method in which molecules are separated by their size, and in some cases molecular weight.[Citation36] Sephadex gels, which are widely used in industrial processes, are prepared by crosslinking dextran with epichlorohydrin.[Citation37,Citation38]
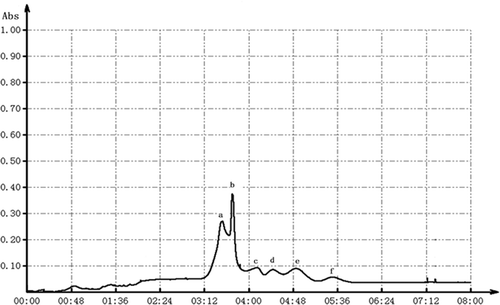
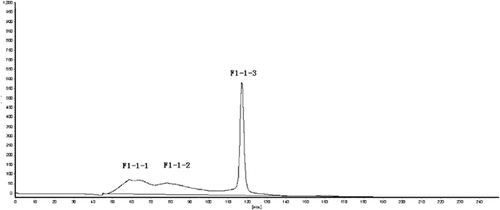
As can be seen in , peptide F1-1 was eluted with six peaks after being separated by Sephadex G-100 gel. Among all six peaks, peak a and b occupied the main parts of the total amounts of peptides. Because the two peaks were not completely separated, Sephadex G-50 gel was used for next filtration of the peak a and b mixture. showed that the peptide mixture was separated into three parts, such as peptide F1-1-1, F1-1-2, and F1-1-3. Peptide F1-1-3 presents a narrow peak with content of 40.52%. And the contents of the other two peptides were 25.28% with F1-1-1 and 27.05%with F1-1-2.
Antioxidant Activity Test of Peptide after Gel Filtration
After gel filtration, three peptides were acquired and had their antioxidant activities tested. Peptide F1-1 was used for gel filtration and had a strong ability to scavenge a hydroxyl radical, but a weak ability to scavenge a superoxide radical. Therefore, the aim of this purification step was to enhance the ability in scavenging hydroxyl radical and obtain the high quality peptide with high purity. The antioxidant activity test of peptide in this step focused on the ability to scavenge the hydroxyl radical.
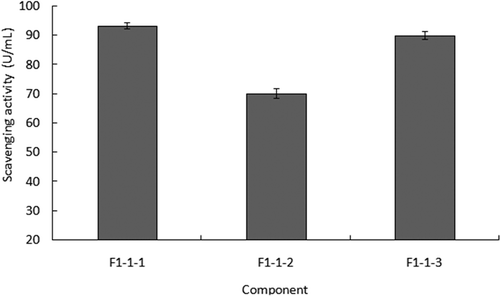
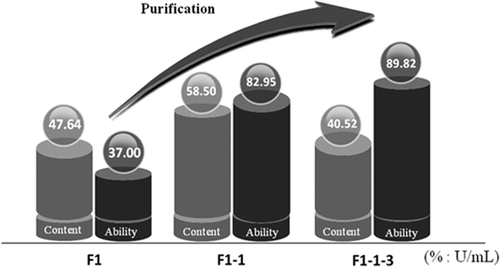
presents the result of the scavenging hydroxyl radical ability test in which peptide F1-1-1, F1-1-2, and F1-1-3 showed an ability of 93.26, 70.04, and 89.82 U/mL, respectively. Peptide F1-1-3 had almost equal ability to F1-1-1, and both of them were higher than that of F1-1-2. demonstrates that through the three steps of purification, such as ultra-filtration, HSCCC, and gel filtration, the antioxidant ability of the purified peptide was actually increasing from 37 to 89.82 U/mL. The contents of peptide in each step of purification occupied the main parts of total amounts.
Figure 3d. Contents and scavenging hydroxyl radical abilities of three peptides in each step of purification.
Activity oriented research was conducted by many investigators. To obtain a peptide with high potential to scavenge hydroxyl radical, Ren et al. completed their works with four steps of purification, including ultra-filtration, ion-exchange chromatography, HSCCC, and gel filtration. Wang et al. prepared antioxidant peptides from Sphyrna lewini muscle by testing the ability to scavenge the DPPH radical.[Citation39] In this article, antioxidant abilities and yields were both taken into consideration. During purification, the abilities were enhanced step-by-step, and at the same time the aim for peptide was always the main part in each step.
Purity Test
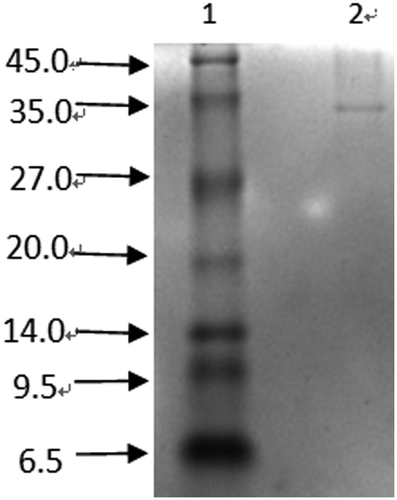
Because of low resolution on separating peptides with low molecular weights, normal SDS-PAGE is not suitable for a purity and molecular weight test.[Citation40] Tricine-SDS-PAGE, which has a better efficiency than SDS-PAGE in peptide separation, has been used widely. The result of Tricine-SDS-PAGE in revealed only a single band, which meant peptide F1-1-3 was pure with electrophoresis. Compared with peptide mark, peptide F1-1-3 was estimated to have a molecular weight of 30 KDa.
Conclusion
The sea cucumber, Apostichopus japonicas, has been a tonic food in China for centuries, and it is also a popular luxury food item with superior prices.[Citation41,Citation42] As a by-product, the egg in Apostichopus japonicus also has a variety of nutrients that are equal to, or even superior to, the body wall. Similar to the polyphenolics extracted from many plants, peptides hydrolyzed from animal proteins usually exhibited antioxidant activities.[Citation43–Citation45] In this study, the antioxidant peptide was prepared step-by-step using different purification methods, and its antioxidant ability was tested during each step at the same time. It could be seen that antioxidant ability was enhanced by a large margin from 37 to 89.82 U/mL. With the result of Tricine-SDS-PAGE, purified peptide F1-1-3 was tested to be pure with a molecular weight of about 30 KDa. The results demonstrated that this peptide from the egg in Apostichopus japonicus had good antioxidant ability, and after purification it could be utilized more efficiently for healthcare and pharmaceutical applications.
Funding
The authors would like to thank the Public Science and Technology Research Funds Projects of Ocean of China (201105029; 201205027), the Earmarked Fund for Modern Agro-Industry Technology Research System in Shandong Province (SDAIT-08), the Technology Development Project in Yantai City (2014ZH081), and the Natural Science Foundation of Shandong Province (ZR2014CP030) for financial support.
Additional information
Funding
References
- Aminin, D.L.; Pinegin, B.V.; Pichugina, L.V.; Zaporozhets, T.S.; Agafonova, I.G.; Boguslavski, V.M.; … Stonik, V.A. Immunomodulatory Properties of Cumaside. International Immunopharmacology 2006, 6, 1070–1082.
- Forghani, B.; Ebrahimpour, A.; Bakar, J.; Abdul Hamid, A.; Hassan, Z.; Saari, N. Enzyme Hydrolysates from Stichopushorrens as a New Source for Angiotensin-Converting Enzyme Inhibitory Peptides. Evidence-Based Complementary and Alternative Medicine 2012, 2012, 1155–1163.
- Liu, H.H.; Ko, W.C.; Hu, M.L. Hypolipidemic Effect of Glycosaminoglycans from the Sea Cucumber Metriatylascabra in Rats Fed a Cholesterol-Supplemented Diet. Journal of Agricultural and Food Chemistry 2002, 50, 3602–3606.
- Kumar, R.; Chaturvedi, A.K.; Shukla, P.K.; Lakshmi, V. Antifungal Activity in Triterpene Glycosides from the Sea Cucumber Actinopygalecanora. Bioorganic & Medicinal Chemistry Letters 2007, 17, 4387–4391.
- Zhang, S.Y.; Tang, H.F.; Yi, Y.H. Cytotoxic Triterpene Glycosides from the Sea Cucumber Pseudocolochirus Violaceus. Fitoterapia 2007, 78, 283–287.
- Mamelona, J.; Pelletier, É.; Girard-Lalancette, K.; Legault, J.; Karboune, S.; Kermasha, S. Quantification of Phenolic Contents And Antioxidant Capacity Of Atlantic Sea Cucumber, Cucumariafrondosa. Food Chemistry 2007, 104, 1040–1047.
- Anderson, E.N. The Food of China; Yale University Press: New Haven, CT, 1988.
- Zhang, E. Chinese Medicated Diet; Publishing House of Shanghai College of Traditional Chinese Medicine (Shanghai, China), 1990.
- Wen, J.; Hu, C.; Fan, S. Chemical Composition and Nutritional Quality of Sea Cucumbers. Journal of the Science of Food and Agriculture 2010, 90, 2469–2474.
- Chen, S.; Xue, C.; Yin, L.A.; Tang, Q.; Yu, G.; Chai, W. Comparison of Structures and Anticoagulant Activities of Fucosylated Chondroitin Sulfates from Different Sea Cucumbers. Carbohydrate Polymers 2011, 83, 688–696.
- Abedin, M.Z.; Karim, A.A.; Ahmed, F.; Latiff, A.A.; Gan, C.Y.; CheGhazali, F.; … Zaidul, M. Isolation and Characterization of Pepsin-Solubilized Collagen from the Integument of Sea Cucumber (Stichopus Vastus). Journal of the Science of Food and Agriculture 2013, 93, 1083–1088.
- Sun, W.H.; Leng, K.L.; Lin, H.; Xing, L.H.; Ning, J.S.; Wang, Z.J.; … Liu, Q. Analysis and Evaluation of Chief Nutrient Composition in Different Parts of Stichopusjaponicus. Chinese Journal of Animal Nutrition 2010, 22, 212–220.
- Zhou, X.; Wang, C.; Jiang, A. Antioxidant Peptides Isolated from Sea Cucumber StichopusJaponicus. European Food Research and Technology 2012, 234, 441–447.
- Collin, P.D. U.S. Patent No. 6,767,890. Washington, DC: U.S. Patent and Trademark Office, 2004.
- Takashi, H.; Nobuhiro, Z.; Kyoko, Y.; Ryoko, N.; Xue, C.; Tatsuya, S. Recent Advances in Researches on Physiologically Active Substances in Holothurians. Journal of Ocean University of China 2005, 4, 193–197.
- Kumar, P.N.; Devaky, K.S.; Sadasivan, C.; Haridas, M. Conformational Analysis of Sea Cucumber (Caudinaarenicola) C Globin 94-97 Fragment, Pro-Glu-Leu-Leu. Protein and Peptide Letters 2002, 9, 403–409.
- Gill, I.; López-Fandiño, R.; Jorba, X.; Vulfson, E.N. Biologically Active Peptides and Enzymatic Approaches to Their Production. Enzyme and Microbial Technology 1996, 18, 162–183.
- Seebach, D.; Albert, M.; Arvidsson, P.I.; Rueping, M.; Schreiber, J.V. From the Biopolymer PHB to Biological Investigations of Unnatural β- and γ-Peptides. CHIMIA International Journal for Chemistry 2001, 55, 345–353.
- Cao, R.; Liu, Q.; Yin, B.Z. Optimization of Enzymatic Hydrolysis Process for Sea Cucumber Gonads by Response Surface Methodology. Food Science (in Chinese) 2012, 33, 29–33.
- Wang G.M.; Zhang, J.; Wang, M.J.; Zhao, Y.P.; Gao, J.Q.; Li, Q. Optimization of the Enzymatic Hydrolysis Condition of Apostichopus Japonicus Spawn. Food Science (in Chinese) 2012, 33, 193–198.
- Ma, Y.; Ito, Y. Recent Advances in Peptide Separation by Countercurrent Chromatography. Analytica Chimica Acta 1997, 352, 411–427.
- Byun, H.G.; Kim, S.K. Purification and Characterization of Angiotensin I Converting Enzyme (ACE) Inhibitory Peptides from Alaska Pollack (Theragra Chalcogramma) Skin. Process Biochemistry 2001, 36, 1155–1162.
- Bougatef, A.; Nedjar-Arroume, N.; Manni, L.; Ravallec, R.; Barkia, A.; Guillochon, D.; Nasri, M. Purification and Identification of Novel Antioxidant Peptides from Enzymatic Hydrolysates of Sardinelle (Sardinella Aurita) by-Products Proteins. Food Chemistry 2010, 118, 559–565.
- Castro, L.; Freeman, B.A. Reactive Oxygen Species in Human Health and Disease. Nutrition 2001, 17, 161–165.
- Leeuwenburgh, C.; Heinecke, J.W. Oxidative Stress and Antioxidants in Exercise. Current Medicinal Chemistry 2001, 8, 829–838.
- Ito, Y. High-Speed Countercurrent Chromatography. Nature 1987, 17, 65–143.
- Weisz, A.; Ito, Y. In I.D. Wilson (Ed.), Encyclopedia of Separation Science, Vol. 6, Academic Press: London, U.K., 2000, p. 2588.
- Kitazume, E. High-Speed Countercurrent Chromatography. Chemical Analysis Series 2000, 132, 415.
- Knight, M. Separations of Hydrophobic Synthetic Peptides in Counter-Current Chromatography. Journal of Chromatography A 2007, 1151, 148–152.
- Oka, H.; Harada, K.I.; Suzuki, M.; Nakazawa, H.; Ito, Y. Foam Counter-Current Chromatography of Bacitracin: I. Batch Separation with Nitrogen and Water Free of Additives. Journal of Chromatography A 1989, 482, 197–205.
- Han, C.; Chen, J.; Liu, J.; Lee, F.S.C.; Wang, X. Isolation and Purification of Pseudostellarin B (Cyclic Peptide) from Pseudostellaria Heterophylla (Miq.) Pax by High-Speed Counter-Current Chromatography. Talanta 2007, 71, 801–805.
- Ren, J.; Zhao, M.; Shi, J.; Wang, J.; Jiang, Y.; Cui, C.; … Xue, S.J. Purification and Identification of Antioxidant Peptides from Grass Carp Muscle Hydrolysates by Consecutive Chromatography and Electrospray Ionization-Mass Spectrometry. Food Chemistry 2008, 108, 727–736.
- Wang, L.P. Production and Physiological Effects of Casein-Derived Peptides Formed with Lactobacillus Helveticus Cells; Beijing Forestry University (Beiing, China), 2008.
- Rajapakse, N.; Mendis, E.; Jung, W.K.; Je, J.Y.; Kim, S.K. Purification of a Radical Scavenging Peptide from Fermented Mussel Sauce and Its Antioxidant Properties. Food Research International 2005, 38, 175–182.
- Kullisaar, T.; Songisepp, E.; Mikelsaar, M.; Zilmer, K.; Vihalemm, T.; Zilmer, M. Antioxidative Probiotic Fermented Goats’ Milk Decreases Oxidative Stress-Mediated Atherogenicity in Human Subjects. British Journal of Nutrition 2003, 90, 449–456.
- Paul-Dauphin, S.; Karaca, F.; Morgan, T.J.; Millan-Agorio, M.; Herod, A.A.; Kandiyoti, R. Probing Size Exclusion Mechanisms of Complex Hydrocarbon Mixtures: The Effect of Altering Eluent Compositions. Energy & Fuels 2007, 21, 3484–3489.
- Porath, J.; Flodin, P. Gel Filtration: A Method for Desalting and Group Separation. Nature 1959, 183, 1657–1659.
- Agrawal, B.B.; Goldstein, I.J. Specific Binding of Concanavalin A to Cross-Linked Dextran Gels. Biochemical Journal 1965, 96, 23contd–23con5c.
- Wang, B.; Li, Z.R.; Chi, C.F.; Zhang, Q.H.; Luo, H.Y. Preparation and Evaluation of Antioxidant Peptides from Ethanol-Soluble Proteins Hydrolysate of Sphyrna Lewini Muscle. Peptides 2012, 36, 240–250.
- Cleveland, D.W.; Fischer, S.G.; Kirschner, M.W.; Laemmli, U.K. Peptide Mapping by Limited Proteolysis in Sodium Dodecyl Sulfate and Analysis by Gel Electrophoresis. Journal of Biological Chemistry 1977, 252, 1102–1106.
- Raison, C.M. Advances in Sea Cucumber Aquaculture and Prospects for Commercial Culture of Holothuriascabra. CAB Reviews 2008, 3, 1–15.
- Purcell, S.W.; Hair, C.A.; Mills, D.J. Sea Cucumber Culture, Farming, and Sea Ranching in the Tropics: Progress, Problems, and Opportunities. Aquaculture 2012, 368, 68–81.
- Uddin Md., N.; Ahmed, N.U.; Rahman Md., A.; Akter, R.; Akter, R. Antioxidative Potential of the Polyphenolics of Stephania Japonica var. Discolor (Blume) Forman: A Chromatographic (High-Performance Liquid Chromatography) and Spectrophotometric Measure. International Journal of Food Properties 2016, 19, 911–928.
- Ramos-Escuderoa, F.; Moralesa, M.T.; Asueroa, A.G. Characterization of Bioactive Compounds from Monovarietal Virgin Olive Oils: Relationship Between Phenolic Compounds-Antioxidant Capacities. International Journal of Food Properties 2015, 18, 348–358.
- Zhang, L.; Liu, Y.Z.; Lu, D.; Han, J.; Lu, X.M.; Tian, Z.H.; & Wang, Z. Angiotensin Converting Enzyme Inhibitory, Antioxidant Activities, and Antihyperlipidaemic Activities of Protein Hydrolysates from Scallop Mantle (Chlamys Farreri). International Journal of Food Properties 2015, 18, 33–42.