ABSTRACT
This study aimed to evaluate the effects of rosemary extract, grown in Iran, on thermoxidative stability of soybean oil. Rosemary extract was added to soybean oil at a concentration of 3000 mg/kg and then heated at 180°C for 20 h. The oxidative stability index, total polar compounds, tocopherol content, and fatty acid profile were measured at intervals of 0, 10, and 20 h. The results compared with synthetic antioxidant tert-butyl hydroquinone at a concentration of 50 mg/kg. Rosemary extract could lead to higher oxidative stability index, lower polar compounds, more retention of tocopherols and the greatest amount of polyunsaturated fatty acids in soybean oil after 20 h of thermoxidation process. The tert-butyl hydroquinone showed weaker antioxidant activity than rosemary extract and there was no synergistic effect between them.
Introduction
Vegetable oils are very susceptible to oxidation due to having a high percentage of unsaturated fatty acids.[Citation1] Therefore, it is necessary to slow this kind of spoilage to increase shelf life of vegetable oils. The best way to do this is to use antioxidants. Antioxidants prevent the progress of oxidation reaction in various ways such as; neutralize free radicals, stabilize singlet oxygen, and chelate pro-oxidant metals.[Citation2,Citation3] Today, synthetic antioxidants are used widely in the food industry.[Citation4] Consumption of these food additives creating concerns from consumers, because studies have shown that long-term use of these chemicals can have carcinogenic potential.[Citation5] These concerns prompted researchers consider the idea of using natural compounds including herbal extracts rather than synthetic compounds to improve the oxidative stability of food. Therefore, extensive research is being carried out with the aim of achieving a natural antioxidant compounds from plant sources that have the ability to replace the synthetic antioxidants in food.[Citation6]
Traditionally, the use of herbs and spices like rosemary (Rosmarinus officinalis L.) in food preparation to enhance their palatability has been common. But, rosemary also has antioxidant properties due to having compounds such as phenolic acids, phenolic triterpenes, diterpenes, and flavonoids.[Citation7] From a variety of antioxidant compounds found in rosemary extract, carnosic acid is the most active compound.[Citation8,Citation9] The structure of this compound may change during the extraction process and turn to carnosol or other phenolic diterpene compounds such as rosmanol and isorosmanol.[Citation10–Citation12] Although derived compounds from carnosic acid have antioxidant properties, their activity is weaker due to lower polarity.[Citation10]
During the process of frying, the activity of antioxidant substances depends on their composition and type of compounds. In this case, the polar antioxidants are more efficient than non-polar types. A proposed explanation is that the polar antioxidants tend to be located in the interface of air and oil, and thus more effectively enhance oxidative stability of the oil, while the non-polar types remain in the oil phase.[Citation13] Some studies[Citation14–Citation16] show that rosemary extract can lead to improved oxidative stability of oil during frying. Even fresh rosemary leaves can be used as an antioxidant in some foods such as; salmon, shrimp, meat, bread, and in some vegetable oils.[Citation17,Citation18] Although the constituents of plant extracts is under the control of genetic processes, but their synthesis is significantly affected by environmental conditions. Environmental factors are causing changes in plant growth, quality, and quantity of its constituents. The aim of this study was to evaluate the effects of extract obtained from rosemary grown under climatic conditions of northern Iran on oxidative stability of soybean oil.
Materials and methods
Materials
In the present study, refined soybean oil without synthetic antioxidant and citric acid was obtained from the Shomal Agro-Industrial Complex Co. located in Behshahr City, Mazandaran Province, Iran. Leaves of rosemary were collected in September 2014 from the Kotra area located around Tonekabon City, Mazandaran Province, Iran. All chemicals and solvents were prepared from Merck (Darmstad, Germany) and Sigma Aldrich (St. Louis, MO, USA) companies.
Methods
Preparation of the rosemary extract
First, fresh rosemary leaves were dried at room temperature away from sunlight and then dried leaves were powdered. Then to obtain the extract, the resulting powder was mixed with ethanol as a solvent at a ratio of 1/10 (powder/solvent) and, subsequently, the mixture was used in the soxhlet apparatus for 2 h. The obtained extract was filtered and then rotary evaporator was used for its solvent recovery (Heidolph, Germany). After this step, the extract was stored at –18°C until the time of testing.[Citation19]
Total phenolic content
The total phenolic content of the rosemary extract was measured spectrophotometrically using Folin–Ciocalteau reagent according to the procedure reported by Shahidi and Naczk.[Citation20] The 0.5 mL of sample was mixed with 2.5 mL of Folin–Ciocalteau reagent 10% (v/v) and then over a period of 0.5 to 8 min, 2 mL of sodium carbonate 7.5% (w/v) was added. The samples were stored at room temperature for 30 min and then their absorption was read at 765 nm by spectrophotometer (Milton Roy 20D) ultraviolet visible (UV-Vis). The total phenolic content of each repetition was reported based on mg gallic acid per gram of extract.
Radical scavenging activity
The stable free radical 2,2’-diphenyl-1-picrylhydrazyl (DPPH•) was used to determine the radical scavenging activity of rosemary extract according to the procedure reported by Erkan et al.[Citation19] For this purpose, different concentrations of the extract in ethanol (0.02, 0.05, and 0.08%) were prepared. Then, 1.5 mL of each of the prepared concentrations were mixed with 1.5 mL of DPPH• solution in methanol (0.2 mmol/L). These solutions were stored in an incubator at 25°C for 30 min away from the light and then their absorbance were read at 517 nm by spectrophotometer (Milton Roy 20D) UV-Vis against a blank. The blank composed of 1.5 mL of methanol without the extract mixed with 1.5 mL of DPPH• solution in methanol (0.2 mmol/L). The radical scavenging activity of each solution was calculated as the inhibition percentage according to the following equation:
where Ablank: the blank absorbance at 517 nm, Asample: the sample absorbance at 517 nm.
In this study, the percentage inhibition of DPPH• by rosemary extract was expressed as IC50. The IC50 value defined as the concentration of antioxidant that could reduce the initial concentration of free radicals to 50%.
Thermoxidation process
The treatments evaluated in this study included: soybean oil without any antioxidant (S); soybean oil with synthetic antioxidant at a concentration of 50 mg/kg (synthetic tert-butyl hydroquinone S-TBHQ); soybean oil with rosemary extract at a concentration of 3000 mg /kg (SR); and soybean oil with a mixture of 3000 mg/kg of rosemary extract and 50 mg/kg of TBHQ (SR-TBHQ). In this research, TBHQ was used in accordance with the prevailing standard concentration usual for soybean oil. The 30 mL of each treatment were poured separately in 50 mL beakers and then the hot plate was used to apply a temperature of 180°C (normal temperature for deep frying). The treatments were heated for 20 h. The heating method was carried out as a batch (10 h per day). Oxidation characteristics of the samples were measured at intervals of 0, 10, and 20 h.
Soybean oil analyses
In this study, total polar compounds, oxidative stability index, tocopherol concentrations, and fatty acid profile were evaluated to determine the antioxidant properties of rosemary extract during the heating process of soybean oil at 180°C for 20 h.
Total polar compounds
The total polar compounds were evaluated according to the method reported by Dobarganes et al.[Citation21] This is a chromatographic method and results were expressed as a percentage. This technique is based on the use of adsorption chromatography to separate the sample into two parts with different polarity. Therefore, these two parts can be measured gravimetrically.
Oxidative stability index
The oxidative stability index was determined according to the Rancimat method reported by Farhoosh.[Citation22] For this purpose, 3 g of each sample was separately analyzed using Rancimat equipment (Metrohm model 734, Herisan, Switzerland) at 120°C and with an air flow rate of 15 L/h.
Tocopherol concentrations
In this study, the high-performance liquid chromatography (Varian Associates, Inc., Walnut Creek, CA) was used to evaluate the concentration of tocopherols according to the AOCS Official Method Ce 8-89.[Citation23] Conditions that were observed in this test: fluorescence detector (TSP brand and FL 2000 model, Varian Associates, Inc.) wit excitation 290 nm and emission 330 nm; analytical column with 4.6 mm × 25 cm and 5-µm particle size; silica (Supelcosil LC-Si, Supelco) for normal phase and C18 (Supelco Discovery C18) for reversed phase; flow rate of 1 mL/min. A mixture of 0.5% isopropanol and 99.5% n-hexane was used as the mobile phase. Four tocopherol isomers were considered as external standards for calculations. Results were expressed as mg/kg.
Fatty acid composition
Fatty acid composition of the samples was evaluated by gas-liquid chromatography. Fatty acid methyl esters were prepared by mixing oil and hexane (0.3 g in 7 mL) with 2 mL of 7 N methanolic potassium hydroxide at 50°C for 15 min. Esters of fatty acids were identified by HP-5890 chromatograph (Hewlett-Packard, CA, USA) equipped with a CP-FIL 88 (Supel Co., Inc., Bellefonte, PA, USA) capillary column of fused silica, 60 m × 0.22 mm I.D., 0.2 µm film thickness, and a flame ionization detector. Helium was used as carrier gas with a flow rate of 0.7 mL/min. The temperature of the oven, the injector and the detector was 198, 280, and 250°C, respectively. Results were expressed based on the relative percentage of areas.[Citation24]
Statistical analysis
All experiments were carried out in a completely randomized design with three replications, four treatments, and three heating times. The data were analyzed by analysis of variance (ANOVA) and the Duncan’s test for the 5% significance level, each of which were done with the SPSS software, Version 21 (IBM, NY, USA).
Results and discussion
Total phenolic content
In accordance with the method used in this study, the total phenolic content of rosemary extract was obtained 81.37 mg of gallic acid per g of extract. Celiktas et al.[Citation25] studied the rosemary extracts obtained from supercritical extraction method at different seasons and regions of Turkey and found very different results (4.1 to 119 mg of gallic acid per g of extract). Also, Mata et al.[Citation26] found 58.4 and 73.5 mg of gallic acid per g of extract for total phenolic compounds of water and ethanolic rosemary extracts, respectively.
Radical scavenging activity
The IC50 value is considered as an indicator to assess the radical scavenging properties of antioxidants. Whatever antioxidants have a higher capacity to inhibit free radicals, the IC50 values associated with them will be smaller. In this study, the highest DPPH• inhibitory activity of rosemary extract was 75.63% and using linear regression with high coefficient of determination (R2 = 0.999) showed that the IC50 of this extract is 42.75 µg/mL. Such strong scavenging free radical activity of rosemary extract is most likely related to the presence of compounds such as carnosic acid and rosmarinic.[Citation26]
Total polar compounds
Hydrolytic, oxidative, or thermal deterioration of oils and fats creates non-volatile compounds which have a higher polarity compared with triacylglycerols. Therefore, the quality of edible oils will decrease by increasing their polar compounds.[Citation27] The maximum allowable initial concentration of total polar compounds in refined edible oils should not be higher than 6.4%.[Citation28] In this study, as can be seen from , the total polar compounds in all treatments of soybean oil in the beginning of the heating process was less than the maximum and also, there was no significant difference between them.
Table 1. Total polar compounds (%) and oxidative stability index (h) of soybean oil treatments during the heating process.
The index increased by increasing the heating time so that all treatments after 10 and 20 h of heating showed significant differences. Although the total polar compounds in antioxidant treatments (S-TBHQ, SR, SR-TBHQ) was less than the control sample (S) throughout heating, but the effect of antioxidants was different compared to each other. At the end of the heating process, S-TBHQ, SR, and SR-TBHQ treatments than S treatment decreased the mean value of total polar compounds by 14.39, 50.64, and 47.82%, respectively. According to the results, it can be said that the use of rosemary extract alone was more effective than when used simultaneously with TBHQ. As shown in , after 10 h of thermal process, the results of S and S-TBHQ treatments were not significantly different from each other. Thus, in the case of SR-TBHQ treatment, rosemary extract had a main role in the emergence of antioxidant properties. At the end of the heating process, this treatment had no significant difference in terms of total polar compounds than SR treatment while in comparison with the S-TBHQ treatment had lower values. Meanwhile, rosemary extract and TBHQ did not have synergistic effect on each other. Reblova et al.[Citation29] studied the relationship between the amount of total polar compounds in canola oil and heating time in the production of French fries and found that the formation of polar compounds will increase significantly with increasing heating time. These researchers applied the rosemary extract as an antioxidant in their study and found that this extract can effectively reduce the formation of polar compounds. Also, Corsini et al.[Citation30] studied the frying process of cassava in sunflower oil and cotton seed oil and found that the polar compounds increased after 25 h of heating. In fact, the intensification of the oxidation reaction caused by rising temperature will lead to an increase in polar compounds and this will reduce the performance of the oil in the frying process. The maximum permissible level for the formation of polar compounds in frying oils in many countries has been set 25%.[Citation31] In this research, only the treatments containing rosemary extract (SR and SR-TBHQ), total polar compounds was less than 25%.
Oxidative stability index
The degree of unsaturation and antioxidants are the two main factors determining the oxidative stability of edible oils and both are affected by heat. Through the analysis of oxidative stability index can be evaluated the stability of edible oils against the oxidation reaction and also assessed the secondary volatile carbonyl compounds resulting from their oxidation.[Citation32] In the present study, with or without the use of antioxidants, soybean oil oxidative stability index decreased significantly with increasing heating time (). Therefore, studied antioxidants were not able to maintain oil stability by increasing the heating time. However, antioxidants effectively improved the oxidative stability index of the treatments after 20 h of heating and rosemary extract had a stronger effect than TBHQ. Oxidative stability index of the TBHQ treatments was significantly less than SR treatments during the thermoxidation process (). Meanwhile, between SR and SR-TBHQ treatments, there was no significant difference during the heating time. So, TBHQ and rosemary extract had no synergistic effect on each other. Merril et al.[Citation33] studied the antioxidant properties of TBHQ, rosemary extract, ascorbyl palmitate, and tocopherols about various oils at 110°C. These researchers reported that in assessing the oxidative stability index of oils, TBHQ, and ascorbyl palmitate showed a synergistic effect, while, antioxidant activity of TBHQ was reduced in the presence of rosemary extract. Ramalho and Jorge[Citation34] reported that the oxidative stability of soybean oil at 100°C increases with the addition of rosemary extract (1000 mg/kg). These researchers had obtained similar results to those found in this study.
Tocopherol concentration
Edible oils have naturally different amounts of various tocopherols. These compounds can affect oil oxidation properties, even as minor components. The loss of tocopherols during different stages of the production process of edible oils can be more than 56% of their initial amount.[Citation35] So, pay attention to the loss of tocopherols in edible oils during the heating process is very important. The standard value for each of the types of tocopherols in soybean oil is: α-tocopherol at 9–352 mg/kg; β-tocopherol at 0–36 mg/kg; γ-tocopherol at 89–2307 mg/kg; δ-tocopherol at 154–932 mg/kg; and total tocopherols at 600–3370 mg/kg.[Citation36] In the present study, with exceptions δ-tocopherol and total tocopherols, the results obtained for the other tocopherols in primary soybean oil were in accordance with the standards of Codex Alimentarius ().
Table 2. Tocopherols (mg/kg) of soybean oil treatments during the heating process.
Different stages of the production process of soybean oil, especially deodorizing stage, may lead to different amounts of total tocopherols. As is seen from , the concentration of tocopherols decreased significantly in all treatments with increasing heating time. Barrera-Arellano et al.[Citation37] found that the total tocopherols in polyunsaturated oils will drop significantly after 10 h of heating and this value about soybean oil was 40%. Moreover, this researchers reported that α-tocopherol had the highest reduction. The loss of tocopherols in all treatments containing rosemary extract decreased significantly during the heating process ().
Figure 1. Evaluation of the effects of thermoxidation process on retention of tocopherols (%) in soybean oil during 20 h of heating at 180°C. S: soybean oil; S-TBHQ: soybean oil + TBHQ (50 mg/kg); SR: soybean oil + rosemary extract (3000 mg/kg); SR-TBHQ: soybean oil + rosemary extract (3000 mg/kg) + TBHQ (50 mg/kg).
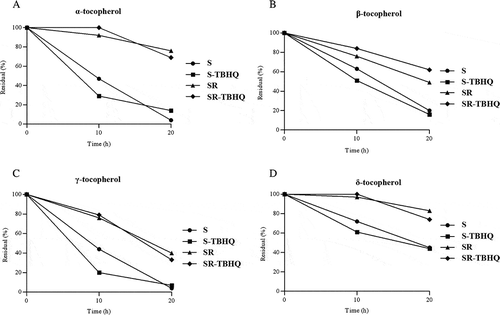
In this study, the addition of rosemary extract caused α-tocopherol more preserved than other tocopherols after 20 h of heating (). This was in accordance with the results reported by Rizner-Hras et al.[Citation38] At the end of 20 h of heating, increasing the percentage of retained tocopherols by adding rosemary extract in SR treatment compared with S treatment was as follows: α-tocopherol from 8 to 68.5%, β-tocopherol from 27 to 41%, γ-tocopherol from 4.4 to 39%, and δ-tocopherol from 45 to 75.5%. Meanwhile, between SR and SR-TBHQ treatments, there was no significant difference in the percentage of retained tocopherols in the end of thermoxidation process and this showed that there was no synergistic effect between TBHQ and rosemary extract in soybean oil. Stability sequences suggested in various references for different fractions of tocopherol is: δ > γ > β > α.[Citation39] However, in the present study, another result (δ > β > α = γ) obtained. Also, Steel et al.[Citation40] observed a higher destruction of γ and α-tocopherol after 10 h of soybean oil heating. Verleyen[Citation41] found that α-tocopherol was quickly destroyed. Moreover, Warner and Gehring[Citation42] found a greater destruction of δ and α-tocopherol after 65 h of soybean oil heating. The stability of the different fractions of tocopherol increased significantly with the addition of rosemary extract to soybean oil. Meanwhile, rosemary extract changed the sequence of stability of the tocopherol fractions as: δ > α > β > γ. According to the reduction of tocopherols losses especially α-tocopherol, TBHQ was slightly weaker than the rosemary extract in the concentrations considered in this study.
Fatty acid profile
Thermal process of fats and oils can lead to changes in their fatty acid composition, therefore, the study of the fatty acid profile of fats and oils can reveal valuable details about the severity of changes in their main fatty acids resulting from this process. Juárez et al.[Citation43] reported that the changes of fatty acids in edible oils are specifically affected by the degree of their unsaturation and found that α-linolenic acid (C18:3) could be reduced from 20 to 25%. In the present study, linoleic acid (C18:2) was identified as the most fatty acid (more than 50%) found in all treatments at the beginning of the thermoxidation process (). This was similar to the results obtained by Kim et al.[Citation44]
Table 3. Fatty acid composition (%) of soybean oil treatments during the heating process.
The type of oil used in the preparation of potato chips could affect its oxidative stability.[Citation45] These researchers found that if the use of sunflower oil compared to partially hydrogenated sunflower oil in the frying process, oxidative stability of this product will decrease significantly. Thus, the fatty acid composition of the oil used in frying process will absolutely affect the oxidative stability of the final product. In the present study, an increase in the time of thermoxidation process led to the decrease of linoleic acid and α-linolenic acid in all treatments. After 20 h of heating, the sequence of reducing the amount of linoleic acid in treatments was as follows: S (9.21%) > SR-TBHQ (8.66%) > S-TBHQ (7.62%) > SR (5.04%). Also, this sequence about α-linolenic acid was as follows: S (27.3%) > S-TBHQ (26.47%) > SR-TBHQ (23.44%) > SR (16.97%). These results showed that the reduction in the amount of α-linolenic acid was more than linoleic acid during the thermoxidation process. Moreover, TBHQ and rosemary extract had no synergistic effect on each other. Velasco et al.[Citation46] reported that after 15 h of frying, reducing the amount of polyunsaturated fatty acids in sunflower and olive oils were 21.71 and 42.86%, respectively. The human body is not able to synthesize α-linolenic and linoleic acids, therefore, these fatty acids are considered essential fatty acids and the reduction in their quantity is as a loss of nutritional value of oil. The results showed that in most treatments, there was no significant change in the amount of stearic acid, arachidic acid, behenic acid, and eicosenoic acid during thermoxidation process. Meanwhile, the amount of Palmitic acid and oleic acid increased slightly (). Gunstone[Citation47] reported that in soybean oil, the amount of saturated, monounsaturated, and polyunsaturated fatty acids are 15, 22, and 61%, respectively. These values are close to the results obtained of the present study. With or without the presence of antioxidants, the amount of saturated and monounsaturated fatty acids increased significantly, while polyunsaturated fatty acids showed a significant decrease after 20 h of heating ().
Table 4. Saturated, monounsaturated, and polyunsaturated (%) of soybean oil treatments during the heating process.
However, the fatty acid composition of soybean oil had more changes in the samples without antioxidants. Oxidation and polymerization of polyunsaturated fatty acids are probably the main factors in their destruction and reduction during thermal processes.[Citation48] With a decrease in polyunsaturated fatty acids, there will be a proportional increase in the amount of saturated fatty acids during frying process.[Citation46] In the present study, the results of fatty acid profiles showed that the fatty acid composition of soybean oil is positively influenced by the rosemary extract. At the end of thermoxidation process, the SR treatment had a higher amount of polyunsaturated fatty acids compared to other treatments, while the amount of saturated and monounsaturated fatty acids was lower in this treatment.
Conclusion
In this study, the results showed that the oxidative stability index, the retention of α-tocopherol and the amount of polyunsaturated fatty acids in soybean oil can be improved with the addition of rosemary extract after 20 h of thermoxidation process at 180°C. The TBHQ and rosemary extract did not have a synergistic effect on each other. Our results also showed that the antioxidant activity of TBHQ is weaker than rosemary extract in the concentrations studied in this research. The rosemary extract can be used as a source of safe and effective natural antioxidant to preserve the edible oils.
Acknowledgments
The authors gratefully acknowledge the laboratory supports by Azad University of Tonekabon.
References
- Farhoosh, R.; Pazhouhanmehr, S. Relative Contribution of Compositional Parameters to the Primary and Secondary Oxidation of Canola Oil. Food Chemistry 2009, 114, 1002–1006.
- Akoh, C.C.; Min, D.B. Food Lipids: Chemistry, Nutrition, and Biotechnology; CRC Press: Boca Raton, FL, 2008; 928.
- Panglossi, H.V. Antioxidants: New Research; Nova Science Publishers: New York, NY, 2006; 211.
- Pokorný, J. Are Natural Antioxidants Better—and Safer—Than Synthetic Antioxidants? European Journal of Lipid Science and Technology 2007, 109, 629–642.
- Venskutonis, P.R. Natural Antioxidants in Food Systems, in Food Oxidants, and Antioxidants: Chemical, Biological, and Functional Properties, Bartosz, G.; Ed.; CRC Press: Boca Raton, FL, 2013; 235–302.
- Moure, A.; Cruz, J.M.; Franco, D.; Domínguez, J.M.; Sineiro, J.; Domínguez, H.; Núñez, M.J.; Parajó, J.C. Natural Antioxidants from Residual Sources. Food Chemistry 2001, 72, 145–171.
- Cordeiro, A.M.T.M.; Medeiros, M.L.; Santos, N.A.; Soledade, L.E,B.; Pontes, L.F.B.L.; Souza, A.L.; Queiroz, N.; Souza, A.G. Rosemary (Rosmarinus Officinalis L.) Extract: Thermal Study and Evaluation of the Antioxidant Effect on Vegetable Oils. Journal of Thermal Analysis and Calorimetry 2013, 113, 889–895.
- Terpinc, P.; Bezjak, M.; Abramovi, H.A. kinetic Model for Evaluation of the Antioxidant Activity of Several Rosemary Extracts. Food Chemistry 2009, 115, 740–744.
- Visentin, A.; Cismondi, M.; Maestri, D. Supercritical CO2 Fractionation of Rosemary Ethanolic Oleoresins as a Method to Improve Carnosic Acid Recovery. Innovative Food Science and Emerging Technologies 2011, 12, 142–145.
- Huang, S.W.; Frankel, E.N.; Schwarz, K.; Aeschbach, R.; German, J.B. Antioxidant Activity of Carnosic Acid and Methyl Carnosate in Bulk Oils and Oil-in-Water Emulsions. Journal of Agricultural and Food Chemistry 1996, 44, 951–956.
- Richheimer, S.L.; Bernart, M.W.; King, G.A.; Kent, M.C.; Bailey, D.T. Antioxidant Activity of Lipid-Soluble Phenolic Diterpenes from Rosemary. Journal of the American Oil Chemists’ Society 1996, 73, 507–514.
- Nogala-Kalucka, M.; Korczak, J.; Dratwia, M.; Lampart-Szczapa, E.; Siger, A.; Buchowski, M. Changes in Antioxidant Activity and Free Radical Scavenging Potential of Rosemary Extract and Tocopherols in Isolated Rapeseed Oil Triacylglycerols During Accelerated Tests. Food Chemistry 2005, 93, 227–235.
- Frankel, E.N.; Huang, S.W.; Aeschbach, R.; Prior, E. Antioxidant Activity of a Rosemary Extract and Its Constituents, Carnosic Acid, Carnosol, and Rosmarinic Acid, in Bulk Oil and Oil-in-Water Emulsion. Journal of Agricultural and Food Chemistry 1996, 44, 131–135.
- Che-Man, Y.; Jaswir, I. Effects of Rosemary and Sage Extracts on Frying Performance of Refined, Bleached, and Deodorized (RBD) Palm Olein During Deep-Fat Frying. Food Chemistry 2000, 69, 301–307.
- Filip, S.; Hribar, J.; Vidrih, R. Influence of Natural Antioxidants on the Formation of Trans-Fatty-Acid Isomers During Heat Treatment of Sunflower Oil. European Journal of Lipid Science and Technology 2011, 113(2), 224–230.
- Lalas, S.; Dourtoglou, V. Use of Rosemary Extract in Preventing Oxidation During Deep-Fat Frying of Potato Chips. Journal of the American Oil Chemists’ Society 2003, 80(6), 579–583.
- Martínez, M.L.; Penci, M.C.; Ixtaina, V.; Ribotta, P.D.; Maestri, D. Effect of Natural and Synthetic Antioxidants on the Oxidative Stability of Walnut Oil Under Different Storage Conditions. LWT–Food Science and Technology 2013, 51, 44–50.
- Chen, X.; Zhang, Y.; Zu, Y.; Yang, L.; Lu, Q.; Wang, W. Antioxidant Effects of Rosemary Extracts on Sunflower Oil Compared with Synthetic Antioxidants. International Journal of Food Science and Technology 2014, 49, 385–391.
- Erkan, N.; Ayranci, G.; Ayranci, E. Antioxidant Activities of Rosemary (Rosmarinus Officinalis L.) Extract, Blackseed (Nigella Sativa L.) Essential Oil, Carnosic Acid, Rosmarinic Acid, and Sesamol. Food Chemistry 2008, 110, 76–82.
- Shahidi, F.; Naczk, M. Phenolic in Food and Nutraceuticals; CRC Press: Boca Raton, FL, 2004; 558.
- Dobarganes, M.C.; Velasco, J.; Dieffenbacher, A. Determination of Polar Compounds, Polymerized, and Oxidized Triacylglycerols and Diacylglycerols in Oils and Fats. Pure and Applied Chemistry 2000, 72, 1563–1575.
- Farhoosh, R. The Effect of Operational Parameters of the Rancimat Method on the Determination of the Oxidative Stability Measures and Shelf-Life Prediction of Soybean Oil. Journal of the American Oil Chemists’ Society 2007, 84, 205–209.
- American Oil Chemist’s Society. (AOCS). Determination of Tocopherols and Tocotrienols in Vegetable Oils and Fats by HPLC, AOCS Official Method Ce 8-89; AOCS International: Urbana, IL, 1998.
- Farhoosh, R.; Niazmand, R.; Rezaei, M.; Sarabi, M. Kinetic Parameter Determination of Vegetable Oil Oxidation Under Rancimat Test Conditions. European Journal of Lipid Science and Technology 2008, 110, 587–592.
- Celiktas, O.Y.; Bedir, E.; Sunkan, F.V. In Vitro Antioxidant Activities of Rosmarinus Officinalis Extracts Treated with Supercritical Carbon Dioxide. Food Chemistry 2007, 101, 1457–1464.
- Mata, A.T.; Proenca, C.; Ferreira, A.R.; Serralheiro, M.L.M.; Nogueira, J.M.F.; Araujo, M.E.M. Antioxidant and Antiacetylcholinesterase Activities of Five Plants Used as Portuguese Food Spices. Food Chemistry 2007, 103, 778–786.
- Gharachorloo, M.; Ghavami, M.; Mahdiani, M.; Azizinezhad, R. The Effects of Microwave Frying on Physicochemical Properties of Frying and Sunflower Oils. Journal of the American Oil Chemists’ Society 2010, 87, 355–360.
- Lumley, I.D. Polar Compounds in Heated Oils. In Frying of Foods: Principles, Changes, New Approaches, Varela, G.; Bender, A.E.; Morton, I.D.; Eds.; Ellis Horwood: Chichester, UK, 1998; 166–173.
- Reblova, Z.; Kudrnova, J.; Trojakova, L.; Pokorny, J. Effect of Rosemary Extracts on the Stabilization of Frying Oil During Deep Fat Frying. Journal of Food Lipids 1999, 6, 13–23.
- Corsini, M.S.; Silva, M.G.; Jorge, N. Loss in Tocopherols and Oxidative Stability During the Frying of Frozen Cassava Chips. Grasas y Aceites 2009, 60, 77–81.
- Angelo, P.M.; Jorge, N. Antioxidant Evaluation of Coriander Extract and Ascorbyl Palmitate in Sunflower Oil Under Thermoxidation. Journal of the American Oil Chemists’ Society 2008, 85, 1045–1049.
- Gamez-Meza, N.; Noriega-Rodriguez, J.A.; Leyva-Carrillo, L.; Ortega-Garcia, J.; Bringas-Alvarado, L.; Garcia, H.S.; Medina-Juarez, L.A. Antioxidant Activity Comparison of Thompson Grape Pomace Extract, Rosemary, and Tocopherols in Soybean Oil. Journal of Food Processing and Preservation 2009, 33, 110–120.
- Merril, L.I.; Pike, O.A.; Ogden, L.V.; Dunn, M.L. Oxidative Stability of Conventional and High-Oleic Vegetable Oils with Added Antioxidants. Journal of the American Oil Chemists’ Society 2008, 85, 771–776.
- Ramalho, V.C.; Jorge, N. Antioxidant Action of Rosemary Extract in Soybean Oil Submitted to Thermoxidation. Grasas y Aceites 2008, 59, 128–131.
- Medina-Juarez, L.A.; Gamez-Meza, N.; Ortega-Garcia, J.; Noriega-Rodriguez, J.A.; Angulo-Guerrero, O. Trans Fatty Acid Composition and Tocopherol Content in Vegetable Oils Produced in Mexico. Journal of the American Oil Chemists’ Society 2000, 77, 721–724.
- Codex Alimentarius. Codex Standard for Named Vegetable Oils, Codex Alimentarius Commission; Codex Stan 210–1999, http://www.fao.org/input/download/standards/336/CXS_210e_2015.pdf (accessed August 31, 2016).
- Barrera-Arellano, D.; Ruiz-Mendez, V.; Velasco, J.; Marquez-Ruiz, G.; Dobarganes, M.C. Loss of Tocopherols and Formation of Degradation Compounds at Frying Temperatures in Oils Differing in Degree of Unsaturation and Natural Antioxidant Content. Journal of the Science of Food and Agriculture 2002, 82, 1696–1792.
- Rizner-Hras, A.; Hadolin, M.; Knez, Z.; Bauman, D. Comparison of Antioxidative and Synergistic Effects of Rosemary Extract with a-Tocopherol, Ascorbyl Palmitate, and Citric Acid in Sunflower Oil. Food Chemistry 2000, 71, 229–233.
- Kamal-Eldin, A.; Appelqvist, L.A. The Chemistry and Antioxidant Properties of Tocopherols and Tocotrienols. Lipids 1996, 31, 671–701.
- Steel, C.J.; Dobarganes, M.C.; Barrera-Arellano, D. The Influence of Natural Tocopherols During Thermal Oxidation of Refined and Partially Hydrogenated Soybeans Oils. Grasas y Aceites 2005, 56, 46–52.
- Verleyen, T. Modeling of a-Tocopherol Loss and Oxidation Products Formed During Thermoxidation in Triolein and Tripalmitin Mixtures. Lipids 2001, 36, 719–726.
- Warner, K.; Gehring, M.M. High-Temperature Natural Antioxidant Improves Soy Oil for Frying. Journal of Food Science 2009, 74, 500–505.
- Juarez, M.D.; Masson, L.; Samman, N. Deterioration of Partially Hydrogenated Soybean Oil Used in Deep Fat Frying of a Meat Food. Grasas y Aceites 2005, 56, 53–58.
- Kim, J.; Kim, D.N.; Lee, S.H.; Yoo, S.H.; Lee, S. Correlation of Fatty Acid Composition of Vegetable Oils with Rheological Behavior and Oil Uptake. Food Chemistry 2010, 118, 398–402.
- Robert, P.; Masson, L.; Romero, N.; Dobarganes, M.C.; Izaurieta, M.; Ortiz, J.; Wittig, E. Industrial Frying of Crisps. Influence of the Unsaturation Degree of Frying Fat on the Oxidative Stability During Storage. Grasas y Aceites 2001, 52, 389–396.
- Velasco, J.; Marmesat, S.; Bordeaux, O.; Marquez-Ruiz, G.; Dobarganes, C. Formation and Evolution of Monoepoxy Fatty Acids in Thermoxidized Olive and Sunflower Oils and Quantitation in Used Frying Oils from Restaurants and Fried-Food Outlets. Journal of Agricultural and Food Chemistry 2004, 52, 4438–4443.
- Gunstone, F.D. Fatty Acid and Lipid Chemistry; Chapman & Hall: London, UK, 1996.
- Warner, K. Oxidative and Flavor Stability of Tortilla Chips Fried in Expeller Pressed Low Linolenic Acid Soybean Oil. Journal of Food Lipids 2009, 16, 133–147.
