ABSTRACT
Avocado fat is a semi-solid substance with potential functional lipid characteristics. A study was carried out to evaluate the effect of addition of palm stearin and cocoa butter on the solidification behavior of avocado fat to formulate a mixture to become similar to lard. A total of three mixtures were prepared: avocado fat:palm stearin:cocoa butter (88:7:5), avocado fat:palm stearin:cocoa butter (86:7:7), avocado fat:palm stearin:cocoa butter (84:7:9; w/w), and identified by the mass ratio of avocado fat to palm stearin and cocoa butter. The fat mixtures were compared with lard in terms of the fatty acid and triacylglycerol compositions using gas chromatography and high-performance liquid chromatography, thermal properties using differential scanning calorimetry and solid fat content using p-nuclear magnetic resonance. Although there were considerable differences between lard and the fat mixtures with regard to fatty acid and triacylglycerol compositions, some similarities were seen with regard to thermal properties and solid fat content profile. Of all the fat mixtures, avocado fat:palm stearin:cocoa butter (84:7:9) displayed closer similarity to lard with respect to thermal transitions at –3.59°C and its solid fat content profile showed the least difference to that of lard throughout the temperature range measured.
Introduction
Lard is a fat substance obtained by rendering of the adipose tissues of swine. Due to its semi-solid nature, lard has found uses as a fat ingredient in bakery products, confectionaries, as well as meat products.[Citation1] According to Cheong et al.,[Citation2] lard remains a major player in the meat product industry due to its positive contributions in flavor and texture. Lard blended with rapeseed oil, for instance, was found to be effective in preserving the sensory quality of pork sausages.[Citation2] Lard is said to be a good binder for meat products and its usage in minced-meat is believed to be due to the binding property resulting from the stickiness behavior.[Citation3] Lard was also used as a bakery shortening agent owing to the relatively easy processing requirements to produce an acceptable consistency. Apart from this, the wide plastic range of lard at room temperature tended to provide good lubrication in the process of dough mixing.[Citation4] These attributes provided good reasons for utilization of swine fat left in the carcass industry. Inclusion of lard in food or any other consumer products, however, has become a growing public concern due to negative implications of animal fats on human nutrition, as well as prohibitions under Halal and Kosher food regulations.
Producing alternatives for lard has been the interest of researchers for several decades.[Citation5–Citation8] According to Ghotra et al.,[Citation4] the first ever attempt to produce a substitute for lard was made in the United States of America by Roudebush in 1873. In the recent past, several researchers looked into partial replacement of lard in various meet product formulations. As Halal and Kosher food regulations demand complete replacement of lard from all food formulations, few recent research studies were dedicated to develop an alternative plant-based fat for lard. For instance, a replacement for lard was investigated using binary fat mixtures of mee fat and palm stearin (PS),[Citation9] as well as binary fat mixtures composed of engkabang fat and canola oil.[Citation10] However, the compatibility of ternary fat blends comprising of avocado (Avo) fat, PS, and cocoa butter (CB) to lard has not been considered in any of the previous studies. According to our previous research, the solid fat content (SFC) curve of Avo was similar in pattern to that of lard (LD), but the values were always lower throughout the temperature range.[Citation6] Hence, incorporation of hard fat components like PS and CB into Avo could enhance the SFC to a level comparable to the SFC values of LD. The SFC value of PS at 25°C was about 86% while the SFC of CB was 19.5% at 25°C.[Citation6] It can be hypothesized that addition of CB into PS would bring down the SFC of PS at 25°C to make it a suitable medium to raise the SFC of Avo to that of LD all thorough out the temperature range. In evaluating ternary fat mixtures for lard, several aspects are needed to be considered. According to the reports of Maw et al.[Citation11] and Nishioka and Irie,[Citation3] physical characteristics including the firmness and stickiness of lard were correlated to its fatty acid composition. However, Campos et al.[Citation12] asserted that physical and textural properties of lard were not solely dependent on composition, they may also be affected by several other factors. Apart from fatty acid and triacylglycerol (TAG) composition, detailed investigations on solidification and melting behavior of formulated fats are, therefore, necessary. Hence, the objective of this study was to compare three ternary plant-based fat blends (Avo fat, PS, and CB) and lard with respect to their chemical composition, DSC thermal properties, and SFC profiles.
Materials and Methods
Materials
Samples of Avo fruits were collected in replicates from trees located in University Agricultural Park, Serdang in West Malaysia. Fruits were immediately cut into pieces without removing the skin and subjected to drying in an oven. A sample of PS was obtained as a generous gift from Malaysian Palm Oil Board (MPOB) while a sample of CB was purchased from the Malaysian Cocoa Board. LD was extracted using adipose tissues of swine collected from local slaughter houses according to the method reported previously by Marikkar and co-workers.[Citation13] All chemicals used in this experiment were of analytical or high-performance liquid chromatography (HPLC) grade.
Fat Extraction
Fat extraction from finely grounded samples (less than 325 mesh) of dried Avo fruits was carried out according to the Soxhlet extraction method using petroleum ether as solvent (40–60°C).[Citation14] The extracted fat was kept in an oven at 60°C for 1 h to expel solvent before storing at –20°C.
Preparation of Ternary Mixture
The fat samples were melted at 70°C for 1 h before mixing. A total of three mixtures were prepared: Avo:PS:CB (88:7:5), Avo:PS:CB (86:7:7), Avo:PS:CB (84:7:9; w/w), and identified by the mass ratio of Avo to PS and CB. All samples were kept under frozen storage at –20°C. Prior to analyses, the fat mixtures were removed from frozen storage, and then left static at room temperature for 1 h before being warmed at 70°C until they became completely molten.[Citation5]
Determination of Slip Melting Point (SMP) and Iodine Value (IV)
SMP and IV of the fat samples were determined according to AOCS method Cc.3.25, and AOCS method Cd Id–92, respectively.[Citation15]
Determination of Fatty Acid Composition
Fatty acid methyl esters were prepared by dissolving 50 mg portion of oil in 0.8 mL of hexane and adding 0.2 mL portion of 1 M solution of sodium methoxide.[Citation16] The top hexane layer was injected on an Agilent 6890N gas chromatograph (Agillent Technologies, Singapore) equipped with a polar capillary column RTX-5 (0.32 mm internal diameter, 30 m length, and 0.25 μm film thickness; Restex Corp., Bellefonte, PA) and a flame ionization detector (FID). Split injection was conducted with a split ratio of 58:1, nitrogen was used as a carrier gas at a flow-rate of 1.00 mL/min. The temperature of the column was 50°C (for 1 min), and programmed to increase to 200°C at 8°C/min. The temperatures of the injector and detector were maintained at 200°C.[Citation17] The identification of the peaks of the samples was done with reference to a chromatographic profile containing a set of fatty acid methyl ester standards. The percentage of fatty acid was calculated as the ratio of the partial area to the total area.[Citation5,Citation6]
Determination of TAG Composition
The TAG compositions of samples were determined according to the method described by Yanty et al.[Citation5] using Waters Model 510 liquid chromatography equipped with a differential refractometer Model 410 as the detector (Waters Associates, Milford, MA). The analysis of TAG was performed on a Merck Lichrosphere RP-18 column (5 μm; 12.5 cm × 4 mm i.d.; Merck, Darmstadt, Germany) which was maintained at 30°. The mobile phase was a mixture of acetone:acetonitrile (63.5:36.5) and the flow rate was 1.5 mL/min. The injector volume was 10 μL of 5% (w/w) oil in chloroform. Each sample was chromatographed three times, and the data were reported as peak area percentages. The identification of the peaks of the samples was done using a set of TAG standards purchased from Sigma-Aldrich (Deisehofen, Germany) as well as the TAG profiles of lard, PS,[Citation9] and Avo fat[Citation5] reported previously.
Thermal Analysis by Differential Scanning Calorimetry (DSC)
Thermal analysis was carried out on a Mettler Toledo differential scanning calorimeter (DSC 823 Model) equipped with a thermal analysis data station (STARe software, Version 9.0x, Schwerzenbach, Switzerland). Nitrogen (99.999% purity) was used as the purge gas at a rate of ~20 mL/min. Approximately, 4–8 mg of melted sample was placed in a standard DSC aluminum pan and then hermetically sealed. An empty, hermetically sealed DSC aluminum pan was used as the reference. The oil/fat samples were subjected to the following temperature program: 70°C isotherm for 1 min, cooled at 5°C/min to –70°C. The samples were held at –70°C isotherm for 1 min, and heated at 5°C/min to reach 70°C.[Citation5]
Determination of SFC
SFC was measured according to AOCS method Cd 16b-93[Citation15] using a Bruker Minispec (Model Mq 20) pulse nuclear magnetic resonance (pNMR) spectrometer (Karlsruhe, Germany). The sample in the NMR tube was melted at 70°C for 15 min, followed by chilling at 0°C for 60 min, and then held at each measuring temperature for 30 min prior to measurement. Melting, chilling, and holding of the samples were carried out in pre-equilibrated thermostatic glycol containing baths, accurate to 0.1°C. SFC measurements were taken at 5°C intervals over the range of 0–70°C.
Statistical Analysis
All analyses were carried out in triplicate and the results were expressed as mean value ± standard deviation. Data were statistically analyzed by one-way analysis of variance (ANOVA), by using Tukey’s Test of MINITAB (version 15) statistical package at 0.05 probability level.
Results and Discussion
SMP and IV
SMP and IV of ternary mixtures of Avo:PS:CB and LD are presented in . The sample of Avo used in this study was appeared to be semi-solid and its SMP value was 30°C. Additions of PS and CB into Avo caused significant (p < 0.05) increases in SMP values (38.25 to 40.5°C) with respect to original Avo and LD (27.5°C). The SMP values of all three blends were found to be higher than that of LD. This could be because of the fact that PS (59.9°C) and CB (35.66°C) were complete solid fats at room temperature and their SMP values were higher than that of Avo. The IV of ternary mixtures of Avo:PS:CB were in a range of 65.47 to 70.27 and showed that all ternary blends displayed IV significantly lower (p < 0.05) than those of the original Avo (84.30) and LD (73.76) samples. According to these results, none of the ternary mixtures of Avo:PS:CB was found to have a IV closely similar to that of LD. It was mainly due to the fact that the degree of unsaturation of PS and CB were relatively low as observed in a number of studies.[Citation6,Citation9]
Table 1. Basic physico-chemical characteristics and fatty acid composition (%) of Avo, PS, CB, Avo:PS:CB mixtures, and LD.1
Fatty Acid Composition
The data of fatty acid composition of ternary mixtures of Avo:PS:CB and LD is presented in . The major fatty acids of Avo were oleic (43.65%), palmitic (30.37%) and linoleic (17.45%) and were in accordance with the fatty acid composition of Malaysian Avo oil reported earlier.[Citation5] However, the relative proportions of these fatty acids of Avo were found to vary based on the variety and geographical origin.[Citation19] The additions of PS and CB into Avo caused slight increment in the proportion of palmitic (from 30.37 to 33.08%), stearic (from 1.30 to 4.57%) and oleic (from 43.64 to 44.04%) acids with concurrent decreases in the amounts of linoleic acids (from 17.45 to 14.28%). The increments of palmitic and stearic acids of the ternary blends could be due to the presence of higher proportions of palmitic and stearic acids in PS[Citation20] and CB,[Citation21] respectively. Although original Avo was not found to possess arachidic acid, the additions of PS and CB into Avo caused the ternary mixtures to have traces of arachidic acid. With the increments of PS and CB in the mixtures, the total saturated fatty acid content was tended to increase from 36.65 to 38.01% while the total unsaturated fatty acid content was found to decrease from 61.99 to 63.35% with respect to original Avo (31.67%). These changes in the degree of unsaturation of fatty acid composition lead to the changes of SMP and IV of these blends as noted in . When compared to LD, the ternary mixtures were found to have significantly greater (p < 0.05) proportions of palmitic and oleic acids, but significantly lower (p < 0.05) proportions of stearic and linoleic acids. On a quantitative basis, none of fatty acid except linoleic in the three ternary mixtures was found to become compatible to those of LD.
TAG Composition
The TAG compositions of ternary mixtures of Avo:PS:CB were compared to that of LD as shown in . Avo composed of dioleoyl-palmitoyl-glycerol (POO) (22.76%), palmitoyl-linoleoyl-oleoyl glycerol (POL) (19.29%), dipalmitoyl-3-oleoyl glycerol (PPO) (12.43%), and trioleoyl glycerol (OOO) (11.42%) as dominant TAG molecules, which was in accordance with the TAG composition of Malaysian Avo oil as reported by Yanty and co-workers.[Citation5] In fact, the proportions of these TAG molecules could vary depending on the cultivar type and geographical origin. After addition of PS and CB into Avo, some of the TAG molecules were increased (e.g., PPO, tripalmitoyl glycerol (PPP), dioeoyl-3-stearoyl glycerol (SOO), palmitoyl-oleoyl-stearoyl glycerol (SPO), and dipalmitoyl-3-stearoyl glycerol (PPS)) while others were tended to decrease (e.g. dilinoleoyl-linoleneoyl glycerol (LLLn), trilinoleoyl glycerol (LLL), oleoyl- dilinoleoyl glycerol (OLL), dilinoleoyl-palmitoyl glycerol (PLL), dioeoyl-3-linoleoyl glycerol (OOL), POL, dipalmitoyl-1-linoleoyl glycerol (PPL), OOO and POO). The observed increments on PPP, PPO, and SPO were due to the fact that they were present in higher proportions in PS[Citation20] and CB.[Citation21] Although TAG molecular species namely, StOSt was not originally present in Avo, it was detected in the ternary mixtures since it was a major TAG molecule present in CB. Among the TAG molecules, StPO (6.41 to 10.56%) was found to show greater increments in the mixtures. As a consequence, the proportions of the di- and tri-saturated TAG molecules were found to increase in the mixtures when compared to the original Avo. For instance, the di-saturated TAG was increased remarkably from 24.89 to 33.61% when compared to that of the original sample of Avo (17.03%). On the other hand, the tri-saturated TAG molecules were found to increase only slightly from 7.80 to 8.00% with respect to that of the original sample of Avo (2.99%). The increasing proportions of di- and tri-saturated TAGs in the fat mixtures could have caused increments in palmitic and stearic acids and a decrease in IV (). When compared to LD, the amount of di- and tri-saturated TAG of the Avo:PS:CB mixtures were found to increase steadily with concurrent decreases of di- and tri-unsaturated TAG molecules. However, di-saturated TAG of Avo:PS:CB (88:7:5) were found to be lower (24.89%) than that of LD (26.60%). Among the mixtures, the amounts of LLLn, LLL, OOL, PPL, and PPSt of Avo:PS:CB (84:7:9) was found to become compatible to those of LD.
Table 2. TAG composition of Avo, PS, CB, Avo:PS:CB mixtures and LD.1
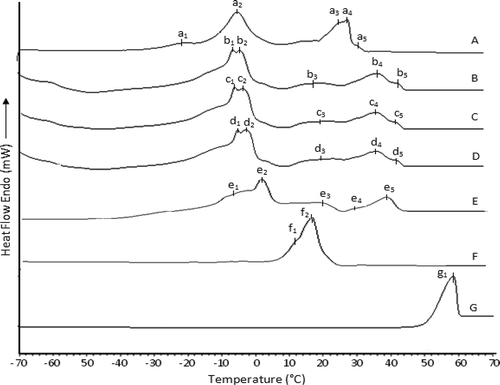
Melting Behavior by DSC
The melting curves of Avo, PS, CB, and Avo:PS:CB mixtures are compared to that of LD as shown in . The pattern of melting behaviors displayed by PS[Citation9] and CB[Citation18] were more or less similar to those described in previous reports. According to , the native Avo sample was also found to have five endothermic transitions (Curve E). The low-melting region consisted of a major sharp peak at around –3.58ºC with a shoulder-peak at around –10.72ºC while the high-melting transition was a minor peak at 40.93ºC with a shoulder-peak at 30.12ºC and a broad peak at 17.95ºC. On the other hand, the DSC melting profiles of Avo:PS:CB mixtures (Curve B, C, and D) were found to display some differences to the melting profile of original sample of Avo. By overall, the addition of PS and CB into Avo was found to affect the melting peaks throughout the temperature region. The two peaks of Avo (Curve E) at the lower temperature (e1 and e2) were tended to become a duplet peak in all three ternary mixtures (). In addition, the peak of Avo at position e3 tended to became broader in all three ternary mixtures. With the disappearance of the peak of Avo at position e4, there appeared a broad peak with a shoulder in the higher temperature region. Other than this, all melting peak-maxima of the transitions were found to have shifted to higher temperature resulting in higher end-set temperature of melting (Tendset). This phenomenon was a direct result of compositional changes, which would cause an increase in saturated fatty acids () and disaturated/trisaturated TAG contents () in the mixtures.[Citation22,Citation23] The overlay of heating curves of the ternary mixtures formed by blending Avo with PS and CB also compared with the melting behavior of LD. The pattern of melting profile displayed by LD was more or less similar to those described in previous reports.[Citation13] On a comparative basis, there were more differences than similarities between the melting transitions of LD and the ternary mixtures. For instance, all three Avo:PS:CB mixtures had higher end-set of melting (Tendset) transition (at around 47°C) than (37.5°C). However, a closer similarity between the mixture Avo:PS:CB (84:7:9) and LD was seen at the peak-maximum of (d1 and d2) and (a2) at -–3.59°C.
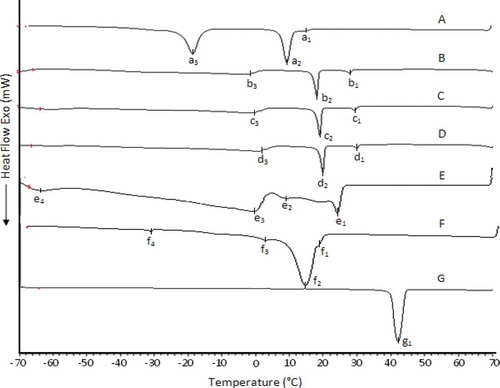
Cooling Behavior by DSC
The cooling curves of Avo, PS, CB, and Avo:PS:CB mixtures are compared to that of LD as shown in . The pattern of the profiles of the cooling curves displayed by PS[Citation9] and CB[Citation18] were more or less similar to those described in our previous reports. According to , the cooling profile of Avo had four transitions; e1, e2, and e3 were in the range of –4 to 25°C while e4 was at –65°C. Addition of PS and CB into Avo was found to cause remarkable changes in the cooling profiles of the mixtures as they were hard solid fats. In all three ternary mixtures, there were three transitions; a major sharp peak at around 18 to 20°C, a broad minor peak at around –2 to 2°C and a minute sharp peak at around 28 to 30°C. Clearly, the onset of crystallization temperatures of all three ternary mixtures was higher than that of Avo. The shifting of the onset of crystallization of the ternary mixture was in accordance with the decreasing degree of unsaturation noted in fatty acid () and TAG compositions () as discussed previously in several reports.[Citation22,Citation23] For instance, there were significant (p < 0.05) increases in palmitic and stearic acid contents and also in the proportions of disaturated and trisaturated TAG molecules in the mixtures. The overlay of cooling curves presented in compares the cooling behavior of lard with those of the ternary mixtures formed by blending Avo with PS and CB. The pattern of cooling behaviors displayed by LD was more or less similar to those described in our previous reports.[Citation13] On a comparative basis, the overlay showed that none of the ternary mixtures displayed any similarity to lard with respect to the positions of thermal transitions. The onset of crystallization (Tonset) of Avo:PS:CB mixtures were also found to be higher (~30°C) than that of LD (~16°C).
SFC
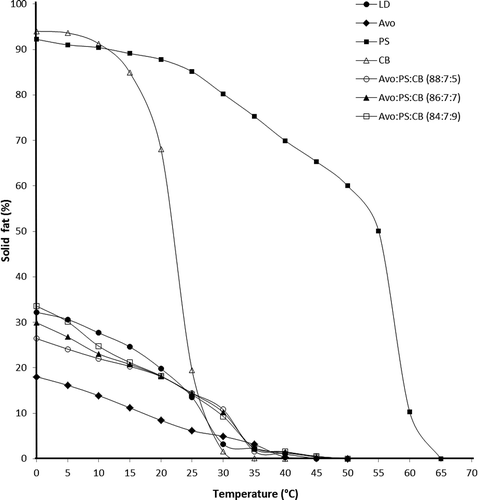
The SFC profiles of Avo, PS, CB, ternary mixtures of Avo:PS:CB and LD are given in . The quantitative data of the SFC profile for individual fat and the ternary mixtures are given separately in for a better comparison. Study on SFC is highly important since it has a great influence on the suitability of fat or fat blend for a particular application.[Citation24] The SFC value of CB was dropped from 94 to 19.4% at 25ºC and tended to become almost 0% at 35ºC. This is in accordance with the findings reported previously by Jayarani and Reddy.[Citation18] In the case of PS, the initial SFC value of 92.5% was observed at 0°C and tended to become 0% at 65ºC. In this case, the solid fat disappeared only at 65ºC, even though the SMP value of PS was 59.9ºC (). This is not unusual as the fats subjected to SMP determination might slip in the capillary where approximately 5 to 8% solid is still present.[Citation15] According to , the SFC of LD and Avo were 32.19 and 18.02%, respectively, at 0ºC and were tended to decrease gradually to 0% at 45 and 50ºC, respectively. As shown in , the SFC values of Avo oil were always lower than those of LD throughout the temperature range. Hence, inclusion of hard butters would be necessary to adjust the SFC profile of Avo to become similar to those of LD. After adding PS and CB into Avo, the SFC values of the fat mixtures were found to increase throughout the temperature region. The increasing SFC values were found to correlate well (r = +0.97; p < 0.05) with the increasing proportion of disaturated/trisaturated TAG molecules in the fat mixtures (). All three fat mixtures showed a lower SFC values than LD at 10, 15, and 20ºC. According to the calculations presented in , Avo:PS:CB (84:7:9) was the mixture that showed the least difference to LD in terms of SFC values throughout the temperature range. This mixture exhibited SFC values closely similar to those of LD at 0, 5, 20, 25, 35, and 45°C and the values were not significantly (p > 0.05) different. Hence, this mixture was the most compatible to LD with regard to solidification behavior within the range of 0 to 45ºC.
Table 3. SFC values of avocado fat, palm stearin, cocoa butter, Avo:PS:CB mixtures, and lard.1
Table 4. Comparing least difference of SFC values of LD, Avo, PS, CB, and Avo:PS:CB mixtures.1
Conclusions
In this study, it was attempted to explore the possibility of producing a fat mixture to mimic the properties of LD by blending Avo, PS, and CB in different ratios. Among the three different mixtures formulated, Avo:PS:CB (84:7:9) was found to have the closest similarity to LD in terms of some compositional parameters and thermal characteristics. In TAG composition, the proportions of OOL, PPL, and PPSt of this mixture were roughly similar to those of LD. Although there were much differences in between the DSC transitions of LD and the mixtures, a closer similarity was seen between Avo:PS:CB (84:7:9) and LD at the peak-maximum of (d1 and d2) and (a2) at –3.59°C. With regard to SFC values, Avo:PS:CB (84:7:9) was the mixture, which showed the least difference to LD at 0, 5, 20, 25, 35, and 45°C temperatures.
Funding
Authors gratefully acknowledge the financial support from a research grant (02-02-12-2036 RU) obtained under the Research University Grants Scheme of Universiti Putra Malaysia.
Additional information
Funding
References
- Muguerza, E.; Fista, G.; Ansorena, D.; Astiasaran, I.; Bloukas, J.G. Effect of Fat Level and Partial Replacement of Pork Backfat with Olive Oil on Processing and Quality Characteristics of Fermented Sausages. Meat Science 2002, 61, 397–404.
- Cheong, L.Z.; Hong, Z.; Yuan, X.; Xuebing, X. Physical Characterization of Lard Partial Acylglycerols and Their Effect on Melting and Crystallization Properties of Blends with Rapeseed Oil. Journal of Agricultural and Food Chemistry 2009, 57, 5020–5027.
- Nishioka, T.; Irie, M. Evaluation of Method for Firmness and Stickiness of Porcine Perirenal Fat. Meat Science 2005, 70, 399–404.
- Ghotra, B.S.; Dyal, S.D.; Narine, S.S Lipid Shortening: A Review. Food Research International 2002, 35, 1015–1048.
- Yanty, N.A.M.; Marikkar, J.M.N.; Long, K. Effect of Varietal Differences on Composition and Thermal Behaviour of Avocado Oil. Journal of the American Oil Chemists’ Society 2011, 88, 1997–2003.
- Yanty, N.A.M.; Marikkar, J.M.N.; Miskandar, M.S. Comparing the Thermo-Physical Properties of Lard and Selected Plant Fats. Grasas Y Aceites 2012, 63, 328–334.
- Raihana, A.R.; Marikkar, J.M.N.; Ismail, A.; Musthafa, S. A Review on Food Values of Selected Fruits’ Seeds. International Journal of Food Properties 2015, 18, 2380–2392.
- Rohman, A.; Windarsih, A.; Riyanto, S.; Sudjadi, Ahmed, S.A.; Rosman, A.S.; Farahwahida M.Y. Fourier Transform Infrared Spectroscopy Combined with Multivariate Calibrations for the Authentication of Avocado Oil. International Journal of Food Properties 2016, 19, 680–687.
- Yanty, N.A.M.; Marikkar, J.M.N.; Shuhaimi, M.; Miskandar, M.S. Composition and Thermal Analysis of Binary Mixture of Mee Fat and Palm Stearin. Journal of Oleo Science 2014, 63, 325–332.
- Nur Illiyin, M.R.; Marikkar, J.M.N.; Shuhaimi, M.; Mahiran, B.; Miskandar, M.S. A Comparison of the Thermo Physical Behavior of Engkabang (Shorea Macrophylla) Seed Fat—Canola Oil Blends and Lard. Journal of the American Oil Chemists’ Society 2013, 90, 1485–1493.
- Maw S.J.; Fowler, V.R.; Hamilton, M.; Petchey, A.M. Physical Characteristics of Pig Fat and Their Relation to Fatty Acid Composition. Meat Science 2003, 63, 185–190.
- Campos, R.; Narine, S.S.; Marangoni, A.G. Effect of Cooling Rate on the Structure and Mechanical Properties of Milk Fat and Lard. Food Research International 2002, 35, 971–981.
- Marikkar, J.M.N.; Lai, O.M.; Ghazali, H.M.; Che Man, Y.B. Detection of Lard and Randomised Lard as Adulterants in RBD Palm Oil by Differential Scanning Calorimetry. Journal of the American Oil Chemists’ Society 2001, 78, 1113–1119.
- AOAC. 2007. Official Methods of Analysis of AOAC International, 18th ed.; Association of Official Analytical Chemists: Gaithersburg, MD.
- AOCS. 1999. Official Methods and Recommended Practices of the American Oil Chemists’ Society, 5th ed.; American Oil Chemists’ Society, Champaign, IL.
- PORIM. 1995. PORIM Test Methods; Palm Oil Research Institute of Malaysia: Kuala Lumpur.
- Nasyrah, A.R.; Marikkar, J.M.N.; Dzulkifly, M.H. Comparative Thermal Characteristics and Fatty Acid Composition of Mono- and Diacylglycerols of Lard and Some Commercial Emulsifiers. International Journal of Food Properties 2014, 17, 1116–1125.
- Jayarani, T.; Reddy, S.Y. Heat-Resistant Cocoa Butter Extenders from Mahua (Madhuca Latifolia) and Koum (Garcinia Indica) Fats. Journal of the American Oil Chemists’ Society 1999, 76, 1431–1436.
- Ozdemir, F.; Topuz, A. Changes in Dry Matter, Oil Content and Fatty Acids Composition of Avocado During Harvesting Time and Post Harvesting Ripening Period. Food Chemistry 2004, 86, 79–83.
- Miskandar, M.S.; Nor Aini, I. Palm Stearin as Low Trans Hard Stock for Margarine. Sains Malaysiana 2010, 39, 821–827.
- Wong, S. The Chocolate Fat from the Borneo Illipe Trees; Vivar Printing Sdn Bhd: Pahang, Malaysia, 1988.
- Tan, C.P.; Che Man, Y.B. Differential Scanning Calorimetric Analysis of Edible Oils: Comparison of Thermal Properties and Chemical Composition. Journal of the American Oil Chemists’ Society 2000, 77, 143–155.
- Marikkar, J.M.N. DSC as a Valuable Tool for the Evaluation of Oils and Fats Adulterations. In Differential Scanning Calorimetry: Applications in Fat and Oil Technology, Chiavaro, E.; Ed.; CRC Press/Taylor & Francis Group: Boca Raton, FL, 2015; 159–178.
- Lipp, M.; Anklam, E. Review of Cocoa Butter and Alternative Fats for Use in Chocolate—Part A. Compositional Data. Food Chemistry 1998, 62, 73–97.