ABSTRACT
Viscoelastic properties for reconstituted A. vera hydrogels were evaluated in different ranges of concentration (0.2–1.6%, w/v) and temperature (30–60°C). The storage (G′) and loss (G″) moduli were well described by power function of the frequency (R2 > 0.92). The combined effect of concentration and temperature was optimized for reconstituted gel formation, targeting maximum viscoelastic moduli (G0′ and G′′0) and minimum slope (n′ and n″) values using response surface methodology. The optimum condition selected was 1.6%, w/v at 30°C having higher gel strength [G′0 (47.8 Pa.sn′) and G″0 (27.4 Pa.sn′)], as well as minimum slope values [n′ (0.24) and n″ (0.08)]. Further, the effect of initial aging time on the viscoelastic moduli of optimized sample (1.6%, w/v at 30 ± 1°C) was noticed by fitting the intermediate creep ringing data to rheological models. The viscoelastic moduli (G′J and G′K) increased with aging time (0–180 min) for optimized A. vera hydrogel, as confirmed by Jeffrey’s and Kelvin-Voigt models. The obtained results suggested that optimized conditions for reconstituted A. vera gel formation could be useful to improve the gel strength which could be useful for various food applications.
Introduction
Aloe vera (A. vera) gel is widely utilized as functional ingredient in food, medicinal, and cosmetics.[Citation1] It is converted into powder in order to preserve its bioactivity for a longer duration.[Citation2] Reconstitution of A. vera powder into solutions, semi-diluted solutions, and gels has been studied by several researchers to regain its viscoelastic behavior prior to dehydration.[Citation3–Citation7] The formation of reconstituted dispersions, with cost effective crude and partially purified powder seems to be very complex due to heterogeneous composition. Nindo et al.[Citation6] studied the effect of different drying methods (spray drying, refractance window, and freeze drying) on viscosity (η) of the reconstituted A. vera solutions obtained from crude powders. However, reconstitution of crude fibrous and partially purified non-fibrous powder into an aqueous dispersion at the critical concentration (i.e., a minimum amount of water incorporated) has been studied by Kiran and Rao.[Citation4] They reported that, partially purified non-fibrous alcohol insoluble residue (NFAIR) was suitable for the formation of frequency dependent sol-gel behavior. Besides, heating protocol of 50ºC and 30 min was standardized for reconstituted A. vera (NFAIR) hydrogel formation based on higher gel strength and network strength.[Citation8] Further detailed structural elucidation of partially purified NFAIR powder has not been attempted earlier. Reconstituted hydrogel formation from A. vera (NFAIR) powder also dependent on external factors such as concentration, test temperature, and aging time.[Citation9,Citation10]
A. vera polymer concentration variation influences the viscoelastic behavior by affecting internal structural rearrangements via hydrogen and hydrophobic interactions and also participates in conformational transition.[Citation4,Citation5,Citation11] However, commonly a decreasing trend of viscoelastic behavior was also noticed while increasing the test temperature for food biopolymers such as A. vera (concentrated juices and dispersions),[Citation4,Citation6,Citation11] alginates,[Citation12] globular proteins,[Citation13] and LM pectin/calcium gels.[Citation14] Besides, Dak et al.[Citation15] mentioned that fluid foods undergoes rheological and structural changes when subjected to various temperature and concentration during processing, storage, transportation, and marketing, etc. On the other hand, combined effects of concentration and test temperature on the viscoelastic behavior of reconstituted A. vera gels were not studied earlier. In turn selection of optimum conditions for reconstituted A. vera hydrogel with higher gel strength could be useful for food formulations, and for incorporation as gelling agents. Response surface methodology (RSM) is most commonly used technique to optimize the process conditions and estimate the empirical correlation between the independent variables and responses of interest.[Citation16] Although number of studies on reconstitution of A. vera powder were available,[Citation3,Citation5–Citation7] optimizing the concentration and test temperature conditions for reconstituted A. vera hydrogels using RSM has not been attempted earlier.
The effect of external parameters viz. concentration, temperature and time, during gelation process of complex biopolymer systems have been extensively studied using non-destructive rheological analysis and corroborated with morphological observations.[Citation17] Rheological tests can provide structural insight into the gelation mechanisms of complex biopolymer materials. Most commonly used tests are small amplitude oscillation (SAOS) and creep analysis,[Citation18] of which, SAOS provides particulars about the viscoelastic behavior during the frequency sweep test. In addition creep analysis of biopolymer gels provides insight into the viscoelastic responses and macroscopic viscosity during undisturbed state of the samples.[Citation19,Citation20]
The present study was carried out to characterize A. vera polysaccharides in NFAIR and identify optimum concentration and temperature for obtaining the high gel strength of reconstituted A. vera fluids. Further the effects of initial aging time by creep analysis on the viscoelastic responses along with morphological observations of NFAIR optimized hydrogels were also evaluated.
Materials and methods
Preparation of NFAIR powder and NMR data distribution
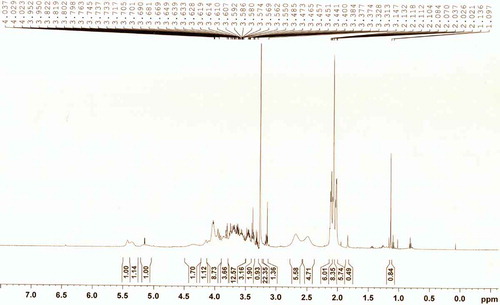
A. vera fresh leaves were collected prior to each experiment from the research farm of Agricultural and Food Engineering Department, IIT Kharagpur, India. Further, the leaves were kept in inverted position to drain out the yellow sap followed by washing with 100 ppm sodium hypochlorite, and then trimming the side edges as well as bottom portion. Finally, the green rind portion was filleted and inner gel portion was extracted. NFAIR powder was prepared by ethanol treatment using the method described earlier by Kiran and Rao.[Citation4] A. vera polysaccharides in NFAIR powder was further characterized using NMR spectrophotometer (Model: AVANCE DAX-400, Make: Bruker, 600 MHz, Sweden) at 600 MHz, in which freeze dried NFAIR sample was solubilized in D2O at 5 mg mL−1 for 1 h and the same has been transferred to 5 mm NMR tube before analysis.
Preparation of reconstituted A. vera hydrogels
Reconstituted hydrogels were prepared by mixing A. vera (NFAIR) powder (0.2–1.6%, w/v concentration) with distilled water (pH 7). Then the vials were stirred at 100 rpm for 2 min by using vortex (Model: Spinex, Make: Tarson, India) to get uniform distribution of the sample. Subsequently, the sample vials were processed by heat treatment at 50°C for 30 min in a constant temperature bath (Model: UD 80 SH-3L, Make: Takashi, Japan) and were left undisturbed at ambient temperature (28 ± 1°C) for 12 h.[Citation8]
Dynamic oscillation measurements of reconstituted A. vera hydrogels
Rheological measurements of reconstituted A. vera hydrogels were carried out using a stress-controlled Rheometer (Model: Bohlin Gemini-200, Make: Malvern, UK) equipped with a cone and plate (CP-1°/40 mm) geometry. A Peltier system with circulating water bath for sample temperature control and software (Bohlin R6.51.0.3) for data acquisition was used. Sample dispersion of 1 ml was carefully placed on the lower plate, where the gap between two plates was 30 µm. A solvent trap was used to avoid solvent evaporation effect. The samples were stabilized with an initial equilibrium time of 30 s before the experimental run. Dynamic oscillatory measurements were carried out for A. vera hydrogels at different concentrations of 0.2–1.6%, w/v. Frequency sweep test (0.1 to 100 rad/s) was conducted in the temperature range of 30–60ºC, at constant stress selected from linear viscoelastic region (LVR) zone. In the present study, Power law constant (slopes) were calculated by relating viscoelastic moduli (G′ and G″) data to angular frequency (ω), using the following equations (Eqs. 1 and 2).
where G′ and G′′ represents storage and loss moduli, respectively, n′ and n′′ are the corresponding slopes, ω is angular frequency and G′0 and G′′0 are the intercepts of the Power law model for frequency sweep. The effect of temperature (30–60°C) at different concentrations (0.2–1.6%, w/v) on G′0 and G′′0 was studied using Arrhenius model.
In Eq. (3), R represents gas constant and T corresponds to absolute temperature (K), where each parameter (A) is modeled by a pre-exponential factor A0 and the activation energy (Ea).
Experimental design
The full factorial design with two independent variables viz., concentration and temperature were used and a quadratic polynomial model (Eq. 4) was developed for individual parameters viz. G′0, G′′0, n′, and n″, which were obtained from Power law model fit:
where Yi is ith response (i = 1 to 4), c0-c5 are the regression coefficients, x1 and x2 represented the dimensions of the coded values for temperature and concentration, respectively. In the polynomial model, the real values expressed in coded values to determine the relative importance of individual parameters and terms (linear, square, and quadratic) affecting response. According to full factorial design a total of 16 experiments were conducted and four responses viz., G′0, G′′0, n′, and n″ were analyzed.
Optimization and validation
Numerical optimization was carried out to optimize the process parameters by using the desirability function as described by Montgomery.[Citation21] Numerical optimization technique was applied to find out the optimum combination of concentration and temperature. The technique was targeted to the maximum value of overall desirability function (D) calculated by the following equation (Eq. 5):
where Di is the desirability index for an ith response (here i = 1 to 4) and r is its relative importance whose value ranges from 1 to 4. Both, overall and individual desirability indices ranged from 0 to 1. As a whole, the target was to maximize the G′0 and G″0 moduli along with the minimum slope values n′ and n″ of reconstituted A. vera hydrogels. Constraints, limits, and relative importance of each parameter were set according to the desirability. Retention of the higher gel strength with minimum slope values was accorded priority for optimization. The predicted numerical optimization values were further validated by performing the experiment at the nearest possible process condition.
Mechanistic and morphological insight of optimized reconstituted A. vera hydrogels
To understand the insight of gelation process, the effect of aging time and morphology of optimized reconstituted hydrogels was studied using creep, and high-resolution transmission electron microscope (HRTEM) analysis.
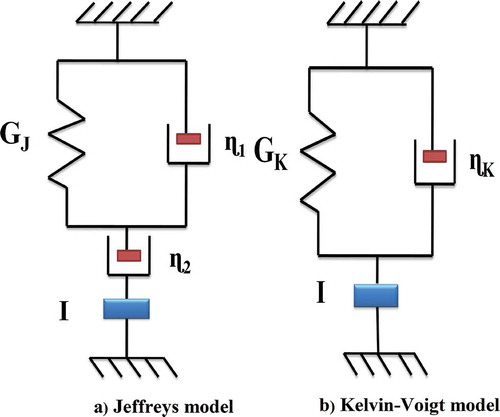
Creep analysis
Reconstituted A. vera hydrogels prepared as described in “Preparation of reconstituted A. vera hydrogels” section and after heating the sample at 50ºC/30 min further cooled under running tap water for 5 min. Then the sample was directly submitted for creep experiments where small stress (σ), i.e., 10 Pa selected from LVR zone was applied. The variation of shear strain (γ) in response to applied stress was measured for 300 s. This test repeated for 30 min of the interval until creep compliance (Jc) of the samples showed minimal changes between consecutive creep tests which took approximately 3 h.[Citation10] The generalized Jeffrey’s and Kelvin-Voigt models were used to calculated the viscoelastic moduli (viz., G′J, G″J for Jeffrey’s, and G′K, G″K for Kelvin-Voigt) behavior with short time creep response of the material from strain data of the shear based creep experiment.[Citation19] Jeffrey model consists of three parameters (one spring and two dashpots) as shown in . In this model single mode viscous element (dashpot) arranged in series with a Kelvin-Voigt model, which consists of an elastic element (a Hookean Spring) in parallel to another dashpot; GJ, η1, and η2. Moreover, addition of fourth element I, i.e., instrument inertia is the source of creep ringing behavior also represented. The creep strain (γ) behavior of reconstituted A. vera gels were fitted to the Jeffrey’s expression (Eq. 6).[Citation19]
Figure 1. Schematic representation of rod and spring for (a) Jeffreys model and (b) Kelvin-Voigt model.
where
where γ (t) is strain of the system, I is the moment of inertia (measuring geometry plus rheometer drive). The parameter B is the combination of form factors (i.e., B = C2/C1) where, each measuring system used in an instrument will be associated to form factors which convert torque and angular velocity to shear stress and shear rate, respectively. The factor C2 = shear rate/angular velocity (dimensionless), and C1 = Shear stress/applied torque (units: m−3). The viscoelastic storage (G′J) and loss (G″J) moduli of Jeffrey’s model were determined using the following expressions.
where,
Here, λ1 and λ2 are considered as relaxation and retardation time, respectively. However, Kelvin-Voigt model consists of two parameters, i.e., spring and dashpots arranged in series as shown in . In this elastic element is a Hookean Spring; Gk (Pa-m) and viscous element is dash pot ηk (Pa-s-m). Moreover, addition of third elements I, i.e., instrument inertia is the source of creep ringing behavior also represented. The creep strain (γ) behavior of reconstituted A. vera gel were further fitted to the following equation (Eq. 7).
The viscoelastic moduli (G′K and G″K) of Kelvin-Voigt model is determined by the following expression.
High resolution transmission electron microscopy (HRTEM) analysis of A. vera xerogel
Xerogel for HRTEM analysis was obtained by placing a fine drop of reconstituted A. vera fluids of 0.2–1.6%, w/v concentrations on a carbon-coated HRTEM copper grid (Quantifoil, Germany) and air dried at room temperature (27 ± 1°C). Further, the specimen was kept for drying in desiccators for 24 h and then transferred to HRTEM (Model: JEM–2100 HRTEM, Make: JEOL, Japan) operating at 20 kV and micrographs were obtained at magnificence of 0.2 μm.[Citation22,Citation23]
Statistical data analysis
Statistical analysis was carried out on the data from duplicate analysis with two replicates (n = 4) was carried out for rheological model parameters. Model significance was tested using the analysis of variance (ANOVA) test at 95% confidence of interval. ANOVA test was performed using Design expert 7.0 (Stat-Ease, Inc., Minneapolis, MN, USA) software, whereas, SPSS-20 software (IMB®, SPSS® Statistics 20, Armonk, NY, USA) was used for statistical significance.
Results and discussion
NMR data distribution of A. vera powder
To understand the structural complexity in partially purified powder, NFAIR was subjected to NMR spectroscopy. In general, 1H NMR technique is being widely utilized to validate the authenticity and quality of A. vera powders, by monitoring the presence of polymeric sugar (i.e., acetylated mannan), organic acids and free sugars.[Citation3,Citation24,Citation25] However, alcohol insoluble residue of A. vera mainly consists of linear polysaccharide, which is composed of partially acetylated mannan with β-(1→4)-linkage.[Citation3] The 1H NMR spectrum identifies characteristic signal at 2.0–2.3 ppm for acetyl groups and mass of signals at 3.4–4.5 ppm assignable to a carbohydrate moiety.[Citation3,Citation25] Besides, the quality of A. vera powder was also determined from the presence of organic acids formed due to microbial contamination, fermentation (viz., lactic acid peak at 1.34, 2.00, 4.27, 11.00 ppm and succinic acid at 2.50, 11.00 ppm), chemical degradation (viz., acetic acid at 2.08, 11.00 ppm) and natural malic acid (3.4 ppm).[Citation24,Citation25] The presence of lactic and succinic acid in A. vera powders indicates the degradation of bioactive acetylated polysaccharides.
In the present context, NMR profile presented in for A. vera (NFAIR) of concentration 5 mg/mL showed signal intensity at 2.0–2.3 ppm indicating the presence of acetylating part due to ethanol treatment. Similar observations were reported earlier for A. vera powders.[Citation3,Citation24,Citation25] Further, low-intensity signals were observed for lactic and succinic acid indicating minimal degradation due to microbial contamination. Moreover, chemical degradation also seems to be absent as peaks associated with the acetic acid were not detected. Natural organic acid such as malic acid peaks were noticed in the powder. This might be due to plants metabolism which helps to form the malic acid via Crassulacean acid metabolic (CAM) pathway. Further, presence of carbohydrate moiety represented the mass of signal peaks in the range of 3.4–4.5 ppm. The presence of glucose moiety was also noticed at 5 and 4.5 ppm peaks.[Citation26] Therefore, obtained results indicated that the A. vera NFAIR powder consisted of acetylated polysaccharide, thus can further be utilized in a wide varieties of food and therapeutic applications.[Citation1,Citation27,Citation28]
Dynamic oscillation behavior of reconstituted A. vera hydrogel
The effect of concentrations (0.2–1.6%, w/v) and temperatures (30–60°C) on the G′0 and G″0 values obtained through Power law model were assessed. Interestingly for all combinations of concentration and temperature of reconstituted A. vera aqueous fluids, higher correlation coefficients for G′0 (R2 > 0.97) and G″0 (R2 > 0.86) were obtained as shown in . Further, test temperatures above 60°C showed deviated viscoelastic moduli, i.e., absence of LVR zone over the selected stress sweep region (0.01 to 100 Pa), which indicated the disruption of internal gel networks.[Citation8,Citation11,Citation29]
Table 1. Power law model parameters (G′0, n′, G″0 and n″) of reconstituted A. vera gels prepared at various concentrations 0.2–1.6 %, w/v and temperatures 30–60°C.
Effect of concentration
The conformational transition for A. vera (NFAIR) samples at various concentrations ranging from 0.2–1.6%, w/v at 30°C were noticed during the frequency sweep test (). At lower A. vera concentration in the range of 0.2–0.4%, w/v throughout the frequency sweep, the dominance of G″ over G′ was observed indicating a solution-like behavior of the gel which might be due to the existence of isolated polymers.[Citation3,Citation7,Citation29] The reconstituted A. vera hydrogels at 0.8%, w/v and low frequency showed solution-like (i.e., Gʹʹ > Gʹ) nature which revealed the presence of entanglement system in the terminal zones. At high frequency, it showed gel-like behavior (i.e., Gʹ > Gʹʹ), which indicated the semi-diluted solution nature ().[Citation30–Citation32] The conformational shift from solution to semi-diluted solutions might be hypothesized due to the collection of polymeric groups cross linked with adjacent counterparts and impurities homogeneously at a higher A. vera concentration. A similar type of behavior was documented for food hydrocolloids such as pectin, gellan, and xanthan, etc.[Citation33,Citation34]
Figure 3. Viscoelastic behavior of reconstituted A. vera hydrogels at various concentration of 0.2-1.6% (w/v) measured at 30°C.
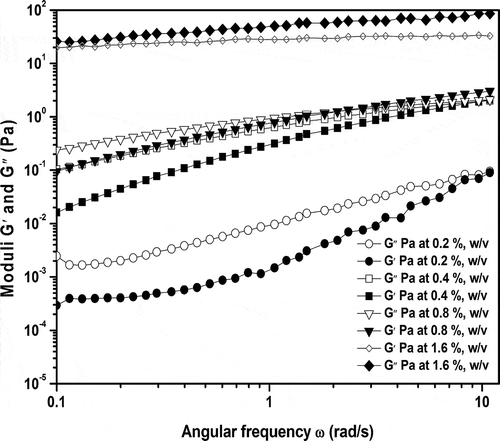
The higher concentration samples viz., 1.6%, w/v throughout the frequency sweep indicated the gel-like network formation as characterized by increase in magnitude of G′. Furthermore, at all concentrations sample started flowing on tilting within 3–15 min, indicating the weak gel behavior.[Citation8] Similar kind of concentration effects were also reported in case of welan, rhamsan, gellan,[Citation33,Citation34] and soya proteins.[Citation35] Moreover, disappearance of terminal regions at low frequency were noticed in case of 1.6%, w/v concentration indicating the conformational transition from disordered semi-diluted solution regime to structured gel-like networks.[Citation33,Citation34,Citation36] The reason may be increase in concentration caused tenuous association of rigid and ordered molecular structures in reconstituted hydrogels by weak forces of interactions such as hydrogen and hydrophobic bonds.[Citation3,Citation27,Citation28] Also the presence of internal trace elements (mainly Ca), as noticed by semi-elemental detection (EDS; data not shown) might be responsible for minor electrostatic interactions with low degree of esterified galacturonic acid moiety.[Citation5,Citation8,Citation37] The obtained results suggested that reconstituted aqueous NFAIR fractions showed semi-diluted solution at lower concentration, whereas, it attained typical gel-like behavior at higher concentration. Therefore, selection of proper concentration might influence the gel strength and conformational transition by creating additional networks.
Effect of temperature
The temperature (30–60°C) dependency on the Power law parameters G′0 and G″0 at various concentrations of A. vera hydrogels (0.2–1.6%, w/v) were also described by Arrhenius model Eq. 3. Moderate correlation coefficient for G′0 (R2 > 0.84) and G″0 (R2 > 0. 85) were obtained except for G″0 at 1.6%, w/v concentration, where R2 > 0.75. The activation energy (Ea) values ranged from 13.08–55.93 kJ/mol and found to be inversely proportional with concentration, indicating probably that high energy barrier required at lower concentration, where less energy barrier required at higher concentration to develop A. vera gelation networks. At lower concentrations dilute polymeric groups might have participated in random arrangements, whereas at higher concentration of 1.6%, w/v internal polymeric units possibly participated in chain-chain interaction by crosslinking with adjacent molecules by weak forces of interactions, thus A. vera gel networks were less affected by thermal treatment.[Citation38,Citation39] The obtained results suggested that increase in concentration on reconstitution promoted the formation of A. vera gel networks.[Citation39,Citation40] Similar type of temperature effects on flow behavior was noticed in case of concentrated A. vera juices.[Citation11]
Combined effect of temperature and concentration using RSM
The combined effects of concentration and temperature on viscoelastic moduli were analyzed using Power law model (), wherein the model parameters viz., G′0, n′, G″0, and n″ as responses were evaluated using the experimental design Eq. (5). The combined effects of concentration and temperature on the above mentioned parameters were assessed using ANOVA test. An empirical quadratic equation was developed for all the selected responses with a good fit (R2 > 0.87) at the coefficients of the empirical equations. The F-value was used to indicate the influence of the model terms on the selected response variables. The constant term was significant (p < 0.05) for each of the model responses. The multivariate analysis showed that the linear and square terms of A. vera concentration significantly influenced (p < 0.05) the viscoelastic moduli, whereas, the change in temperature (linear and square terms), and its interaction term with concentration were not statistically significant for all the responses (p > 0.1).
Localization of optimization zone for power law fitted viscoelastic parameters
The developed polynomial models for G′0, n′, G″0, and n″ described their dependency on two independent variables viz., temperature and concentration including linear, interaction and square terms. To visualize the interaction between two independent parameters affecting the response, contour plots were generated from the empirical equation.
Power law parameters
Intercepts of storage (G′0) and loss (G″0) moduli
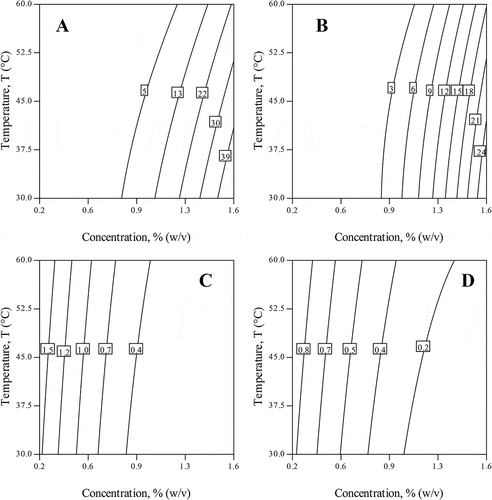
The linear and interaction terms of temperature and concentration, and square term of concentration (p < 0.05) were significantly affected both G′0 and G″0. The F-value for concentration term was found to be higher for G′0 (411.4) and for G″0 (641.7) than its interaction and square terms, which suggested that concentration was a major contributing factor for the reconstituted hydrogel formation. The contour plots of G′0 and G″0 (in and ) for reconstituted fluids indicated that above 0.8%, w/v concentration, the moduli values increased possibly due to internal polymer association.[Citation3,Citation4] The contour also showed the antagonistic effect between temperature and concentration on viscoelastic moduli. Further, lower temperature (30°C) and higher concentration (1.6%, w/v) favored increase in viscoelastic moduli (G′0 and G″0) due to internal network formation of adjacent polymeric chains by hydrogen and hydrophobic interactions. Besides, moduli values decreased with an increase in temperature over the frequency range studied, which, might be due to disruption of junction zones stabilized by hydrogen, hydrophobic and electrostatic interactions.[Citation38,Citation39] Similarly effect of temperatures on the flow behavior was also noticed in case of clear juices,[Citation29] concentrated juices[Citation11] and reconstituted fractions of A. vera.[Citation4]
Slopes of storage (n′) and loss (n″) moduli
The linear and square terms of concentration were significantly (p < 0.05) affected both slopes n′ and n″ values, respectively; whereas, temperature associated terms were not significant (p > 0.1). The F-value for concentration (linear term) was found to be higher for n′ (65.8) and for n″ (113.6) than temperature (linear, interactions and square terms) indicating concentration was a major contributing factor for reconstituted A. vera gel formation. According to Ferry,[Citation41] decrease in slope (n′) toward zero indicates rubbery elastic material property, whereas n′ values approaching toward 2 indicates liquid behavior. In general, the formation of 3D networks is found when slope values are near to zero. The contour plot on concentration-temperature landscape was showed in and . With decrease in concentration the n′ values varied between 0.3 to 1.5 and n″ values from 0.2 to 0.8. At lower concentration (<0.6%, w/v) the counters for n′ were higher and almost linear with temperature axis indicating additive effect on n′. This indicated that, below <0.6%, w/v concentration, there was existence of isolated polymers which were unable to participate in network formation indicating solution.[Citation30] The contour plot, at higher concentration (>0.6%, w/v) and low temperature showed decrease in slope (n′) values, which might be due to the cumulative effects of weak forces of interaction that participate in chain-chain interaction with the adjacent polymer units which in turn helps in 3D network formation.[Citation2,Citation30,Citation42]
Optimization and validation
The optimization for formation of reconstituted A. vera hydrogel generally demands higher concentration, minimum temperature in order to avoid internal bond dissociation. The processing conditions were optimized based on maximum viscoelastic values and minimum slope values for attainment of 3D network formation (). The optimized conditions through numerical optimization suggested that concentration of 1.6%, w/v and temperature of 30°C was found to be best combination with a maximum desirability of 0.99. Further, model validation was attempted at optimized condition (1.6%, w/v and 30°C). Predicted results were compared with the actual data at optimized condition. It was found that prediction corresponding to each response was in good agreement with the experimental data, where the average absolute relative deviation was 7.91% (). From the obtained RSM results it was observed that concentration was a major contributing factor in comparison with temperature (linear, interaction, and square terms), for increasing moduli (G′0 and G″0) and minimizing the slope (n′ and n″) values.
Table 2. The set of constrains for various parameters targeting the high quality gel through combination of concentration and temperature and experimental data validation of optimized gel responses.
Mechanistic and morphological insight of reconstituted A. vera hydrogels
Effect of creep time on viscoelastic moduli during initial aging of reconstituted A. vera gel
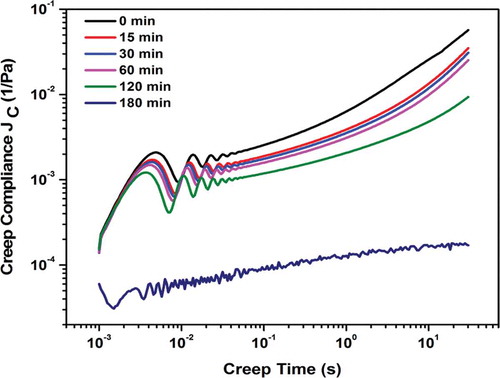
The effect of creep compliance (Jc) as a function of initial aging time (t) during undisturbed conditions on the optimized reconstituted gel was noticed at 1.6%, w/v with different time intervals (0–180 min) as shown in . The creep behavior at all time periods (0–180 min) showed damping oscillation which might be due to coupling between the instrument inertia and viscoelastic nature of the hydrogel.[Citation19,Citation43] This phenomenon, called creep ringing, has been observed for biopolymer gels such as Bovine serum albumin (BSA),[Citation44] mixture of agar and carrageenan,[Citation45] and silk fibroin gels.[Citation10] Further, damped oscillation strain response was found to be increased with gelation time between 0 and 180 min for 1.6%, w/v (). This data was further fitted to Jeffrey’s and Kelvin-Voigt models for calculation of viscoelastic moduli from creep compliance (Jc) as stated in Eqs. (6) and (7). All models showed that gel strength was increased with time during free oscillation, due to internal network re-association of A. vera polysaccharide ().[Citation8,Citation27,Citation28] Similar observations were also reported for protein-surfactant mixtures,[Citation44] and silk fibroin solution.[Citation10] Among the two models fitted, Jeffrey’s model was found to be the best (R2 > 0.96), to determine viscoelastic moduli (G′J and G″J) as represented in . The model fitted to the creep ringing data can be applicable for short gelation time scale (i.e., up to 120 min) to calculate the viscoelastic moduli in reconstituted A. vera hydrogels. However, after 180 min the damped oscillation increased to a larger extent which might be due to faster gelation, thus further restricted the model fitting. The storage modulus (G′J) increased for short gelation time scale from 0–120 min (i.e., 45 to 80 Pa). This indicates gel strength increased with initial aging time during undisturbed condition, as 3D network were expected to develop and later reinforced by intermolecular forces. The results suggested that reconstituted hydrogels left for 2 h helps in attainment of higher gel strength. In the present study only short time gelation kinetics using free oscillation were attempted due to the instrument limitation and sample history over long time periods. Further, detailed investigations on the effect of long time using creep (free) and time sweep (forced) oscillation are needed to understand the gelation mechanism of reconstituted A. vera hydrogel.
Table 3. Jeffrey’s and Kelvin-Voigt models parameters of optimized reconstituted A. vera hydrogels at different aging time intervals of 0–120 min.
HRTEM morphology of reconstituted hydrogel
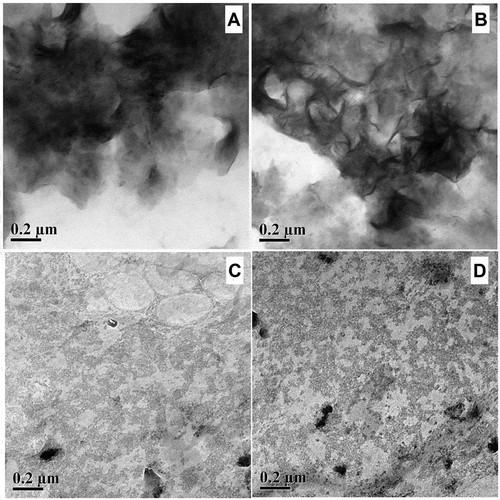
HRTEM micrograph images of reconstituted A. vera xerogel with 0.2–1.6%, w/v concentration showed in . At lower concentration of 0.2–0.4%, w/v, presence of dense entanglements and randomly organized networks were noticed at both magnification of 0.2 μm, which might be due to insufficient polymeric units to interact with the surrounding aqueous medium ( and ). At higher concentration of 0.8–1.6%, w/v, distinct surface morphology with the existence of distributed network-like pattern was noticed ( and ). The reason might be functional groups participated in chain-chain interaction with adjacent polymeric units that in turn gives network like appearance. The dense entanglements further dispersed into aqueous medium by enhancing the biopolymer concentration, and formed soluble aggregates above 0.4%, w/v concentration.[Citation46] In addition, individual circular patches filled with distributed networks were noticed for 1.6%, w/v (). The reason may be intermolecular hydrogen bonding between water and polymer chains at molecular levels leading to greater network formation.[Citation27,Citation28,Citation47] It is fairly predictable that increase in concentration helps in the formation of internal networks which in turn enhances the gel strength.[Citation8]
Conclusions
The presence of bioactive acetyl rich polysaccharides in NFAIR powder were analyzed using NMR. Conformation transition from semi-diluted solution nature to typical gel-like nature was noticed by varying concentrations from 0.2 to 1.6%, w/v. The effect of different temperatures on Power law parameters (viz., G′0 Pa.sn′ and G′′0 Pa.sn″) were evaluated by Arrhenius type equation and the activation energy (Ea) values, were found to be inversely proportional to concentration. Multivariate analysis indicated that concentration had the most significant effect on rheological attributes such as (G′0, G″0, n′, and n″) followed by temperature and their interaction. Numerical optimization suggested that 1.6%, w/v at 30°C was found to be the desirable combination for attaining higher gel strength and minimum slope values of reconstituted A. vera hydrogel. Viscoelastic moduli (G′J and G″J) increased from 45 to 80 Pa with initial aging time (0–120 min) as demonstrated by damping oscillatory response of the creep compliance. The obtained results suggested that reconstituted A. vera hydrogels with optimized conditions (1.6%, w/v at 30°C) found to impart higher gel strength with network formation, indicating its possible application as an food hydrocolloid for enhancing the texture of gel-like products.
Funding
The first author thankfully acknowledges partial support by National Agricultural Innovation Project (NAIP), Indian Council of Agricultural Research (ICAR), New Delhi, India for this research.
References
- Eshun, K.; He, Q. Aloe vera: A Valuable Ingredient for the Food, Pharmaceutical and Cosmetic Industries—A Review. Critical Reviews in Food Science and Nutrition 2004, 44(2), 91–96.
- Ramachandra, C.T.; Rao, P.S. Processing of Aloe Vera Leaf Gel: A Review. American Journal of Agricultural and Biological Sciences 2008, 3(2), 502–510.
- Campestrini, L.H.; Silveira, J.L.M.; Duarte, M.E.R.; Koop, H.S.; Noseda, M.D. NMR and Rheological Study of Aloe Barbadensis Partially Acetylated Glucomannan. Carbohydrate Polymers 2013, 94(1), 511–519.
- Kiran, P.; Rao, P.S. Rheological and Structural Characterization of Prepared Aqueous Aloe Vera Dispersions. Food Research International 2014, 62, 1029–1037.
- McConaughy, S.D.; Stroud, P.A.; Boudreaux, B.; Hester, R.D.; McCormick, C.L. Structural Characterization and Solution Properties of a Galacturonate Polysaccharide Derived from Aloe Vera Capable of in Situ Gelation. Biomacromolecules 2008b, 9(2), 472–480.
- Nindo, C.I.; Powers, J.R.; Tang, J. Thermal Properties of Aloe Vera Powder and Rheology of Reconstituted Gels. Transactions of the ASABE 2011, 53(4), 1193–1200.
- Cervantes-Martínez, C.V.; Medina-Torres, L.; González-Laredo, R.F.; Calderas, F.; Sánchez-Olivares, G.; Herrera-Valencia, E.E.; Gallegos Infante, J.A.; Rocha-Guzman, N.E.; Rodríguez-Ramírez, J. Study of Spray Drying of the Aloe Vera Mucilage (Aloe Vera Barbadensis Miller) as a Function of Its Rheological Properties. LWT–Food Science and Technology 2014, 55(2), 426–435.
- Kiran, P.; Rao, P.S. Development and Characterization of Reconstituted Hydrogels Prepared from Aloe Vera (Aloe Barbadensis Miller) Powder. Journal of Food Measurement and Characterization 2016, 10(3), 411–424. DOI:10.1007/s11694-016-9320-5
- Axelos, M.A.V.; Thibault, J.F. The Chemistry of Low-Methoxyl Pectin Gelation. In The Chemistry and Technology of Pectin; Walter, R.H.; Ed.; Academic Press: San Diego, CA, 1991; 109–118.
- Nagarkar, S.; Patil, A.; Lele, A.; Bhat, S.; Bellare, J.; Mashelkar, R.A. Some Mechanistic Insights into the Gelation of Regenerated Silk Fibroin Sol. Industrial & Engineering Chemistry Research 2009, 48(17), 8014–8023.
- Swami Hulle, N.R.; Kiran, P.; Rao, P.S. Rheological Properties of Aloe Vera (Aloe Barbadensis Miller) Juice Concentrates. Journal of Food Process Engineering 2014, 37(4), 375–386.
- Andresen, I.L.; Smidsørod, O. Temperature Dependence of the Elastic Properties of Alginate Gels. Carbohydrate Research 1977, 58(2), 271–279.
- Richardson, R.K.; Ross-Murphy, S.B. Mechanical Properties of Globular Proteins Gels: 1. Incipient Gelation Behaviour. International Journal of Biological Macromolecules 1981, 3(5), 315–322.
- Donato, L.; Garnier, C.; Novales, B.; Doublier, J.L. Gelation of Globular Protein in Presence of Low Methoxyl Pectin: Effect of Na+ and/or Ca2+ Ions on Rheology and Microstructure of the Systems. Food Hydrocolloids 2005, 19(3), 549–556.
- Dak, M.; Verma, R.C.; Jaaffrey, S.N.A. Effect of Temperature and Concentration on Rheological Properties of “Kesar” Mango Juice. Journal of Food Engineering 2007, 80(4), 1011–1015.
- Ahmed, J.; Ramaswamy, H.; Ngadi, M. Rheological Characteristics of Arabic Gum in Combination with Guar and Xanthan Gum Using Response Surface Methodology: Effect of Temperature and Concentration. International Journal of Food Properties 2005, 8(2), 179–192.
- Thakur, B.R.; Singh, R.K.; Handa, A.K.; Rao, M.A. Chemistry and Uses of Pectin—A Review. Critical Review in Food Science and Nutrition 1997, 37(1), 47–73.
- Menard, K.P. Dynamic Mechanical Analysis: A Practical Introduction, 2nd ed.; CRC Press/Taylor and Francis Group: New York, NY, 2008; 1–240.
- Ewoldt, R.H.; McKinley, G.H. Creep Ringing in Rheometry or How to Deal with Oft-Discarded Data in Step Stress Tests! Rheology Bulletin 2007, 76(1), 4–24.
- Ahmed, J.; Thomas, L. Effect of β-Glucan Concentrate on the Water Uptake, Rheological and Textural Properties of Wheat Flour Dough. International Journal of Food Properties 2015, 18(8), 1801–1816.
- Montgomery, D.C. Design and Analysis of Experiments; John Wiley & Sons, Inc.: U.K., 2010.
- Patra, T.; Pal, A.; Dey, J. Birefringent Physical Gels of N-(4-N-Alkyloxybenzoyl)-L-Alanine Amphiphiles in Organic Solvents: The Role of Hydrogen-Bonding. Journal of Colloid and Interface Science 2010, 344(1), 10–20.
- Agoda-Tandjawa, G.; Durand, S.; Gaillard, C.; Garnier, C.; Doublier, J.L. Rheological Behaviour and Microstructure of Microfibrillated Cellulose Suspensions/Low-Methoxyl Pectin Mixed Systems. Effect of Calcium Ions. Carbohydrate Polymers 2012, 87(2), 1045–1057.
- Davis, B.; Goux, W.J. Single-Laboratory Validation of an NMR Method for the Determination of Aloe Vera Polysaccharide in Pharmaceutical Formulations. Journal of AOAC International 2009, 92(6), 1607–1616.
- Ray, A.; Aswatha; Shashaank, M. An Analysis of the Influence of Growth Periods on Physical Appearance, and Acemannan and Elemental Distribution of Aloe Vera L. Gel. Industrial Crops and Products 2013, 48, 36–42.
- Bozzi, A.; Perrin, C.; Austin, S.; Arce Vera, F. Quality and Authenticity of Commercial Aloe Vera Gel Powders. Food Chemistry 2007, 103(1), 22–30.
- Chokboribal, J.; Tachaboonyakiat, W.; Sangvanich, P.; Ruangpornvisuti, V.; Jettanacheawchankit, S.; Thunyakitpisal, P. Deacetylation Affects the Physical Properties and Bioactivity of Acemannan, an Extracted Polysaccharide from Aloe Vera. Carbohydrate Polymers 2015, 133, 556–566.
- Lim, Z.X.; Cheong, K.Y. Effects of Drying Temperature and Ethanol Concentration on Bipolar Switching Characteristics of Natural Aloe Vera-Based Memory Devices. Physical Chemistry Chemical Physics 2015, 17(40), 26833–26853.
- Lad, V.N.; Murthy, Z.V.P. Rheology of Aloe Barbadensis Miller: A Naturally Available Material of High Therapeutic and Nutrient Value for Food Applications. Journal of Food Engineering 2013, 115(3), 279–284.
- Alonso-Mougán, M.; Meijide, F.; Jover, A.; Rodrı́guez-Núñez, E.; Vázquez-Tato, J. Rheological Behaviour of an Amide Pectin. Journal of Food Engineering 2002, 55(2), 123–129.
- Lopes da Silva, J.A.; Gonçalves, M.P.; Doublier, J.L.; Axelos, M.A.V. Effect of Galactomannans on the Viscoelastic Behaviour of Pectin/Calcium Networks. Polymer Gels and Networks 1996, 4(1), 65–83.
- Picout, D.R.; Ross-Murphy, S.B. Rheology of Biopolymer Solutions and Gels. The Scientific World Journal 2003, 3, 105–121.
- Morris, E.R.; Gothard, M.G.E.; Hember, M.W.N.; Manning, C.E.; Robinson, G. Conformational and Rheological Transitions of Welan, Rhamsan, and Acylated Gellan. Carbohydrate Polymers 1996, 30(2), 165–175.
- Morris, E.R.; Nishinari, K.; Rinaudo, M. Gelation of Gellan—A Review. Food Hydrocolloids 2012, 28(2), 373–411.
- Chronakis, I.S. Network Formation and Viscoelastic Properties of Commercial Soy Protein Dispersions: Effect of Heat Treatment, pH and Calcium Ions. Food Research International 1996, 29(2), 123–134.
- Fitzsimons, S.M.; Mulvihill, D.M.; Morris, E.R. Co-Gels of Whey Protein Isolate with Crosslinked Waxy Maize Starch: Analysis of Solvent Partition and Phase Structure by Polymer Blending Laws. Food Hydrocolloids 2008, 22(3), 468–484.
- Rodríguez-González, V.M.; Femenia, A.; González-Laredo, R.F.; Rocha-Guzmán, N.E.; Gallegos-Infante, J.A.; Candelas-Cadillo, M.G.; Ramírez-Baca, P.; Simal, S.; Rosselló, C. Effects of Pasteurization on Bioactive Polysaccharide Acemannan and Cell Wall Polymers from Aloe Barbadensis Miller. Carbohydrate Polymers 2011, 86(4), 1675–1683.
- Da Silva, J.A.L.; Rao, M.A. Pectins: Structure, Functionality, and Uses. In Food Polysaccharides and Their Applications; Alistair, M.S.; Glyn, O.P.; Peter, A.W.; Eds.; CRC Press/Taylor & Francis Group: London/New York, NY, 2006; 353–411.
- Alvarez, M.D.; Canet, W. Dynamic Viscoelastic Behavior of Vegetable-Based Infant Purees. Journal of Texture Studies 2013, 44(3), 205–224.
- Lopes da Silva, J.A.; Rao, M.A. Pectins: Structure, Functionality, and Uses. In Food Polysaccharides and Their Applications; Stephen, A.M.; Philips, G.O.; Williams, P.A.; Eds.; CRC Press Inc: Boca Raton, FL/New York, NY, 2006; 353–411.
- Ferry, J.D. Viscoelastic Properties of Polymers, 3rd ed.; John Wiley & Sons: New York, NY, 1980; 1–641.
- Georgopoulos, T.; Larsson, H.; Eliasson, A.-C. A Comparison of the Rheological Properties of Wheat Flour Dough and Its Gluten Prepared by Ultracentrifugation. Food Hydrocolloids 2004, 18(1), 143–151.
- Baravian, C.; Quemada, D. Using Instrumental Inertia in Controlled Stress Rheometry. Rheologica Acta 1998, 37(3), 223–233.
- Jaishankar, A.; Sharma, V.; McKinley, G.H. Interfacial Viscoelasticity, Yielding, and Creep Ringing of Globular Protein-Surfactant Mixtures. Soft Matter 2011, 7(17), 7623–7634.
- Norziah, M.H.; Foo, S.L.; Karim, A.A. Rheological Studies on Mixtures of Agar (Gracilaria Changii) and κ-Carrageenan. Food Hydrocolloids 2006, 20(2–3), 204–217.
- Walkenström, P.; Hermansson, A.-M. Mixed Gels of Gelatin and Whey Proteins, Formed by Combining Temperature and High Pressure. Food Hydrocolloids 1997, 11(4), 457–470.
- Souza, C.J.F.; Garcia Rojas, E.E.; Melo, N.R.; Gaspar, A.; Lins, J.F.C. Complex Coacervates Obtained from Interaction Egg Yolk Lipoprotein and Polysaccharides. Food Hydrocolloids 2013, 30(1), 375–381.