Abstract
Black-eyed pea starch was hydrolyzed using concentrated HCl (36% by weight) at different levels (10–60 mL) in the presence of methanol and the physico-chemical properties of native and acidified methanol modified black-eyed pea starches were compared. Results revealed high recovery (>93%) of acid-alcohol treated black-eyed pea starch upon modification. A drop in swelling power and increase in solubility was also observed during acidified methanol treatment. Acid hydrolysis increased paste clarity and the freeze–thaw stability; however, the trend was found to vary at a specific modification level. Acid thinning of starch revealed significant decrease in gel consistency, sediment volume, water, and oil binding capacity. Disruption of interactions between amylose chains resulted in increased amylose leaching on modification. The X-ray diffraction pattern of black-eyed pea starch was of C-type obtained and with increase in acid concentration the intensity of peak was found to be increased. Increased acid concentration showed significant increase in crystallinity; however, marked loss of crystallinity was observed at an acid level of 40 mL which further increased on acid concentrations of 50 and 60 mL. Also, acid concentration showed significantly improved post-reaction color difference (∆E) of the modified starches.
INTRODUCTION
In recent years, the demand of modified starch has increased due to their wide usage in food and non-food applications.[Citation1] Extensive research has already been conducted on cereal, potato, and sweet potato and cassava starches; however, availability of these resources to starch industries is decreasing rapidly due to their increased demand in the production of breakfast cereals and snacks.[Citation2,Citation3] Legumes in contrast are a great source for the isolation of edible protein with starch as the major by-product.[Citation4] Furthermore, the use of legume starch in food formulation has been assumed to be of great importance and also gained considerable attention of food processors, marketers, and consumers,[Citation5] due to their wide range functionality in products such as breads, cakes, and biscuits.[Citation6,Citation7] Moreover, current opinion on healthy eating habits may result in an increase in the proportion of legume starches as an alternative to cereals.[Citation8] Therefore, considering the above facts for profitable protein production; legume starch can be utilized for diverse industrial applications which will eventually lessen the over-dependence on the more familiar starch sources. Black-eyed pea (Vigna unguiculata) member of family Leguminosae, native to Asia and Africa, has a number of commonly used names, e.g., southern pea, cow pea, and crowder pea,[Citation9] is a relatively inexpensive legume with high protein (19-40%) and carbohydrate (50–65%) contents. Also, black-eyed pea is drought-tolerant, shade tolerant, and a warm-weather crop that grows well in poor soils and adds nitrogen to it. Therefore, for protein production to be economical and as an alternative source for starch production, black-eyed pea has a potential to be utilized as an alternative starch source for diverse industrial applications. Native starches have many limitations such as low shear resistance, thermal resistance, a high tendency toward retro-gradation, thermal decomposition and high syneresis, less stability toward extreme processing conditions such as pH, temperature, etc., that reduce their use at the industrial level.[Citation10] High amylose content of legume starches also has a tendency to confer different functional properties in various food applications as compared to popular starch sources.[Citation11] However, the diversity of the modern food industry and the enormous variety food products require starch to be modified in such a way that it is able to tolerate a wide range of processing techniques as well as various distribution, storage, and final preparation conditions.[Citation12] With the advances made so far, it is possible to restructure native starch by means of modifying agents that involves the alteration of its physico-chemical properties by affecting the hydrogen bond in a controllable manner. Use of acid-alcohol treatment for producing thin-boiling starches is gaining popularity owing to higher recovery of modified starch and use of less acid as compared to acid alone.[Citation13] Limited attempts have been made so far to access the functional properties of acidified methanol modified black-eyed pea starches. Therefore, the present investigation was carried out to study the effect of acid-alcohol modification on the functional properties of black-eyed pea starch in order to provide an innovative specialty starch to meet various specific and targeted use in the food industry.
MATERIALS AND METHODS
Materials
The black-eyed pea sample was procured from a local market of Amritsar. Sodium hydrogen sulphite, hydrochloric acid, methanol, ethanol, potassium iodide, iodine, and potassium hydroxide were procured from Thermo Scientific India Ltd., Mumbai. Amylose (859656, amylose from potatoes) was procured from Sigma Aldrich St. Louis, MO, USA. De-ionized water and acid washed glassware were used throughout the experiments.
Starch Isolation
Starch was isolated from black-eyed pea seeds by following the method of Singh et al.[Citation14] Seeds (300 g) were steeped in deionized water containing 0.16% sodium hydrogen sulphite for 12 h at 50°C in an oven (Universal hot air oven Narang Scientific Instruments, Ambala). The steep water was decanted and softened legumes were ground in an auto-mix blender (50C/S, Remi Anupam Mixie Ltd., Bombay) for 3 min in de-ionized water at slow speed. The ground slurry was sieved through nylon cloth (100 mesh). The material left over the nylon cloth was washed thoroughly with de-ionized water. The filtrate was allowed to stand for 1 h. The supernatant was discarded and the settled starch layer was re-suspended in de-ionized water and centrifuged at 3200 × g for 10 min. The upper non-white layer was scraped off. The white layer was re-suspended in distilled water and re-centrifuged at 3200 × g for 10 min and the step was repeated three times. Starch was then collected and dried in an oven at 40°C for 12 h.
Acid-Alcohol Modification
Isolated starch was modified following the method of Lin et al.[Citation13] with slight modifications. Starch (25 g) was suspended in 100 mL methanol in a 250 mL glass stopper flask. The suspension was stirred using a magnetic stirrer (Spinot MC 02, Tarsons Products Pvt. Ltd.; Kolkata, India) for 30 min and maintained at 25°C. Reaction was started by adding 10–60 mL of concentrated hydrochloric acid (36% by weight). The reaction was stopped after 1 h by adding 14 mL of 1 M sodium bicarbonate and then cooled in an ice bath. The starch was centrifuged at 3500 × g (Kubota Corporation, Gyeonggi, South Korea) for 5 min and washed four times with 50% ethanol. The white precipitates were dried in an oven at 40°C for 12 h and ground to a fine powder using a mortar and pestle.
Amylose Content
Amylose content of the isolated starch was determined as described by Williams et al.[Citation15] Iodine stock solution and iodine reagent were prepared. For the stock solution, 20 g potassium iodide was weighed into 100 mL beaker, together with 2 g resublimed iodine. The reagents were dissolved in de-ionized water and carefully diluted to 100 mL into volumetric flask. For iodine reagent stock solution (10 mL) was pipetted into a 100 mL volumetric flask and diluted to 100 mL using distilled water. Starch sample (20 mg) was weighed into a 100 mL beaker. Exactly 10 mL of 0.5N KOH solution was added and stirred with a glass rod until fully dispersed. The dispersed samples were transferred into a 100 mL volumetric flask and diluted to mark with de-ionized water. An aliquot of test starch solution (10 mL) was pipetted into 50 mL volumetric flask and 5 mL of 0.1 N HCl was added, followed by 0.5 mL of iodine reagent. The volume was diluted to 50 mL and absorbance was measured at 625 nm after 5 min. The amylose content was determined using a standard curve.
Amylose Leaching
Starch suspensions prepared by dissolving 20 mg of starch samples in excess water were heated in sealed tubes at 60°C for 30 min. The tubes were then cooled at ambient temperature (25–27°C) followed by centrifugation at 2000 × g for 10 min. One milliliter of supernatant was withdrawn and its amylose content was determined as described by Hoover and Ratnayake.[Citation16]
Swelling Power and Solubility Index
Swelling power and solubility were determined using the method of Leach et al.[Citation17] Starch slurry (2%) was prepared by heating at 90°C for 30 min with constant stirring and then cooled at room temperature (i.e., 30°C). Samples were poured in each pre-weighed culture tube and centrifuged at 3200 × g for 30 min. Supernatant and sediments were separated. Sediment was weighed for calculation of swelling power and supernatant was poured in pre-weighed Petri plates to evaporate the moisture at 110°C for 24 h. After this, the Petri plates were subjected to weighing for calculation of solubility (%).
Gel Consistency
Gel consistency of both the starch samples was determined by the following method as described by Balasubaramanian et al.[Citation18] Starch samples (0.1 g, dry basis) were wetted in a test tube (16 × 150 mm) with 0.2 mL of 95% ethanol containing 0.025% bromothymol blue and dispersed in 2 mL of 0.2 N KOH. The tubes were heated in boiling water bath for 8 min. Then it was cooled at room temperature for 5 min followed by cooling in an ice water bath for 20 min and laid down horizontally for 1 h at room temperature. The longer the gel traveled within the tube, the lower its consistency.
Sediment Volume
Sediment volume was determined using the method of Tessler et al.[Citation19] Slurry was prepared by dissolving 1 g of starch in 95 mL of distilled water. The pH was adjusted to 7.0 using 5% NaOH or 5% HCl followed by cooking the slurry in a boiling water bath for 15 min. Distilled water was then added to make up the total weight to 100 g. The slurry was transferred to a 100 mL-graduated cylinder and was kept covered at room temperature for 24 h. The volume of the sediment, consisting of starch granules was then measured.
Water Binding and Oil Binding
Water and oil binding capacities of native and acidified methanol modified starches were determined according to the method described by Adeleke and Odedeji.[Citation20] Water and oil absorption was determined by mixing 1 g of starch with 15 mL deionized water and soya bean oil, respectively, in a pre-weighed centrifuge tubes. After holding for 30 min, the tubes were centrifuged for 10 min at 3000 × g. The supernatant was discarded and tubes were then weighed again. Water absorption capacity and oil absorption capacity were expressed as grams of water and grams of oil retained per gram of starch, respectively.
Freeze–Thaw Stability
The freeze–thaw stability of native and acidified methanol modified starches was evaluated by following the method described by Hoover and Ratnayake.[Citation16] Native and acidified methanol modified starch gels (6% db) were subjected to cold storage at 4°C for 16 h (to increase nucleation) and then frozen at –16°C. To measure freeze–thaw stability, the gels frozen at –16°C for 24 h, were thawed at 25°C for 6 h and then refrozen at –16°C. Five cycles of freeze–thaw were performed. The excluded water was determined by centrifuging the tubes at 1000 × g for 20 min after thawing.
Light Transmittance
Light transmittance of starch suspensions was measured by a modified method of Perera and Hoover[Citation21] for measuring turbidity. An aqueous starch suspension (2%) was heated in a boiling water bath for 1 h with constant stirring. The suspension was cooled for 1 h at 30°C and then stored at 4°C. Light transmittance was determined at 640 nm against a distilled water blank for 6 days at an interval of 24 h using an ultraviolet (UV) spectrophotometer (Shimadzu UV-VIS 2600).
Color
Hunter lab color flex colorimeter (Hunter Associates Laboratory Inc., rest on USA) was used to measure the degree of change in color of both the starches (native and acidified methanol modified). The color coordinates of this meter were L* = whiteness; a* = redness to greenness; and b* = yellowness to blueness. The instrument was standardized with standard reference tile, coordinates for the tile were L* = 50.83 to 93.00; a* = 0.92 to –26.27; and b* = 1.70 to 12.12.
X-Ray Diffraction (XRD) Measurements
Starch granules were exposed to 100% relative humidity for 3 days and wide angle X-ray diffractograms were recorded by an analytical diffractometer (Pan Analytical, 117 Phillips, Holland) using Cu-Kα radiation, selected by means of quartz monochromator with a wavelength of 0.154 nm operating at 40 kV, 118 and 85 mA. XRD diffractograms were acquired at 25°C scattering between 2Ө range of 4 to 30° with a step size of 0.02° and sampling interval of 10 s following the method of Ikawa et al.[Citation22] at goniometer scanning speed of 4° 2Ө/min.
Statistical Analysis
Means and standard error mean (SEM) were calculated using Microsoft Excel, 2007 (Microsoft Corp., Redmond, WA). Significant difference between starch samples was verified by one-way analysis of variance and comparison between means was made by critical difference value as described by Snedecor and Cochran.[Citation23]
RESULTS AND DISCUSSION
Recovery Yield (%)
A detailed study on acid alcohol modification of black-eyed pea starch was carried out to elaborate the functional properties of starch. The study was planned with a view to optimize the concentration of the reactants to obtain the desired properties of the starch. Recovery yield of acidified methanol (0.36% HCl) starch were quite high, ranged between 93.18 to 95.48%. Higher yields were attributed to limited solubilization of black-eyed pea starch during acid-methanol hydrolysis.
Amylose Content and Amylose Leaching
Amylose content of both the starches is depicted in . Acid alcohol modification significantly resulted in breakdown of amylopectin chains by acid that adds up to amylose[Citation24] as compared to native starch. Dutta et al.[Citation25] observed similar trends on acid alcohol modification for various durations of jackfruit starch. Another possibility for the amylose values on addition of 40 mL of acid could be that the degraded amylose fractions re-crystallized themselves in a form that was more resistant to the acid attack. However, further increase in acid concentration (50–60 mL) led to degradation of the released amylose chains hence again resulted in decreased amylose content.
FIGURE 1 (a) Amylose content of native and acidified methanol modified black-eyed pea starch; (b) Amylose leaching of acid alcohol modified black-eyed pea starch. Data are presented as means ± SEM (n = 3).
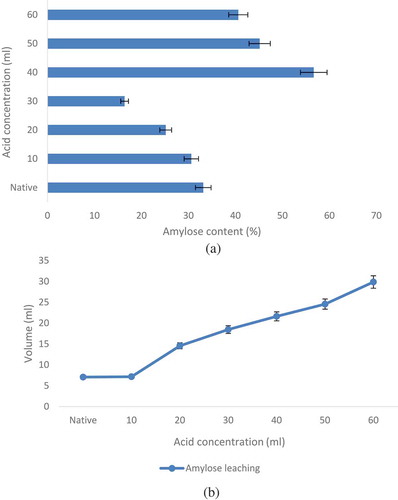
The acidified methanol modified starch samples showed higher amylose leaching compared to native starch (). Pulse starches exhibited no measurable amylose leaching at temperatures below 60°C which might be due to their high amylose content packed closely in the amorphous region.[Citation26] However, with increasing concentration of acid, the strong interactions (via hydrogen bonding) between amylose-amylose and amylose-amylopectin chains (within the granule interior) would be disrupted resulting in pronounced increase in amylose leaching.
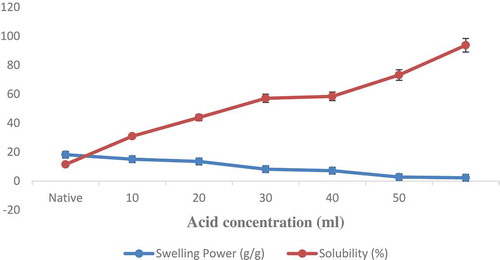
Swelling Power and Solubility
Swelling power and solubility are significant functional properties and important pre-requisite for other functional properties of the starch. Acidified methanol modification led to significant decrease in swelling power and increase in solubility as depicted in (). This decrease in swelling power was attributed by loosening of granular structure and presence of large number of crystallites (formed by association between long amylopectin chains). Crystallite formation would increase the granular stability, thereby reducing the extent of granular swelling.[Citation16] The increase in solubility values may be accredited to the weakening of hydrogen bonds or due to degradation of amylopectin upon modification which resulted in increased number of exposed hydroxyl groups which are responsible for greater water absorption, eventually the starch granules swell and disrupt resulting in increased solubility.[Citation27–Citation29] As the solubility of modified starch was significantly higher (p < 0.05), therefore, it could be used in enhancement of potential functional property in sausage and meat emulsions, providing a unique texture to these foods.[Citation30] Solubility of modified starch at an acid concentration of 60 mL was found to be higher than 90% making the starch “cold soluble.” Moreover, the starch granules were nearly fully dissolved making it a potential component in sausage and meat emulsions, as these properties are very important for texture in these foods.
Gel Consistency and Sediment Volume
Results of gel consistency of native and modified starch are presented in . It can be inferred from the results that gel consistency of acidified methanol modified starch decreased with increased acid concentration. Modified starch traveled a longer distance as compared to native starch up to an acid treatment of 30 mL which signifies lower gel consistency, i.e., gel formation weakens with increase in hydrolysis. Acid modification at a level of 40 mL showed an increase in gel consistency value. It was reported that the acid catalyzed hydrolysis of starch as opposed to enzymatic (α-amylase) hydrolysis can take place at branch points, thus reducing the degree of branching and increasing the percentage of linear segments and gel consistency.[Citation18,Citation31]
FIGURE 3 (a) Gel consistency of native and acidified methanol black-eyed pea starch; (b) Sediment volume of native and acid alcohol modified black-eyed pea starch. Data are presented as means ± SEM (n = 3).
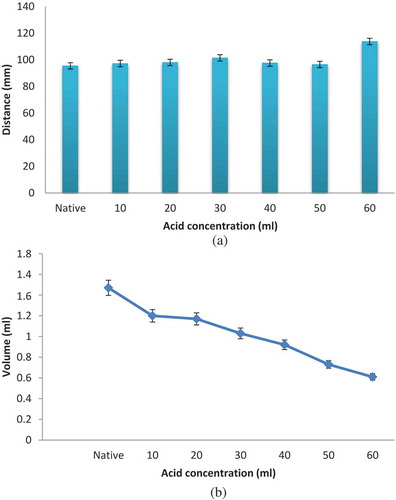
Acidified methanol modified starch showed a significantly (p < 0.05) lower sediment volume in comparison with native starch (). This decrease in sediment volume might be due to decreased interaction between the starch molecules on acid hydrolysis, thus inhibited swelling and hence, resulted in reduced sedimentation.[Citation1] A similar trend was reported by Balasubramanian et al.[Citation18] for acid modified pearl millet and finger millet starches.
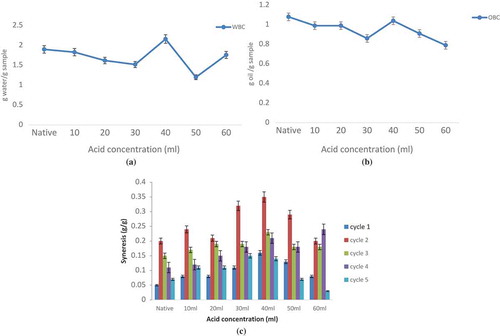
Water-Binding and Oil-Binding Capacities
Water binding capacity, an important functional trait for many food products, refers to the amount of water held by starch in a food matrix. The results for water-binding capacity (WBC) of native and acidified methanol modified starch are shown in . The acid thinning reduced both the water and oil binding capacity of starch following an analogous trend with the increasing acid concentration. The results are in agreement with the findings of[Citation32] in sweet potato starch. This decrease might be due to increase in crystalline region and decrease in amorphous region in starch granules as a result of acid attack that reduced the number of available binding sites, thus lowering the water and oil binding capacity.[Citation33,Citation34] However, at an acid concentration of 50 mL, an increase in the water and oil binding capacity was observed due to the degradation of crystalline region into amorphous region which released the hydroxyl groups from hydrogen and covalent bonding for water and oil binding.[Citation1,Citation35] Degradation by acid also resulted in loose association of amylose and amylopectin as a consequence of which water and oil binding capacity improved.[Citation36] By controlling the water binding capacity of starch-based materials, snacks can have a high degree of mouth melt, less waxiness, improved texture, and increased crispiness.
Freeze–Thaw Stability
Retention of stability of the gelatinized starch after repeated cycles of freezing and thawing is a desired property for the use of starch by the industries involved in the manufacture of frozen foods.[Citation37] Rapid syneresis was observed in the first two freeze–thaw cycles followed by reduction in the next three cycles () for all modified samples. Similar findings for waxy maize, amaranth, wheat, corn, rice, potato starches have been reported.[Citation37–Citation39] Acid-alcohol modification resulted in increased syneresis and decreased freeze–thaw stability until an acid treatment of 40 mL was reached. This increase in syneresis might be due to the stronger physical damage to the bonding patterns of starch gel resulting in lowering of the water holding capacities by the granules during continuous freezing and thawing.[Citation3] Increasing the concentration of acid beyond 40 mL decreased the syneresis which might have occurred due to the re-association of linear chains of amylose. Lowest rate of syneresis after five freeze–thaw cycle of acid alcohol treated starch (60 mL) makes it suitable for use in frozen products that are thawed before consumption.
Light Transmittance
Starch gel clarity directly influences brightness and opacity in foods that contain it as a thickener. Light transmittance provides information on the clarity of starch paste that in turn depends on the proportion of swollen and non-swollen granule remnants in the starch matrix. Acid thinning caused a significant increase in starch paste clarity (% transmittance; ) which may be due to the little light refraction from its swollen granule remnants.[Citation40] However, the percentage transmittance of the native, as well as the modified starch pastes, decreased with the time of storage at 4°C. The leaching of amorphous regions during acid thinning enhances the bond formation between the amylopectin molecules,[Citation33] and thus it can decrease its light transmittance upon storage at 4°C. This decrease might be a result of improved bond formation between the leached amylose and amylopectin molecules providing stability to the starch structure after modification and causing decreased tendency toward retrogradation.[Citation33] Starch gel clarity was observed highest at 40 mL modification level, thus has great potential to be incorporated into foods requiring transparency, e.g., juices, jellies, fruit pastes, mayonnaises, and sausages.
TABLE 1 Light transmittance of native and acidified methanol modified black-eyed pea starches
Color
Color is an important criterion while modification of different types of starch. The color of native and acidified methanol modified starches was determined by Hunter lab coordinates. It is evident from that acid alcohol modification significantly affected (p < 0.05) the color parameters (i.e., L*-, a*-, and b*-values) of starch in comparison with native starch. However, acid modification resulted in non-significant differences in L*-value with increased acid concentration. The L*-value for all samples was more than 94 which may be interpreted as more or less perfect white. Yellowness, indicated by positive value of b*-hue, decreased on modification and redness, i.e., a*-chroma (green/red) increased on modification. This increase in whiteness and decrease in yellowness may be due to the washing out of residual starch pigments upon acid treatment,[Citation41] as well as the washing steps followed to neutralize the acid. ΔE showed that there was a significant difference (p < 0.05) in the color of native and acidified methanol modified starches. The above trend might be due to the lipid–amylopectin complexation on modification that was difficult to remove after exhaustive washes and centrifugation during the starch isolation process.[Citation42]
TABLE 2 Color estimation of native and acidified methanol modified black-eyed pea starches
X-Ray Diffraction
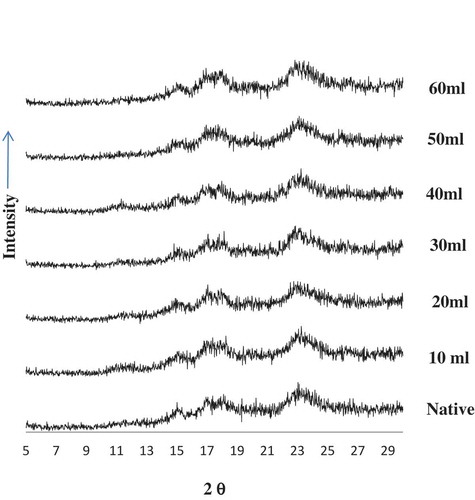
Plant starches are generally classified into A-, B-, and C-types as per the X-ray diffraction pattern revealed by their respective amylopectin crystalline lattices. All starches showed a “C” type X-ray pattern () which is a mixture of A-type and B-type (Donald, 2004)[Citation43] with a single peak at 15.2 and 23.2° 2Ө, and dual peaks at 17–18.1° 2Ө.peaks at I5°, 17°, and 23.4° (20) typical for cereal starches. The treatments varied the peak intensities but the pattern remained practically unchanged which shows that the structure of black-eyed pea starch is relatively unaltered under the modification conditions. Double peaks represented two transitions, the melting of B polymorphs followed by A polymorphs.[Citation42] Quantitatively, sharpness in XRD peaks is directly proportional to the crystalline nature and mean crystallite size of that material and larger crystallite materials. With increasing acid concentration, crystalline structure increased but there was marked loss of crystallinity at an acid level of 40 mL which further increased on acid concentrations of 50 and 60 mL, which is well in agreement with amylose content as it occupies the crystalline parts of starch. Kainuma and French[Citation44] suggested that cleavage of starch chains in the amorphous regions allows extensive reordering of the chain segments to give a more crystalline structure with a sharper X-ray pattern. The final value is in agreement with amylose content of starch of legumes seed powder and amylose occupies the crystalline parts of starch.[Citation45]
CONCLUSION
The physico-chemical properties of native and acidified methanol modified black-eyed pea starch have been studied. The black-eyed pea starch was hydrolyzed using HCl (36% by weight) at various concentrations (10–60 mL). Acid alcohol treated starch resulted in more than 93% recovery of starch. Functional properties of acidified methanol modified starch were significantly different from native starch. Decreased swelling power and increase in solubility were also observed upon acid alcohol treatment. Modification of black eye pea starch led to significant increase in paste clarity, water binding capacity, and oil binding capacity was observed. Crystals in black-eyed pea starch, determined by X-ray diffraction were of C-type, which is the characteristic pattern of legume starches. Acid concentration showed effect on post-reaction color difference (∆E). The information thus generated can be used in producing acidified methanol modified black-eyed pea starch after critically selecting the concentration of acid for hydrolysis to overcome the shortcomings of native starch. In addition, as black-eyed pea is a low glycemic index food, therefore, it may have potential application in producing hydrolyzed starches for diabetic patients.
FUNDING
The support by Department of Food Science and Technology, Guru Nanak Dev University Amritsar is gratefully acknowledged.
Additional information
Funding
REFERENCES
- DAS, A.B.; Singh, G.; Singh, S.; Riar, C.S. Effect of Acetylation and Dual Modification on Physico-Chemical, Rheological and Morphological Characteristics of Sweet Potato (Ipomoea Batatas) Starch. Carbohydrate Polymers 2010, 80, 725–732.
- Singh, H.; Sodhi, N.S.; Singh, N. Structure and Functional Properties of Acid Thinned Starch. International Journal of Food Properties 2009, 12(4), 713–725.
- Sodhi, N.S.; Singh, N. Characteristics of Acetylated Starches Prepared Using Starches Separated from Different Rice Cultivars. Journal of Food Engineering 2005, 70, 117–127.
- Bornet, F.R.J.; Fontvieille, A.M.; Rizkalla, S.; Colonna, P.; Blayo, A.; Mercier, C.; Slama, G. Insulin and Glycemic Responses in Healthy Humans to Native Starches Processed in Different Ways: Correlation with in vitro Alpha Amylase Hydrolysis. American Journal of Clinical Nutrition 1989, 50, 315–323.
- Boye, J.; Zare, F.; Pletch, A. Pulse Proteins: Processing, Characterization, Functional Properties and Applications in Food and Feed. Food Research International 2010, 43, 414–431.
- Anton, A.A.; Ross, K.A.; Lukow, O.M.; Fulcher, R.G.; Arntfield, S.D. Influence of Added Bean Flour (Phaseolus vulgaris L.) on Some Physical and Nutritional Properties of Wheat Flour Tortillas. Food Chemistry 2008, 109, 33–41.
- Benitez, V.; Cantera, S.; Aguilera, Y.; Molla, E.; Esteban, R.M.; Diaz, M.F.; Cabreja, M.A.M. Impact of Germination On Starch, Dietary Fiber and Physicochemical Properties in Non-Conventional Legumes. Food Research International 2013, 50, 64–69.
- Tharanathan, R.N.; Mahadevamma, S. Grain legumes—A Boon to Human Nutrition. Trends in Food Science & Technology 2003, 14(12), 507–518.
- Prinyawiwatkul, W.; McWatters, K.H.; Beuchat, L.R.; Phillips; R.D. Functional Characteristics of Cowpea (Vigna unguiculata) Flour and Starch as Affected by Soaking, Boiling, and Fungal Fermentation Before Milling. Food Chemistry 1997, 58, 361–372.
- Luo, Z.G.; Fu, X.; Gao, Q.Y.; Yu, S.J. Effect of Acid Hydrolysis in the Presence of Anhydrous Alcohols on the Structure, Thermal and Pasting Properties of Normal, Waxy and High-Amylose Maize Starches. International Journal of Food Science 2011, 46, 429–435.
- Nagasawan, Y.T.; Kume, T.; Yoshii, F. Radiation Crosslinking of Carboxymethyl Starch. Carbohydrate Polymers 2004, 58, 109–113.
- Whistler, R.L.; Daniel, J.R. Carbohydrates. In Food Chemistry, 2nd Ed; Fennema, O.R. Ed.; Marcel Dekker Inc./Wiley Inc.: New York, NY, 1985; pp. 69–125, 142.
- Lin, J.H.; Lee, S.Y.; Chang, Y.H. Effect of Acid–Alcohol Treatment on the Molecular Structure and Physicochemical Properties of Maize and Potato Starches. Carbohydrate Polymers 2003, 53, 475–482.
- Singh, N.; Sandhu, K.S.; Kaur, M. Characterization of Starches from Indian Chickpea (Cicer arietinum L.) Cultivars. Journal of Food Engineering 2004, 63, 441–449.
- Williams, P.C.; Kuzina, F.D.; Hlynka, I. A Rapid Calorimetric Procedure for Estimating the Amylose Content of Starches and Flours. Cereal Chemistry 1970, 47, 411–420.
- Hoover, R.; Ratnayake, W.S. Starch Characteristics of Black Bean, Chick Pea, Lentil, Navy Bean and Pinto Bean Cultivars Grown in Canada. Food Chemistry 2002, 78, 489–498.
- Leach, H.W.; McCowen, L.D.; Schoch, T.J. Structure of the Starch Granule. Swelling and Solubility Patterns of Various Starches. Cereal Chemistry 1959, 36, 535–545.
- Balasubramanian, S.; Sharma, R.; Kaur, J.; Bhardwaj, N. Isolation, Modification and Characterization of Finger Millet (Eleucinecoracana) Starch. Journal of Food Science and Engineering 2011, 1, 339–347.
- Tessler, M.M. Process for Preparing Cross-Linked Starches. US Patent 4098997, 1978.
- Adeleke, R.O.; Odedeji, J.O. Functional Properties of Wheat and Sweet Potato Flour Blends. Pakistan Journal of Nutrition 2010, 9(6), 535–538.
- Perera, C.; Hoover, R. Influence of Hydroxypropylation on Retrogradation Properties of Native, Defatted and Heat-Moisture Treated Potato Starches. Food Chemistry 2010, 64, 361–375.
- Ikawa, Y.; Glover, D.V.; Sugimoto, Y.; Fuwa, H. Some Structural Characteristics of Starches of Maize Having a Specific Genetic Background. Starch 1981, 33, 9–13.
- Snedecor, G.W.; Cochran, W.G. Statistical Methods, 8th Ed; 1989 Affiliated East-West Press/Iowa State University Press: Ames, IA, 1994.
- Luo, Z.G.; Fu, X.; Gao, Q.Y.; Yu, S.J. Effect of Acid Hydrolysis in the Presence of Anhydrous Alcohols on the Structure, Thermal and Pasting Properties of Normal, Waxy and High-Amylose Maize Starches. International Journal of Food Science 2011, 46, 429–435.
- Dutta, H.; Paul, K.S.; Kalita, D.; Mahanta C.L. Effect of Acid Concentration and Treatment Time on Acid–Alcohol Modified Jackfruit Seed Starch Properties. Food Chemistry 2011, 128, 284–291.
- Hoover, R.; Sosulski, F. Studies on the Functional Characteristics and Digestibility of Starches from Phaseolus vulgaris Biotypes. Starch 1985, 37, 181–191.
- Tester, R.F.; Karkalas, J. Swelling and Gelatinization of Oat Starches. Journal of Cereal Chemistry 1996, 73, 271–273.
- Atlay, F.; Gunasekaran, S. Influence of Drying Temperature, Water Content, and Heating Rate on Gelatinization of Corn Starches. Journal of Agriculture and Food Chemistry 2006, 54, 4235–4245.
- Mohamed, A.; Jamilah, B.; Abbas, K.A.; Abdul Rahman, R.; Roselina, K. A Review on Physicochemical and Thermos Rheological Properties of Sago Starch. American Journal of Agricultural and Biological Sciences 2008, 3(4), 639–646.
- Carballo, J.; Barreto, G.; Jimenez, C.F. Starch and Egg White Influence on Properties of Bologna Sausage as Related to Fat Content. Journal of Food Science 1995, 60, 673–677.
- Ozturk, S.; Koksel, H.; Perry, K.W.N. Production of Resistant Starch from Acid-Modified Amylotype Starches with Enhanced Functional Properties. Food Engineering 2011, 103, 156–164.
- Tian, S.; Kyle, W.S.A.; Small, D.M. Pilot Scale Isolation of Proteins from Field Peas (Pisum Sativum L.) for Use as Food Ingredients. International Journal of Food Science Technology 1999, 34, 33–39.
- Lawal, O.S. Composition, Physicochemical Properties and Retrogradation Characteristics of Native, Oxidized, Acetylated and Acid-Thinned New Cocoyam (Xanthosoma sagittifolium) Starch. Food Chemistry 2004, 87, 205–218.
- Kaur, M.; Oberoi, D.P.S.; Sogi, D.S.; Gill, B.S. Physicochemical, Morphological and Pasting Properties of Acid Treated Starches from Different Botanical Sources. Journal of Food Science 2011, 48(4), 460–465.
- Adebooye, O.C.; Singh, V. Physico-Chemical Properties of the Flours and Starches of Two Cowpea Varieties (Vignaunguiculata (L.) walp). Innovative Food Science and Emerging Technologies 2008, 9, 92–100.
- Wootton, M.; Bamunuaeachchi, A. Water Binding Capacity of Commercial Produced Native and Modified Starches. Starch 1978, 30(9), 306–309.
- Jobling, S.A.; Westcott, R.J.; Tayal, A.; Jeffcoat, R.; Schwall, G.P. Production of a Freeze-Thaw-Stable Potato Starch by Antisense Inhibition of Three Starch Synthase Genes. Nature Biotech 2002, 20, 295–299.
- Baker, L.A.; Rayas, P.D. Freeze-Thaw Stability of Amaranth Starch and the Effects of Salt and Sugars. Cereal Chemistry 1998, 75(3), 301–307.
- Yuan, R.C.; Thompson, D.B. Freeze-Thaw Stability of Three Waxy Maize Starch Pastes Measured by Centrifugation and Calorimetry. Cereal Chemistry 1998, 75, 571–573.
- Craig, S.A.S.; Maningat, C.C.; Seib, P.A.; Hoseney, R.C. Starch Paste Clarity. Cereal Chemistry 1989, 66, 173.
- Mukprasirt, A.; Sajjaanantakul, K. Physico-Chemical Properties of Flour and Starch from Jackfruit Seeds (Artocarpusheterophyllus Lam.) Compared with Modified Starches. International Journal of Food Science 2004, 39, 271–276.
- Bogracheva, T.Y.; Morris, V.J.; Ring, S.G.; Hedley, C.L. The Granular Structure of C-Type Pea Starch and Its Role in Gelatinization. Biopolymers 1998, 45, 323–332.
- Donald, A.M. Understanding starch structure and functionality. In Starch in Food: Structure, Function and Applications; Eliassion, A. C.; Ed. Woodhead Publishing Limited/CRC Press LLC: New York, 2004; 156–184.
- Kainuma, K.; French, D. Naegeli Amylodextrin and Its Relationship to Starch Granule Structure. Preparation and Properties of Amylodextrins from Various Starch Types. Biopolymers 1971, 10, 1673–1680.
- Marimuthu, M; Gurumoorthi, P. Structural Properties, Starches from Indian Wild Jack Bean (Canavalia ensiformis) Using X-Ray Diffraction. International Journal of Pharmaceutical Chemical and Biological Sciences 2013, 3(2), 320–324.