ABSTRACT
Polyphenol oxidase properties and anti-xanthine oxidase, anti-urease, and antioxidant activity were investigated in Pyrus elaeagnifolia subsp. elaeagnifolia Pallas harvested from Antalya, Turkey. Optimum pH and temperature were 7.0 and 30°C, respectively. Selected kinetic properties of polyphenol oxidase was evaluated.The Km and Vmax-values, using 4-methylcatechol as substrate, were calculated as 3.57 mM and 4781 U/mg protein, respectively. IC50-values ranged from 0.0036 to 4.0231 mM against sodium metabisulphite, ascorbic acid, sodium azide, and benzoic acid, while ascorbic acid was the strongest inhibitor. Aquatic extracts of the sample exhibited strong antioxidant capacity and substantial xanthine oxidase and urease inhibition, with IC50-values of 10.75 ± 0.11 and 0.97 ± 0.03 mg/mL, respectively.
Introduction
Pyrus elaeagnifolia subsp. elaeagnifolia Pallas is a wild pear species which is a member of the Rosaceae family, commonly grown in cities in Turkey such as Amasya, Ankara, Antalya, Bolu, Kahramanmaraş, Kayseri, Konya, Mersin, Sivas, and Van. It is usually known by the common name of Ahlat. After ripening and harvesting, the fruit becomes soft and edible.[Citation1] It is widely consumed as preserves and occasionally pickled and dried. The fruit is also used in folk medicine, primarily in the treatment of diarrhea and detoxification of poisonous snake bites.
Polyphenol oxidase (PPO; EC 1.10.3.1) is a copper-containing enzyme responsible for the hydroxylation of monophenols to o-diphenols and oxidation of o-diphenols to o-diquinones.[Citation2] PPO is compartmentalized in plastids and its phenolic compounds are located in vacuoles in most plant tissues. Action of PPO only occurs when this compartmentation is disrupted after tissues are wounded, as observed in diseased tissues or cell disruption caused by processing and storage. The products of the browning reaction may modify plant proteins and be more toxic to potential phytopathogens. The reactions also produce undesirable blackening in the products during food processing, or in post-harvest of plant products resulting in a reduction of their sensory properties and nutritional value.[Citation3] For this reason, PPO has been characterized in various fruits, vegetables, and fungi such as pears,[Citation1,Citation4–Citation7] hemşin apples,[Citation2] medlar fruits,[Citation8] potatoes,[Citation9] jackfruits,[Citation10] persimmons,[Citation11] yomra apples,[Citation12] mangoes,[Citation13] strawberries,[Citation14] and peaches.[Citation15]
Xanthine oxidase (XO) belongs to the molybdenum-protein family containing one molybdenum, which catalyses the conversion of both hypoxanthine to xanthine and xanthine to uric acid while reducing O2 to O2−• and H2O2. Elevated concentrations of uric acid in the bloodstream of the human body lead to the formation of gout, characterized by hyperuricaemia and recurrent attacks of arthritis.[Citation16] Allopurinol is a potent inhibitor of XO which has been widely used to treat gout and hyperuricaemia. However, severe toxicity of allopurinol has been reported, causing effects such as vasculitis, rash, eosinophilia, hepatitis, hypersensitivity problem, Stevens–Johnson syndrome, renal toxicity, and fatal liver necrosis.[Citation17] Thus, safe and effective XO inhibitors are still needed for hyperuricaemia treatment. As natural products are versatile in providing drugs, the exploration of potential XO inhibitors from fruit was attempted in this study.
Urease is a metalloenzyme containing nickel that catalyses the hydrolysis of urea into ammonia and carbamate. Ureases producing excess ammonium are also involved in the development of urolithiasis, pyelonephritis, hepatic encephalopathy, hepatic coma urolithiasis, and urinary catheter encrustation in humans and animals. The urease activity of Helicobacter pylori plays an important role in the pathogenesis of gastric and peptic ulcers. Acetohydroxamic acid has been clinically used for the treatment of urinary tract infections by urease inhibition.[Citation18] However, it exhibits severe side effects such as breathing difficulties and swelling of the face, lips, tongue, or throat. There is also a resistance problem caused by inappropriate and extensive use of such drugs,[Citation19] so there is a need to explore more effective antiulcer agents and urease inhibitors possessing enhanced efficacy against microorganisms while exhibiting less toxicity to human cells.
This work covers investigations of enzyme inhibitory and antioxidant properties, characterization of PPO, effects of pH and temperature on PPO activity and stability, effects of inhibitors on PPO activity, and substrate specificity of PPO from the Ahlat pear. The research could lead to understanding the properties of PPO that catalyses the browning reaction during storage. The results would provide information and effective methods for controlling browning and extending shelf life.
Materıals and methods
Materials and chemicals
P. elaeagnifolia subsp. elaeagnifolia Pallas pear was collected directly from the Berem High Plateau, Gazipaşa (Antalya, Turkey) at 3000 m altitude in September. P. elaeagnifolia fruit was stored at −30°C until used. All chemicals used in the study were purchased from Sigma Chemical Co. (St. Louis, MO, USA).
Preparation of crude enzyme extract
P. elaeagnifolia pears (Pep; 100 g) were placed in a Dewar flask under liquid nitrogen for 15 min and then homogenized by using a blender in 100 mL of acetate buffer (pH 7.0, 50 mM) containing 2 mM EDTA, 1 mM MgCl2, 1 mM phenylmethylsulphonyl fluoride (PMSF) and 6% (w/v) Triton X-114 for 20 min. After the homogenate was filtered using three layers of cheesecloth, the filtrate was centrifuged at 10,000 rpm for 30 min at 4°C. An equal volume of cold acetone was added to the supernatant and the mixture was incubated overnight at 4°C for precipitation of proteins. After centrifugation at 10,000 rpm for 20 min at 4°C, the precipitate was redissolved in an appropriate volume of 50 mM phosphate buffer (pH 7.0) and this supernatant was used as crude enzyme extract and continued PPO activity during storage (2 months) at +4°C.
PPO activity assay
The enzyme activity was determined using different mono- or di-phenolic compounds, by measuring the increase in absorbance at 494 nm for 4-methylcatechol and 500 nm for all other substrates in a ultraviolet (UV)/visible spectrophotometer (1601UV, Shimadzu, Australia).[Citation20] The assay mixture, containing 100 µL substrate (stock 100 mM), an equal volume of 10 mM 3-methyl-2-benzothiazolinone hydrazone (MBTH) and 20 µL dimethylformamide (DMF), was diluted to 980 µL with 50 mM phosphate buffer (pH 7.0). After that, 20 µL of crude enzyme extract was added. The reference cuvette included all the reactants except the crude enzyme. One unit of PPO activity was defined as the amount of enzyme causing 0.001 increase of absorbance per minute in 1000 µL of reaction mixture.
Protein content
Protein content was determined according to the Lowry method using bovine serum albumin as standard.[Citation21]
Substrate specificity and enzyme kinetics
Substrate specificity was determined using six substrates: catechol, 4-methylcatechol (4-MTC), L-3,4-dihydroxyphenylalanine (L-DOPA), and 3-(3,4-dihydroxyphenyl) propionic acid (DHPPA) as diphenolic substrates, L-tyrosine and 3-(4-hydroxyphenyl) propionic acid (PHPPA) as monophenolic substrates and gallic acid as triphenolic substrate in 50 mM phosphate buffer (pH 7.0). P. elaeagnifolia PPO (PePPO) activity was measured with 4-MTC as the reference (100%). The kinetic data for 4-MTC substrate were plotted as reciprocals of activities versus substrate concentrations. Michaelis–Menten constants (Km) and maximum velocity (Vmax) were measured from the linear regression curve.
Effect of inhibitors and metal ions
Ascorbic acid (1.25–20 µM), sodium azide (1.25–10 mM), benzoic acid (0.1–1.6 mM), and sodium metabisulphite (3.2–51.2 µM) were screened for their effectiveness as inhibitors of PePPO activity, in the presence of 4-MTC. The concentration of inhibitor giving 50% inhibition (IC50) was calculated from a plot of remaining activity against inhibitor concentration. PePPO activity was measured in the presence (1 mM final concentration) of various ionic compounds under standard assay conditions. K1+, Na1+, Fe2+, Hg2+, Mg2+, Co2+, Sr2+, Zn2+, Ni2+, Cd2+, Ca2+, Mn2+, Ba2+, and Al3+ were used as metal ions to measure PPO activity. The percentage activity remaining was expressed by comparison with a standard assay mixture with no metal ion added.[Citation22] Percentage inhibition was calculated using the following equation:
where A0 is initial PPO activity (without inhibitor) and Ai is PPO activity with inhibitor.
pH optimum and stability
PPO activity, as a function of pH, was determined using 4-MTC as substrate (0.1 M stock concentration). The buffers used were: acetate (pH 4.0 and 5.0), phosphate (pH 6.0 and 7.0) and Tris–HCl (pH 8.0 and 9.0). PPO activity was assayed by preparing in a buffer solution at various pH-values of MBTH and DMF. For determining PePPO pH stability, 0.010 mL of crude enzyme solution was added to 0.990 mL buffer solution, ranging from pH 4.0 to 9.0, for 15 d at 4°C. Residual PPO activity was determined in the form of percentage residual PePPO activity at the optimum pH by mixing 0.1 mL of 100 mM 4-MTC as substrate, 0.1 mL of 10 mM MBTH and 0.02 mL DMF with the incubated crude enzyme solution.[Citation22]
Thermal activity and stability
To determine the optimum temperature of the enzyme, PePPO activity was measured at different temperatures in the range from 0 to 80°C at 10°C increments by using 4-MTC as substrate. In order to determine the thermal stability of PePPO, the enzyme solution in 50 mM phosphate buffer, pH 7.0, in Eppendorf tubes, was incubated in a thermoblock at temperatures of 40, 50, 60, 70, and 80°C for 6 h, rapidly cooled in an ice bath for 5 min, and then brought to 25°C. After the mixture reached room temperature, the enzyme activity was assayed under the standard assay conditions. The percentage residual PPO activity was calculated by comparison with unincubated enzyme.
Native polyacrylamide gel electrophoresis
According to Laemmli,[Citation23] polyacrylamide gels were adjusted to 8% for native conditions. After running, gels were incubated in 15 mM L-DOPA in 0.1 M phosphate buffer (pH 7.0) at 30°C for 30 min. Then, an appropriate amount of 1 mM ascorbic acid solution was added to the gel to detect isoenzyme bands.
Preparation of aquatic extract of Pyrus elaeagnifolia pear for use in antioxidant and enzyme inhibition studies
Approximately 30 g of Pep was blended and extracted with 150 mL bidistilled water and shaken in an ultrasonicator bath (Heidolph Promax 2020, Schwabach, Germany) for 6 h at room temperature. The crude mixture was then centrifuged at 10,000 rpm for 30 min at 4°C to remove solid particles, and the filtrates were lyophilized (Christ-Alpha 1-4 LD plus, Osterode am Harz, Germany). The lyophilized filtrate was dissolved in 20 mL distilled water. The aquatic extract of P. elaeagnifolia pear (AePep) was used for antioxidant and enzyme inhibition studies.
Determination of antioxidant capacity
Total phenolic content (TPC) of AePep was analyzed using the Folin–Ciocalteu assay[Citation24] with gallic acid as the standard. For this, 680 µL distilled water, 20 µL aquatic extract and 400 µL of 0.2 N Folin–Ciocalteu reagent were mixed and then vortexed. After 2 min, 400 µL Na2CO3 (7.5%) was added and the mixture was incubated for 2 h at room temperature. Absorbance was then measured at 760 nm. The concentration of TPC was calculated as mg of gallic acid equivalent (GAE) per g of fresh weight (FW), using a standard curve for gallic acid in the concentration range between 0.03125 and 1.0 mg/mL (R2 = 0.998).
The cupric reducing antioxidant capacity (CUPRAC) of AePep was determined according to the method in the literature.[Citation25,Citation26] Trolox® (Sigma Chemical Co, USA) was also tested under the same conditions as a standard antioxidant compound. The standard curve was linear between 8 mg/mL and 0.0625 mg/mL Trolox® (R2 = 0.998). CUPRAC values were expressed as mg Trolox® equivalent per g of FW.
2,2-diphenyl-1-picrylhydrazyl (DPPH) radical scavenging activity of AePep was measured using the method in the literature.[Citation27] Radical scavenging activity was measured by using butylated hydroxy toluene (BHT) as standard and all values are expressed as SC50, the concentration of the samples that caused 50% scavenging of DPPH radical. The ability of AePep to scavenge ABTS•+ (2,2’-azino-bis(3-ethylbenzothiazoline-6-sulphonic acid)) radical was determined according to the literature.[Citation26,Citation27] All determinations were carried out three times, using BHT as standard.
Anti-XO and anti-urease activity of Pyrus elaeagnifolia pallas
The XO inhibition of AePep was measured by a UV spectroscopy technique at 295 nm which monitors uric acid released from xanthine. The inhibitory activity of the extract was determined using the reference methods.[Citation28] A well-known XO inhibitor (XOI), allopurinol, was used as a positive control for the inhibition test. The assay was done in triplicate. The IC50-value was determined as the concentration of compound that gave 50% inhibition of maximal activity. Urease is an enzyme that catalyses the hydrolysis of urea into carbon dioxide and ammonia. The production of ammonia was measured by the indophenol method and used to determine the urease inhibitory activity.[Citation29] The percentage inhibition was calculated from the formula:
Thiourea was used as the standard inhibitor.
Results
Substrate specificity and enzyme kinetics
PePPO relative activity, concerned with the differences in absorption at the wavelength maximum of the final product, was correlated with the activity in the presence of 4-MTC as 100% (). There was no significant oxidation of monophenolic substrates. The inability of the enzymes to oxidize those phenolic compounds suggests that they lacked monophenolase activity and could be diphenolases. Also, Km and Vmax-values, using 4-MTC as substrate, were calculated as 3.57 mM and 4781 U/mg protein by Lineweaver–Burk curve, respectively.
Table 1. Substrate specificity of the PePPO.
Native polyacrylamide gel electrophoresis
Crude enzyme extracted from P. elaeagnifolia Pallas was investigated to characterize the properties of PPO activity. Two PPO isoforms were evaluated by native electrophoresis by using L-DOPA substrate ().
pH optimum and stability
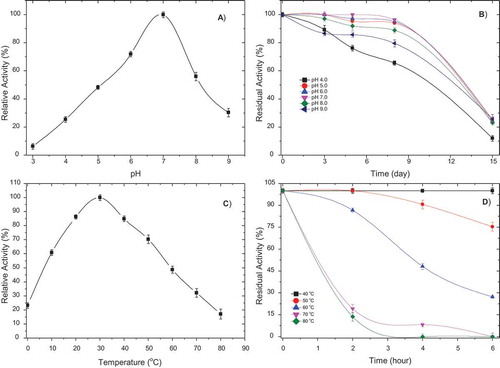
The pH optimum profile for PePPO was found to be bell-shaped for 4-MTC substrate and the optimum pH was found to be 7.0 (). The pH stability was examined by incubating the crude enzyme eluate at different pH-values at 4°C for 15 days (). PePPO was extremely stable, retaining at least 95% of its original activity in the range pH 5.0–8.0 after 72 h of incubation at 4°C.
Thermal activity and stability
The thermal activity of crude PePPO is presented in . The optimum temperature for PePPO activity was 30°C. The PePPO thermal stability profile is shown in , with the residual percentage activity. Highly stable activity was seen in the crude enzyme for 6 days at 40–50°C and moderately stable activity for only 2 days at 60°C.
Effect of inhibitors and metal ions
The behavior of PePPO toward general PPO inhibitors was examined by treatment with four inhibitors, namely ascorbic acid, sodium azide, benzoic acid, and sodium metabisulphite (). Each inhibitor was tested over a wide range of concentrations sufficient to reduce PPO activity by 50% or more. Inhibition data indicate that the binding affinity of ascorbic acid is the greatest, with an IC50-value of 0.0036 ± 0.0002 mM, when compared with the others. The effects of K1+, Na1+, Fe2+, Hg2+, Mg2+, Co2+, Sr2+, Zn2+, Ni2+, Cd2+, Ca2+, Mn2+, Ba2+, and Al3+ on PePPO activity are shown in . While PePPO activity was inhibited by Sr2+, Cd2+, Na1+, Fe2+, and Hg2+, it was stimulated by Ca2+, Mn2+, Ba2+, K1+, Mg2+, Co2+, Zn2+, Ni2+, and Al3+.
Table 2. The IC50 values of some inhibitors.
Table 3. Effect of various metal ions on PePPO activity.
Antioxidant, anti-urease, and anti-XO activity
Only the antioxidant properties of Ahlat pear were investigated as a test of biological activity in this study. Various techniques are used to measure the antioxidant capacity of natural products. However, TPC, CUPRAC, DPPH, and ABTS are the most widely employed tests, and are also sensitive, reliable and easy to perform. CUPRAC and TPC values were found to be 104.05 ± 2.47 mg TEAC/g FW and 4.98 ± 0.06 mg GAE/g FW, respectively (). SC50-values were determined as 3.49 ± 0.07 and 5.01 ± 0.03 mg/mL for AePep using the DPPH and ABTS methods. AePep was evaluated for bovine milk XO activity. The results showed that AePep had promising activity to inhibit XO up to 98.51 ± 0.69% at a concentration of 62.50 mg/mL, with an IC50-value of 10.75 ± 0.11 mg/mL (). Also, AePep exhibited highly efficient anti-urease activity, with an IC50-value of 0.97 ± 0.03 mg/mL ().
Table 4. Antioxidant activity values of AePep.
Table 5. Each enzyme IC50 and % inhibition values of the AePep samples.
Discussion
PePPO activity was characterized using crude enzyme preparations extracted from P. elaeagnifolia fruit. In this study, six widely used substrates (catechol, 4-MTC, L-DOPA, DHPPA, gallic acid, L-tyrosine, and PHPPA) were selected for substrate specificity of PePPO. The inability of the enzymes to oxidize those phenolic compounds suggests that the enzymes lacked monophenolase activity and could be diphenolase. The highest activity was observed in the presence of 4-MTC. The Km and Vmax-values, using 4-MTC as substrate, were calculated as 3.57 mM and 4781 U/mg protein by Lineweaver–Burk curve, respectively. Although PePPO did not show an ability to oxidize the monohydroxyphenols, it was suitable for the dihydroxyphenols, as shown in previous studies for PPO from other plant sources.[Citation8,Citation10,Citation13,Citation30] It was found that 4-MTC, followed by catechol and dopamine, was the most readily oxidized substrate of PPO from pear cultivars.[Citation7] The large range in the apparent Km-values of PPO reported may be due to the different assay methods used, different varieties, different origins of the same variety and different extraction pH.[Citation9]
Two electrophoresis bands were indicated for different isoforms of PePPO. Besides these two bands, third and fourth PPO isoenzyme bands have been detected in the Ankara pear,[Citation5] persimmons,[Citation11] and the Bartlett pear.[Citation1] The pH-values for crude enzyme had a typical bell-shaped curve, with pH 7.0 as the optimum activity for the 4-MTC substrate. Different optimum pH-values for PPO obtained from various sources are reported in the literature. It is around pH 6–7 for PPO from potatoes,[Citation9] yomra apples,[Citation12] jackfruits,[Citation10] pears,[Citation5] hemşin apples,[Citation2] wild pears,[Citation4] chestnut kernels,[Citation31] and Agaricus bisporus.[Citation32] The pH stability was examined by incubating the pure enzyme eluate at different pH-values at 4°C for 15 days. PePPO was extremely stable, retaining at least 95% of its original activity in the range pH 5.0–8.0 after 72 h of incubation at 4°C. The enzyme shows suitable behaviour in the range pH 4–9.
The optimum temperature for PePPO activity was found to be 30°C. When comparing other results for PPO activity, the optimum temperatures for A. bisporus,[Citation32] Ankara pears,[Citation5] wild pears,[Citation4] mangoes,[Citation13] and yomra apples[Citation12] were 20, 35, 35, 20–70, and 40°C, respectively. Also, nearly 50% of PPO activity was lost at 60°C. Inhibition of PePPO in the presence of ascorbic acid, sodium azide, benzoic acid, and sodium metabisulphite was examined, to determine their potential for inhibition of 4-MTC oxidation by PePPO. The inhibition mechanisms could be changed by the inhibitor used. A previous study explained general PPO inhibition mechanisms.[Citation33] Inhibition data indicate that ascorbic acid had the greatest inhibitory effect on PePPO, with an IC50-value of 0.0036 ± 0.0002 mM. However, it was reported that L-cysteine was a more effective inhibitor of tea leaf[Citation30] and some pear cultivars, namely d’Anjou and Bartlett.[Citation7]
Antioxidants may help the body to protect itself from various types of oxidative damage which are linked to aging and diseases such as cancer, diabetes, and cardiovascular disorders.[Citation34] In vitro antioxidant activity of Pep was studied, and the aquatic extract was found to be the most potent DPPH radical scavenger, with IC50-values of 3.49 ± 0.07 mg/mL. In the present investigation, TPC was 4.98 ± 0.06 mg GAE/g FW. Phenolic compounds, especially flavonoids, were found as a large chemical class with XOI properties, and their role has been thoroughly evaluated.[Citation35] Hyperuricaemia is a common metabolic disorder disease in humans, which is caused by uric acid formed from xanthine in the presence of XO. The increasing risk of hyperuricaemia has also been linked with the development of hypertension, hyperlipidaemia, cancer, diabetes, and obesity. The XOI effect of Pep was also investigated, and the IC50-value was found to be 10.75 ± 0.11 mg/mL. It is also emphasized in the literature that distilled water extract of Carica papaya leaves showed promising XOI activity compared to the seeds, petioles, flowers, unripe fruit and unripe fruit peel.[Citation36] Umamaheswari et al.[Citation37] determined that the IC50 of hydromethanolic extract and its fractions of Eucalyptus stricta ranged between 21.2 and 100 µg/mL, and the IC50 of the standard drug allopurinol was 6.1 ± 0.3 µg/mL.
Plants have always been the main source of new drugs and folk medicines. Plants are well-known to contain active metabolites which are useful in treating various infectious diseases with little or no toxicity. Several naturally occurring medicinal plants, herbs, and fruit extracts have been shown to possess antimicrobial activity against H. pylori. Also, polyphenols, especially flavonoids, have been pointed out as notable H. pylori urease inhibitors.[Citation38] The ethnomedicinal use of plants to treat chronic gastritis, ulcers and related gastroduodenal disorders, diseases that can be caused by H. pylori, is widely reported.[Citation39] Studies carried out with several plant extracts allowed for the identification of urease inhibitors that may be useful for the control of H. pylori strain growth.[Citation40] Plant juices obtained from Allium sativum (garlic), A. cepa (yellow and white onion), A. porrum (leek), Brassica oleracea var. apitate (cabbage; Brassicaceae), and B. oleracea var. gemmifera (Brussels sprouts) were also effective urease inhibitors.[Citation41] The urease inhibition effect of AePep was also investigated, and the IC50-value was found to be 0.97 ± 0.03 mg/mL.
Conclusion
PPO activity of P. elaeagnifolia subsp. elaeagnifolia Pallas has been characterized for the first time. In addition, two activity bands were detected in the P. elaeagnifolia extract assayed by native gel electrophoresis, suggesting for the first time the presence of at least two PPO isoenzymes in P. elaeagnifolia fruit. In this study, the results of XO and urease inhibitory and antioxidant activity provided strong evidence that extracts of Pep and its by-products may be developed as natural XO and urease inhibitors. In this study, Pep and its by-product aquatic extract was confirmed to be effective antioxidants, urease, and XO inhibitors, indicating their potential use as functional food ingredients.
Nomenclature
| XO | = | Xanthine oxidase |
| XOI | = | Xanthine oxidase inhibitor |
| Pep | = | Pyrus elaeagnifolia pears |
| PMSF | = | Phenylmethylsulphonyl fluoride |
| DMF | = | Dimethylformamide |
| 4-MTC | = | 4-Methylcatechol |
| L-DOPA | = | L-3,4-Dihydroxyphenylalanine |
| DHPPA | = | 3-(3,4-Dihydroxyphenyl) propionic acid |
| PHPPA | = | 3-(4-Hydroxyphenyl) propionic acid |
| MBTH | = | 3-Methyl-2-benzothiazolinone hydrazone hydrochloride hydrate |
| DPPH | = | 2,2-Diphenyl-1-picrylhydrazyl |
| ABTS | = | 2,2’-Azino-bis(3-ethylbenzothiazoline-6-sulphonic acid) |
| PPO | = | Polyphenol oxidase |
| PePPO | = | Pyrus elaeagnifolia PPO |
| AePep | = | Aquatic extract of Pyrus elaeagnifolia pear |
| TPC | = | Total phenolic content |
References
- Rivas, N.J.; Whitaker, J.R. Purification and Some Properties of Two Polyphenol Oxidases from Bartlett Pears. Plant Physiology 1973, 52, 501–507.
- Aydin, B.; Gulcin, I.; Alwasel, S.H. Purification and Characterization of Polyphenol Oxidase from Hemşin Apple (Malus Communis L.). International Journal of Food Properties 2015, 18, 2735–2745.
- Guo, L.; Ma, Y.; Shi, J.; Xue, S. The Purification and Characterisation of Polyphenol Oxidase from Green Bean (Phaseolus Vulgaris L.). Food Chemistry 2009, 117, 143–151.
- Yerliturk, F.U.; Arslan, O.; Sinan, S.; Gencer, N.; Ozensoy, G.O. Characterization of Polyphenoloxidase from Wild Pear (Pyrus Elaegrifolia). Journal of Food Biochemistry 2008, 32, 368–383.
- Ziyan, E.; Pekyardimci, S. Purification and Characterization of Pear (Pyrus Communis) Polyphenol Oxidase. Turkish Journal of Chemistry 2004, 28, 547–557.
- Nishimura, M.; Fukuda, C.; Murata, M.; Homma, S. Cloning and Some Properties of Japanese Pear (Pyrus Pyrifolia) Polyphenol Oxidase, and Changes in Browning Potential During Fruit Maturation. Journal of the Science of Food and Agriculture 2003, 83, 1156–1162.
- Sidding, M.; Cash, J.N. Physico-Chemical Properties of Polyphenol Oxidase from d’Anjou and Bartlett Pears (Pyrus Communis L.). Journal of Food Processing and Preservation 2000, 24, 353–364.
- Dincer, B.; Colak, A.; Aydin, N.; Kadioglu, A.; Güner, S. Characterization of Polyphenoloxidase from Medlar Fruits (Mespilus Germanica L., Rosaceae). Food Chemistry 2002, 77, 1–7.
- Niphadkar, S.S.; Vetal, M.D.; Rathod, V.K. Purification and Characterization of Polyphenol Oxidase from Waste Potato Peel by Aqueous Two-Phase Extraction. Preparative Biochemistry & Biotechnology 2015, 45, 632–649.
- Tao, Y.M.; Yao, L.Y.; Qin, Q.Y.; Shen, W. Purification and Characterization of Polyphenol Oxidase from Jackfruit (Artocarpus Heterophyllus) Bulbs. Journal of Agricultural and Food Chemistry 2013, 61, 12662–12669.
- Navarro, J.L.; Tárrega, A.; Sentandreu, M.A.; E. Sentandreu, Partial Purification and Characterization of Polyphenol Oxidase from Persimmon. Food Chemistry 2014, 157, 283–289.
- Can, Z.; Dincer, B.; Sahin, H.; Baltas, N.; Yildiz, O.; Kolayli, S. Polyphenol Oxidase Activity and Antioxidant Properties of Yomra Apple (Malus Communis L.) from Turkey. Journal of Enzyme Inhibition and Medicinal Chemistry 2014, 29, 829–835.
- Palma-Orozco, G.; Marrufo-Hernández, N.A.; Sampedro, J.G.; H. Nájera, Purification and Partial Biochemical Characterization of Polyphenol Oxidase from Mango (Mangifera Indica cv. Manila). Journal of Agricultural and Food Chemistry 2014, 62, 9832–9840.
- Terefe, N.S.; Yang, Y.H.; Knoerzer, K.; Buckow, R.; Versteeg, C. High Pressure and Thermal Inactivation Kinetics of Polyphenol Oxidase and Peroxidase in Strawberry Puree. Innovative Food Science and Emerging Technologies 2010, 11, 52–60.
- Liu, L.; Cao, S.; Yang, H.; Qi, X. Pectin Plays an Important Role on the Kinetics Properties of Polyphenol Oxidase from Honeydew Peach. Food Chemistry 2015, 168, 14–20.
- Kostic, D.A.; Dimitrijevic, D.S.; Stojanovic, G.S.; Palic, I.R.; Dordevic, A.S.; Ickovski, J.D. Xanthine Oxidase: Isolation, Assays of Activity, and Inhibition. Journal of Chemistry 2015. DOI:10.1155/2015/294858
- Kim, S.C.; Newcomb, C.; Margolis, D.; Roy, J.; Hennessy, S. Severe Cutaneous Reactions Requiring Hospitalization in Allopurinol Initiators: A Population-Based Cohort Study. Arthritis Care & Research 2013, 65, 493–424.
- Sahu, J.K.; Ganguly, S.; Kaushik, A. Triazoles: A Valuable Insight into Recent Developments and Biological Activities. Chinese Journal of Natural Medicines 2013, 11, 456–465.
- Maddila, S.; Pagadala, R.; Jonnalagadda, S.B. 1,2,4-Triazoles: A Review of Synthetic Approaches and the Biological Activity. Letters in Organic Chemistry 2013, 10, 693–714.
- Espin, J.C.; Trujano, M.F.; Tudela, J.; Garcia-Canovas, F. Monophenolase Activity of Polyphenoloxidase from Haas Avocado. Journal of Agricultural and Food Chemistry 1997, 45, 1091–1096.
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein Measurement with the Folin Phenol Reagent. The Journal of Biological Chemistry 1951, 193, 265–275.
- Liu, N.; Liu, W.; Wang, D.; Zhou, Y.; Lin, X.; Wang, X.; Li, S. Purification and Partial Characterization of Polyphenol Oxidase from the Flower Buds of Lonicera Japonica Thunb. Food Chemistry 2013, 138, 478–483.
- Laemmli, U.K. Cleavage of Structural Proteins During the Assembly of the Head of Bacteriophage T4. Nature 1970, 227, 680–685.
- Singleton, V.L.; Rossi, J.L. Colorimetry of Total Phenolics with Phosphomolybdic Phosphotungstic Acid Reagents. American Journal of Enology and Viticulture 1965, 16, 144–158.
- Apak, R.; Guclu, K.; Ozyurek, M.; Karademir, S.E. Novel Total Antioxidant Capacity Index for Dietary Polyphenols and Vitamins C and E, Using Their Cupric Ion Reducing Capability in the Presence of Neocuproine: CUPRAC Method. Journal of Agricultural and Food Chemistry 2004, 52, 7970–7981.
- Menteşe, E.; Yilmaz, F.; Baltaş, N.; Bekircan, O.; B. Kahveci, Synthesis and Antioxidant Activities of Some New Triheterocyclic Compounds Containing Benzimidazole, Thiophene, and 1,2,4-Triazole Rings. Journal of Enzyme Inhibition and Medicinal Chemistry 2015, 30, 435–441.
- Usta, A.; Yilmaz, F.; Kapucu, G.; Baltaş, N.; Menteşe, E. Synthesis of Some New Benzimidazole Derivatives with Their Antioxidant Activities. Letters in Organic Chemistry 2015, 12, 227–232.
- Kantar, G.K.; Baltaş, N.; Menteşe, E.; S. Şaşmaz, Microwave-Assisted Synthesis and Investigation of Xanthine Oxidase Inhibition of New Phthalonitrile and Phthalocyanines Containing Morpholino Substituted 1,2,4-Triazole-3-One. Journal of Organometallic Chemistry 2015, 787, 8–13.
- Menteşe, M.Y.; Bayrak, H.; Uygun, Y.; Mermer, A.; Ulker, S.; Karaoglu, S.A.; Demirbas, N. Microwave Assisted Synthesis of Some Hybrid Molecules Derived from Norfloxacin and Investigation of their Biological Activities. European Journal of Medicinal Chemistry 2013, 67, 230–242.
- A. Altunkaya, Partial Purification And Characterization of Polyphenoloxidase from Turkish Tea Leaf (Camellia Sinensis L.). International Journal of Food Properties 2014, 17, 1490–1497.
- Gong, Z.; Li, D.; Liu, C.; Cheng, A.; Wang, W. Partial Purification and Characterization of Polyphenol Oxidase and Peroxidase from Chestnut Kernel. LWT–Food Science and Technology 2015, 60, 1095–1099.
- Wu, J.; Gao, J.; Chen, H.; Liu, X.; Cheng, W.; Ma, X.; Tong, P. Purification and Characterization of Polyphenol Oxidase from Agaricus Bisporus. International Journal of Food Properties 2013, 16, 1483–1493.
- Nirmal, N.P.; Benjakul, S. Use of Tea Extracts for Inhibition of Polyphenoloxidase and Retardation of Quality Loss of Pacific White Shrimp During Iced Storage. LWT–Food Science and Technology 2011, 44, 924–932.
- Shobha, R.I.; Rajeshwari, C.U.; Andallu, B. Oxidative Stress and Antioxidant Herbs and Spices in Cancer Prevention. Cancer 2014, 9, 91–100.
- Lespade, L.; Bercion, S. Theoretical Study of the Mechanism of Inhibition of Xanthine Oxidase by Flavonoids and Gallic Acid Derivatives. Journal of Physical Chemistry 2010, 114, 921–928.
- Azmi, S.M.N.; Jamal, P.; Amid, A. Xanthine Oxidase Inhibitory Activity from Potential Malaysian Medicinal Plant as Remedies for Gout. International Food Research Journal 2012, 19, 159–165.
- Umamaheswari, M.; Asokkumar, K.; Sivashanmugam, A.T.; Remyaraju, A.; Subhadradevi, V.; Ravi, T.K. In Vitro Xanthine Oxidase Inhibitory Activity of the Fractions of Erythrina Stricta Roxb. Journal of Ethnopharmacology 2009, 124, 646–648.
- Modolo, L.V.; Souza, A.X.; Hort, L.P.; Araujo, D.P.; Fatima, A. An Overview on the Potential of Natural Products as Ureases Inhibitors: A Review. Journal of Advanced Research 2015, 6, 35–44.
- Toyang, N.J.; Verpoorte, R. A Review of the Medicinal Potentials of Plants of the Genus Vernonia (Asteraceae). Journal of Ethnopharmacology 2013, 146, 681–723.
- Amin, M.; Anwar, F.; Naz, F.; Mehmood, T.; Saari, N. Anti-Helicobacter Pylori and Urease Inhibition Activities of Some Traditional Medicinal Plants. Molecules 2013, 18, 2135–2149.
- Olech, Z.; Zaborska, W.; Kot, M. Jack Bean Urease Inhibition by Crude Juices of Allium and Brassica Plants. Determination of Thiosulfinates. Food Chemistry 2014, 145, 154–160.