ABSTRACT
The volatile profiles of Vitis vinifera cv. Cabernet Gernischet wines in vintages 2010 and 2011 from rain-shelter cultivation and open-field cultivation were compared and detected by headspace solid phase micro-extraction with gas chromatography-mass spectrometry. The results showed that the number and concentration of volatile compounds quantified in wine were lower under rain-shelter cultivation compared with open-field cultivation. The strength of the fruity, fatty, caramel, and floral aromas of the 2010 vintage wines was lower under rain-shelter cultivation condition, and the compounds contributing to herbaceous aroma series were also lower with rain-shelter cultivation in the 2011 vintage wines. However, the strength of fruity, fatty, caramel, floral, and chemical aromas was higher with rain-shelter cultivation in vintage 2011. The cultivations in the two vintages could be clearly divided into three groups by principal component analysis. The difference between two treatments was significant in vintage 2010, whereas there was no significant difference in vintage 2011. The present work reveals the effects of rain-shelter cultivation on Cabernet Gernischet wine volatile compounds and explains that the technique is helpful in improving the wine quality in some rainy regions.
Introduction
With increasing production and consumption, the Chinese wine industry has developed with unprecedented speed in recent years, and the grape planting area in China has increased rapidly. It reached 680 mha in 2013, ranking fourth in the world, according to the International Organization of Vine and Wine (OIV) statistical report on world vitiviniculture.[Citation1] Most of China enjoys a marked continental monsoonal climate, characterized by hot and rainy summer–autumns and cold and dry winter–springs. These climatic characteristics lead to bad grape growth, sugar accumulation, organic acid degradation, and phenolic compound formation. This seriously hinders the development of the grape and wine industry in China and the improvement of China’s wine quality.
To overcome the disadvantages caused by local climatic conditions, protected cultivation was introduced to viticulture in China in the 1980s. Protected cultivation is a fruit culture practice featuring an artificial microclimate that meets fruit growth requirements in some installations in the case of an unfavorable natural environment for fruit growth.[Citation2] In the south of China, rain-shelter cultivation is usually implemented in the production of table grape berries.[Citation3] It has been indicated that the incidence of some major diseases, such as downy mildew (Plasmopara viticola), powdery mildew (Uncinula necator), botrytis (Botrytis cinerea), rip rot (Glomerella cingulata), and sour rot (imperfect yeasts), can be effectively reduced by keeping rainwater away from leaves and fruits in rain-shelter cultivation,[Citation4] and the technique also reduced the use of pesticides in vineyards.[Citation5]
The aroma is one of the important sensory characteristics of wines and it influences the consumers’ overall acceptability.[Citation6–Citation8] As reported, there were more than 800 volatile compounds found in grapes and wines, and their concentration ranged from hundreds of mg/L to the μg/L or ng/L level with different cultivars.[Citation9–Citation11] There are three main groups of wine flavors that originated from varietal, fermentative, and wine ageing aromas.[Citation12] The concentration of these volatile compounds changes with grape variety, environmental conditions, cultural techniques, grape maturity state, and winemaking and ageing techniques.[Citation13–Citation15]
It has been reported that rain-shelter cultivation effectively delayed the maturation of grape berries,[Citation3,Citation16] slowed sugar accumulation by reducing photosynthetically active radiation (PAR), increased berry and cluster weight, and improved economic returns.[Citation17] During the past several decades, although rain-shelter cultivation has been studied for its commercial value on table grapes and other fruits,[Citation4,Citation17–Citation19] few studies have focused on wine grapes and wine. To our knowledge, there has been no published literature on the aroma profiles in the wines of Cabernet Gernischet grape (Vitis vinifera L.) cultured under a rain-shelter model.
In view of the preceding discussion, the purpose of the present study was to compare the aroma profiles of Cabernet Gernischet wine whose grapes cultured under rain-shelter and open-field conditions. From a practical standpoint, this study was carried out to provide sufficient experimental evidence for further application and diffusion of rain-shelter cultivation. Based on the experiment, the cultivation of Cabernet Gernischet grapes and development of the wine industry with regard to organic production system is expected.
Materials and methods
Plant materials and vinification
The two-year study (2010 and 2011) was conducted at the Grape Demonstration Base of the College of Enology, Northwest A&F University, Jingyang County of Shaanxi Province, China (34°40’56’’ N, 108°38’53’’ E). The mesoclimate of the vineyard during the growing season (from April to September) of the two vintages is shown in . The own-rooted vines of Cabernet Gernischet (Vitis vinifera L.) were planted in 2006 and were oriented north-south on flat terroir with sandy soil. The vines were spaced at a distance of 1.8 × 2.7 m, spur-pruned and trained to a bilateral cordon 0.8 m above the ground; the heights of the grapevines’ canopies were from 0.8 to 1.8 m above the ground, and the shoots were trained upwards, and each vine carried ca. 20 grape clusters. The vertical shoot-positioned canopies were uniformly managed. All vines were divided into two groups. Group one was cultured with rain-shelter cultivation technology. Shelters were built along with vine rows before berry coloration and were 2.2 m high, 1.7 m wide and covered with colorless and transparent polyethylene film. Group two was the control and was cultured in an open field (open-field cultivation).
Table 1. Mesoclimate of vineyard during the growing season of the 2010 and 2011 vintages.
Small-scale wine making was conducted in triplicate using the following protocol: control and rain-shelter cultivation fruit (20 kg for each of the three replicates per treatment) were harvested at their physiological maturity, the harvest date was August 22 for open-field cultivation and September 18 for rain-shelter cultivation in vintage 2010, and that was August 30 and September 15 for each in vintage 2011 (). The grapes were crushed, destemmed, and placed separately in stainless steel tanks immediately after transported to the lab. Fifty mg/L sulfur dioxide and 25 mg/L pectinase (LALLZYME EX, Lallemand Company, France) were added to the musts, and the contents were mixed by hand. Considering the low acid content in grape must in both years, tartaric acid was added to the must. The must acid content was 8 g/L in each treatment, according to OIV.[Citation20] After maceration of the musts for 24 h, 200 mg/L active dry wine yeast (Saccharomyces cerevisiae strain RC212, Lavlin, France) was added to the musts according to commercial specifications.
Table 2. Physicochemical parameters of must and wine in the winemaking phases of different treatments.
Alcoholic fermentation was carried out at 22 to 25°C to dryness (reducing sugar < 4 g/L). Throughout this period, three mass homogenizations were performed per day to dissolve the cap of the wine. Temperature and density were also recorded three times per day to evaluate fermentation arrests. At the end of alcoholic fermentation, the wines were separated from pomace and added with SO2 at 50 mg/L. After fermentation, the wine samples were bottled and stored at 4°C prior to analysis (for physicochemical parameters, see ). The aromatic compounds were detected 4 months after the wines were well fermented in each year.
Physicochemical parameter analysis
The total reducing sugars, ethanol, pH, and total acidity were determined according to the National Standard of the People’s Republic of China-GB/T 15038-2006.[Citation21] The total reducing sugars were detected by Fehling’s solution reaction, and the sugars were represented by glucose.
Wine tasting
A wine tasting experiment was held when the wine samples were well done and stored at 4°C for 3 months. The tasting team was composed of 10 professional panelists. Each test session consisted of six wine samples coded with random numbers from 1 to 6; the order of the samples was randomized in each test session. Wine samples were stored at 4°C and presented at 20°C (ca 30 mL) for tasting. The tasting scores were defined according to the methods of Li[Citation22] and Tao.[Citation23]
Gas chromatography-mass spectrometry (GC‐MS) analysis of volatile compounds in wines
The extraction and analysis of wine volatile compounds were based on available methods developed by the Center for Viticulture and Enology, China Agricultural University.[Citation24–Citation26] In this method, volatile thiols and volatile phenols were not measured but they can have a huge impact in red wines. And the method of detecting these two kinds of volatile compounds is developing in our lab. Five milliliters of wine with 10 μL of internal standard 4-methyl-2-pentanol (4M2P, 1.0018 g/L) and 1 g of NaCl added were blended in a 15-mL sample vial tightly capped with a PTFE-silicon septum containing a magnetic stirrer. Afterwards, the vial containing the sample was equilibrated at 40°C for 30 min while being stirred. Then the pretreated solid-phase micro-extraction (SPME) fiber (50/30-μm DVB/Carboxen/PDMS, Supelco, Bellefonte, PA, USA) was then inserted into the headspace, and extracted for 30 min with continued heating and agitation of the sample. After that, the fiber was immediately desorbed in the GC injector for 8 min. The analysis of volatile compounds was performed on an Agilent 6890 GC equipped with an Agilent 5975 MS fitted with a 60 m × 0.25 mm HP-INNOWAX capillary column with 0.25 μm film thickness (J&W Scientific, Folsom, CA, USA). The flow rate of helium was 1 mL/min. The oven temperature was programmed at the successive temperature and time points as follows: 50°C for 1 min, increased to 220°C at a rate of 3°C/min and held at 220°C for 5 min. The ion source temperature was 230°C and the MS transfer-line temperature was 280°C. Electron ionization mass spectrometric data from m/z 30-350 were collected. The ionization voltage was 70 eV.
Data management and analysis were performed using ChemStation Software (Agilent Technologies, Inc.). The retention indices of reference standards and mass spectra matching in the standard NIST 08 library were used to identify the volatile compounds. A comparison of retention indices with those reported in the literature was processed when standards were unavailable. For quantification, a synthetic model wine solution was prepared in distilled water containing 14% vol ethanol (high-performance liquid chromatography [HPLC] quality); and 5 g/L tartaric acid, and the pH was adjusted to 3.8 with 5 M NaOH solution. 4-Methyl-2-pentanol (10 μL added before detection) was employed as the internal standard. The standard solution was diluted into eight levels successively, and volatile compounds of each level were extracted and analyzed using the same method as that used for the wine samples. Volatile compounds without chemical standards were estimated with equations of the compounds that had the same functional group and/or similar numbers of C atoms.
Reagents and standards
NaOH, NaCl and tartaric acid were purchased from Beijing Chemical Works. Ethanol was purchased from Sigma-Aldrich; 4-Methyl-2-pentanol (98.0%, Sigma-Aldrich, Milwaukee, WI) was used as internal standard. The manufacturers and purity information of the chemical standards used for identification and quantification of volatile compounds in this experiment is listed in , and the information of curves of the standards is listed in the additional materials.
Table 3. The parameters of volatile compounds identified in the Cabernet Gernischet wines.
Odor activity values (OAVs) and aroma series
The OAVs of each volatile compound were calculated to estimate the sensory contribution of these odorants to the general flavor of wine. The OAVs were calculated by dividing the concentration of each compound in the sample by its odor threshold value of the compound in water/ethanol solution.[Citation27,Citation28] To predict the overall aroma profile of the wine in the whole size from the data obtained from GC-MS analysis, nine aroma series were grouped based on similar odor descriptors of the aroma active compounds. The aroma series were modelled from the wine aroma wheel[Citation29] and literature (: fruity:1, floral: 2, herbaceous (or vegetal, fresh): 3, nutty: 4, caramel (or sweet): 5, earthy: 6, chemical: 7, fatty: 8, and roasted: 9). Due to the high complexity of olfactive perceptions, some volatile compounds were included in two or more aroma series according to some authors.[Citation30] In this study, except for the compounds without thresholds, the total intensities for each aroma series were calculated as the sum of the OAVs (∑OAV) of each compound assigned to this series list in according to some studies.[Citation24,Citation31–Citation34]
Statistical analysis
Statistical analysis was performed using the SPSS 18.0 software application (Chicago, IL, USA). Duncan analysis was used to assess statistically significant differences in the content of various volatile compounds between wines of the treatments with three replications of the same sample. Significant differences were calculated at 0.05 levels. Principal component analysis (PCA) was also used in the experiment. Origin 8.1 (Systat Software Inc., Richmond, CA, USA) and Excel 2010 (Microsoft Corp., Redmond, WA, USA) were used to draft the graph.
Results and discussion
Physicochemical parameters
To avoid the damage caused by diseases, when the berries were harvested, they may not have attained the optimal maturity. The oenological parameters () show that good ripeness and uniformity were achieved in the grapes. However, it should be noted that, to overcome the diseases of grape berries in vintage 2011, the treatment under open-field cultivation was harvested earlier than the other (rain-shelter cultivation). Except for the total sugar content of the open-field cultivation in vintage 2011, there were no significant differences in the total reducing sugars in the must, total acidity before fermentation and alcohol content of the wines for all treatments ().
Effects of rain-shelter cultivation on volatile compounds and their OAVs
In this study, a total of 57 volatiles were detected in all samples, in vintage 2010, 53 and 51 free volatile compounds were identified in rain-shelter cultivation (T1) and open-field cultivation (T2), respectively, whereas the number was 37 for both in vintage 2011. There were 44 and 45 free volatile compounds quantified, respectively, in rain-shelter cultivation (T1) and open-field cultivation (T2) in vintage 2010; there were 27 and 28, respectively, in vintage 2011. In the two vintages, no significant difference in the total concentration of volatile compounds between the two treatments of wines was found. lists all the volatile compounds identified and their aroma descriptors and thresholds. The quantitative results and OAVs of these compounds are shown in .
Table 4. The concentration of volatile compounds and their odor activity values (OAVs) at the end of alcoholic fermentation (AF) in two vintages.
Alcohols
Alcohols are released as secondary products of yeast metabolism. They can be produced by yeast through either the anabolic pathway from glucose or the catabolic pathway from corresponding amino acids.[Citation31] They are the largest group of free volatile compounds in Cabernet Gernischet wines and account for 51.5–77.1% of the volatile content (). This is consistent with the literature.[Citation11,Citation28,Citation35,Citation36] In the wine of the two vintages, the total content of alcohols was less in rain-shelter cultivation, and the difference was insignificant (p > 0.05). There were 23 and 22 alcohols identified in rain-shelter cultivation (T1) and open-field cultivation (T2) wines, respectively, in vintage 2010, whereas there were 14 and 13, respectively, in vintage 2011. The main compounds accounting for the difference were 1-propanol, 1-butanol, isobutanol, 3-methyl-3-buten-l-ol, 1-heptanol, 1-octanol, 2-phenylethanol, and (S)-3-ethyl-4-methylpentanol, in which the content of 1-propanol, 1-heptanol, (S)-3-ethyl-4-methylpentanol, and 1-octanol was observably higher in rain-shelter cultivation (T1) in vintage 2010, whereas the content of 1-butanol, isobutanol and 3-methyl-3-buten-l-ol was significantly higher in open-field cultivation (T2). For the 2011 vintage wines, the concentration of 1-pentanol and 2-phenylethanol was obviously higher under rain-shelter cultivation (T1) and open-field cultivation (T2), respectively.
Esters
Esters in wine, the primary source of fruity aromas,[Citation37] are the second major volatile constituent. Most esters are secondary metabolites produced by yeast during alcoholic fermentation.[Citation38] According to the pathway the esters produced, esters can be divided into two main groups. Acetate esters came from the reaction of acetyl-CoA with higher alcohols formed by the degradation of amino acids or carbohydrates.[Citation37,Citation38] Fatty acid esters, another important group of esters in wine, are produced during yeast fermentation through ethanolysis of acyl-CoA, which is formed during fatty acid synthesis or degradation.[Citation37,Citation38] In this study, in vintage 2010, 20 and 17 esters were detected, in rain-shelter cultivation (T1) and open-field cultivation (T2), respectively, whereas both were 17 in vintage 2011. Of all esters detected, there were 15 and 14 quantified in vintage 2010 in rain-shelter cultivation (T1) and open-field cultivation (T2), respectively, and both were 9 in vintage 2011. In the two treatments of the two vintages, esters accounted for 15.4-30.0% of the volatile compounds (). In vintage 2010, the content of ethyl acetate, ethyl 9-decenoate and ethyl octanoate was significantly higher in the wine from rain-shelter cultivation (T1), whereas the content of ethyl lactate and diethyl butanedioate was higher in wine from open-field cultivation (T2), and the difference was remarkable. In vintage 2011, the content of phenethyl acetate, ethyl decanoate, ethyl ester, and ethyl laurate was highest in the wine from rain-shelter cultivation (T1), and the content of ethyl 9-decenoate and isoamyl acetate was significantly higher in the wine from rain-shelter cultivation (T1). The content of ethyl benzenepropanoate was higher in the wine from open-field cultivation (T2), and the difference was most remarkable. In the two vintages, the total content of esters was higher in rain-shelter cultivation (T1), but the difference was insignificant.
Acids
Six volatile organic acids were detected in this study, of which 5 and 6 were found in rain-shelter cultivation (T1) and open-field cultivation (T2) in vintage 2010, respectively, and 5 was in vintage 2011. The kind of aromas were produced by yeast and bacteria during fatty acid metabolism.[Citation38] They were described by various fatty, pungent, rancid, fruity, or cheesy odors (). No difference was found in the content of acids between the wine of the two treatments in both vintages, and the total content was slightly higher in open-field cultivation (T2).
Terpenes and others
Terpene and norisoprenoids are two classes of important volatile compounds in wine. They originate from grape berries and are transferred to the wine during fermentation. This group of compounds belongs to the secondary plant constituents and is produced from glucose by acetyl-coenzyme A during grape ripening.[Citation27,Citation39] Terpene compounds have a low olfactory threshold and are generally associated with floral and citric aromas;[Citation40] yeast metabolism has little effect on them during fermentation, so they are good indicators for the variety and quality of grapes.[Citation41] Two terpenes were totally detected in the two vintage wine samples, and both were quantified in rain-shelter cultivation (T1) and open-field cultivation (T2). The content of β-damascenone and linalool was higher in open-field cultivation (T2), and the difference was more significant. Three further compounds were detected in this study—benzaldehyde, 4-methyl-1 -pentene and butylated hydroxytoluene[Citation42,Citation43]—and the content of all were less than their threshold.
Volatile thiols and volatile phenols
Volatile thiols, may play a major role in the aroma of many fresh plants, vegetables, fruit, or processed products (roasted coffee, wine, etc.).[Citation44–Citation46] However, the fact that these substances are present in these complex natural products in trace amounts makes it difficult to identify them and measure their concentrations. Volatile phenols also play an important role in the wine aroma, and they contribute to the overall aroma.[Citation47] The volatile thiols and volatile phenols were not detected by the method used in the current article, while the new method to detect them was building in our lab.
Influence of rain-shelter treatments on aroma profiles
The total OAVs (∑OAV) for each series were calculated by adding the OAVs for each individual compound assigned to this series as listed in ( and ). The ∑OAV of series 4 (nutty) and 6 (earthy) were not shown in because of their low contribution to the overall wine aroma (∑OAV < 1). The application of aroma series makes it possible to correlate quantitative information obtained by chemical analysis and sensory characters of the samples. This has been used by many authors in recent years.[Citation24,Citation31–Citation34]
Figure 1. The total OAVs (∑OAV) of aroma series and tasting scores about volatile compounds in wines. The total OAVs (∑OAV) of aroma series in wines in 2010 (A) and 2011(B); the tasting scores about volatile compounds in wines of 2010(C) and 2011(D). T1: Rain-shelter cultivation; T2: Open-air cultivation.
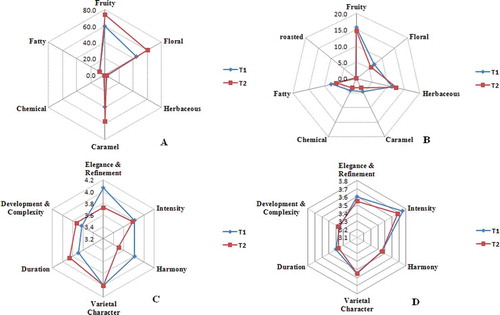
The fruity series had the highest intensity in all treatments. The major contributors to this series were four esters (ethyl hexanoate, ethyl octanoate, isoamyl acetate, and ethyl acetate) and one norisoprenoid (β-damascenone), all of which had an OAV greater than 1 (). The influence of rain-shelter treatment on this aroma series differed between the two vintages. In vintage 2010, wine under rain-shelter cultivation (T1) led to a significant decrease (20%) in ∑OAV for the fruity aroma series, whereas it had a slight increase (6%) in vintage 2011.
In this study, caramel and floral series were two other major aroma series. For the caramel series, the ∑OAV was dominated by four compounds (isopentanol, phenethyl acetate, linalool, and β-damascenone), and the floral series was dominated by seven compounds (1-octanol, 2-phenylethanol, phenethyl acetate, ethyl octanoate, linalool, β-damascenone, and geraniol). Similar to the fruity aroma series, the caramel and floral series were reduced by rain-shelter cultivation (T1) in vintage 2010 and increased in vintage 2011.
The fatty aroma series mainly consisted of four organic acids (propanoic acid, n-decanoic acid, hexanoic acid, and octanoic acid) and three alcohols (isopentanol, 1-heptanol, and (Z)-3-hexen-1-ol). The influence of rain-shelter treatment on this aroma series differed between the two vintages. In vintage 2010, wine under rain-shelter cultivation (T1) decreased in ∑OAV for the fatty aroma series, and the difference was not significant, whereas it increased in vintage 2011.
Herbaceous and chemical aroma series are usually considered as negative factors to wine aroma. They could enhance the complexity of wine aroma at low concentrations, whereas at high concentrations they destroy the fruity note. In this study, four alcohols (1-hexanol, (Z)-3-hexen-1-ol, isobutanol, and 3-methyl-1-pentanol) contributed to the herbaceous series, and four alcohols (1-butanol, isobutanol, isopentanol, and 3-methyl-1-pentanol) contributed to the chemical series. The herbaceous aroma series was decreased by rain-shelter cultivation (T1); the results were similar in both vintages, and the difference was insignificant. Whereas the chemical series was increased by rain-shelter cultivation (T1) in vintage 2011, the difference was not remarkable. From the results above, it is clear that rain-shelter cultivation (T1) reduced ∑OAVs for the volatile compounds in all categories in vintage 2010, whereas the influence was opposite except for herbaceous categories in vintage 2011. The result of vintage 2011 is consistent with previous studies in other quality indexes by other researchers.[Citation3,Citation48] A wine tasting experiment was held to compare the total behavior of wine under different cultivations in different vintages ( and ). From , wine from rain-shelter cultivation in vintage 2010 has higher scores of harmony, elegance and refinement than wine of open-field cultivation, whereas in vintage 2011, there was little difference between the two wines.
PCA
To reveal the influence of rain-shelter cultivation on aromatic constituents, PCA was performed. shows the loading plot on the plane defined by the first and second principal components and the corresponding projection of samples. The first principal component (PC1) describes 70.7% of the variability in the data, and the second (PC2) describes 19.2%. PC1 is correlated highly with all of the aromatic compounds except for V29 (ethyl hexoate), V32 (ethyl lactate) and V47 (hexanoic acid), whereas PC2 is correlated highly with V4 ((E)-2-hexen-1-ol), V15 (2-ethyl-1-hexanol), V16 (2-nonanol), V18 (1-dodecanol), V22 ((S)-3-ethyl-4-methylpentanol), V24 (1,9-nonanediol), V25 (3-methyl-1,5-Pentanediol), V29 (ethyl hexoate), V32 (ethyl lactate), V36 (methyl salicylate), V37 (ethyl decanoate), V41 (n-hexyl isobutyrate), V43 (ethyl octadecanoate), V44 (ethyl hydroxycinnamate), V45 (ethyl eicosanoate), V46 (propanoic acid), V55 (β-damascenone), V56 (4-methyl-1-pentene), and V57 (butylated hydroxytoluene). The cultivations in the two vintages could be clearly divided into three groups according to their relationships between scores and their aromatic constituents. The first group was composed of rain-shelter cultivation in vintage 2010 (T1-10) with the higher positive value of PC1 and PC2, owing to its higher values of V4 ((E)-2-hexen-1-ol), V16 (2-nonanol), V24 (1, 9-nonanediol), V25 ((3-methyl-1, 5-Pentanediol), and V29 (ethyl hexoate).
Figure 2. Distribution of wine samples and loadings of volatile compounds in the first two PCs of volatile compounds. T1-10 Rain-shelter cultivation wine in vintage 2010; T2-10: Open-air cultivation wine in vintage 2010. T1-11: Rain-shelter cultivation wine in vintage 2011. T2-11: Open-air cultivation wine in vintage 2011. V1-V57: The volatile compounds in from 1-hexanol to butylated hydroxytoluene in order.
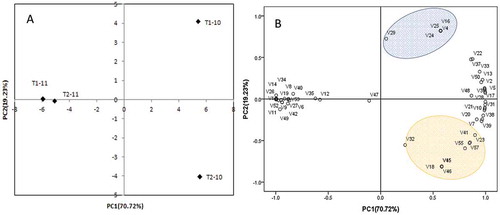
The second group was open-field cultivation in vintage 2010 (T2-10) with higher positive and negative values of PC1 and PC2, respectively. It could be characterized by V18 (1-dodecanol), V23 3-(methylthio)-1-propanol), V32 (ethyl lactate), V41 (n-hexyl isobutyrate), V45 (ethyl eicosanoate), V46 (propanoic acid), V55 (β-damascenone), and V57 (butylated hydroxytoluene). The third group was composed of rain-shelter cultivation and open-field cultivation in vintage 2011 (T1-11 and T2-11) with the higher negative value of PC1. There was little difference between the two treatments.
Conclusions
The volatile components in wine were influenced by a lot of aspects, such as the variety of grape,[Citation49–Citation51] the position of berry in the same variety,[Citation49,Citation52,Citation53] the sugar concentration of berry and wine,[Citation54–Citation56] the effect of fungicides,[Citation57–Citation61] the yeast stains used,[Citation60,Citation62] and the agronomic and technological practices during grape growing and winemaking.[Citation3,Citation50] In this study, the influence of rain-shelter cultivation on volatile compounds of Cabernet Gernischet wines was investigated and compared with those of open-field cultivation. The results showed that the number and concentration of volatile compounds quantified in wines decreased under rain-shelter cultivation compared with that of open-air cultivation. The fruity, fatty, caramel, and floral aromas also decreased in rain-shelter cultivation in vintage 2010, whereas the compounds contributing to fruity and fatty, caramel and floral aroma series increased by rain-shelter cultivation in vintage 2011. In the two vintages, these differences were closely related to climate alterations[Citation3] in the vineyards (). In vintage 2011, the average temperature in the vine stage was lower, and the rainfall was higher than that of vintage 2010, which was bad for grape growth in open-field cultivation.[Citation3] Lower temperatures and heavier rainfall caused serious diseases among the grape berries. The bad quality and lower maturity of grape berries thus influenced the wine quality.[Citation63] To overcome this, the application of rain-shelter cultivation in wine grapes is urgently needed. And the content of volatile thiols and volatile phenols were not detected in this study, while the method for them was building. In some researches,[Citation57–Citation61] the application of fungicides increased the strength of fruity aroma due to several ethyl esters, and reduced the varietal aroma of wines. But the problem of fungicide residual is very serious. The application of rain-shelter can keep rainwater away from leaves and fruits, and decreased the diseases of fruit. Thus the use of fungicides can be avoided, and makes the cultivation of grape Cabernet Gernischet and wine industry in an organic production system.
Nomenclature
SPME-GC/MS Solid-phase micro-extraction gas chromatography mass spectrometry
GC-MS Gas chromatography mass spectrometry
OAVs Odor activity values
OIV International Organization of Vine and Wine
T1 Rain-shelter cultivation
T2 Open-field cultivation
LJFP_1174711_Supplementary_File.zip
Download Zip (19.7 KB)Acknowledgments
The authors would like to thank the Center for Viticulture and Enology, China Agricultural University for technical assistance in the completion of the GC/MS experiments. The authors also like to thank Bao Jiang in Weinan Testing and Inspection and Research Center of Agricultural Products and Food, Weinan Vocational and Technical College, and Jiang-Fei Meng in the college of Enology, Northwest A&F University, for their critical review of the manuscript. The American Journal Experts helped with the editing of the English language throughout the article.
Funding
This study was funded by a grant from the National Technology System for Grape Industry (CARS-30-zp-9).
Additional information
Funding
References
- OIV. Statistical report on World vitiviniculture 2013. http://www.oiv.int/oiv/cms/index?rubricId=44538631-44538637ad44538632-44538649cb-44539710-ad44538635b44957296c44538637/EN/Report.pdf ( accessed on May 15, 2013).
- He, P.C.; Luo, G.G. Ampeliology; China Agriculture Press: Beijing, China, 1999; pp. 54–76.
- Meng, J.F.; Ning, P.F.; Xu, T.F.; Zhang, Z.W. Effect of Rain-Shelter Cultivation of Vitis Vinifera cv. Cabernet Gernischet on the Phenolic Profile of Berry Skins and the Incidence of Grape Diseases. Molecules 2012, 18(1), 381–397.
- Chavarria, G.; Santos, H.P.D.; Zanus, M.C.; Marodin, G.A.B.; Zorzan, C. Plastic Cover Use and Its Influences on Physical-Chemical Characteristics in Must and Wine. Revista Brasileira de Fruticultura 2011, 33(3), 809–815.
- Cortell, J.M.; Kennedy, J.A. Effect of Shading on Accumulation of Flavonoid Compounds in (Vitis Vinifera L.) Pinot Noir Fruit and Extraction in a Model System. Journal of Agricultural and Food Chemistry 2006, 54(22), 8510–8520.
- Gómez-Míguez, M.J.; Cacho, J.F.; Ferreira, V.; Vicario, I.M.; Heredia, F.J. Volatile Components of Zalema White Wines. Food Chemistry 2007, 100(4), 1464–1473.
- Rocha, S.M.; Coutinho, P.; Coelho, E.; Barros, A.S.; Delgadillo, I.; Coimbra, M.A. Relationships Between the Varietal Volatile Composition of the Musts and White Wine Aroma Quality. A Four Year Feasibility Study. LWT–Food Science and Technology 2010, 43(10), 1508–1516.
- Zea, L.; Moyano, L.; Ruiz, M.; Medina, M. Odor Descriptors and Aromatic Series During the Oxidative Aging of Oloroso Sherry Wines. International Journal of Food Properties 2013, 16(7), 1534–1542.
- Aznar, M.; Lopez, R.; Cacho, J.F.; Ferreira, V. Identification and Quantification of Impact Odorants of Aged Red Wines from Rioja. GC-Olfactometry, Quantitative GC-MS, and Odor Evaluation of HPLC Fractions. Journal of Agricultural and Food Chemistry 2001, 49(6), 2924–2929.
- Tao, Y.; Li, H.; Wang, H.; Zhang, L. Volatile Compounds of Young Cabernet Sauvignon Red Wine from Changli County (China). Journal of Food Composition and Analysis 2008, 21(8), 689–694.
- García-Carpintero, E.G.; Sánchez-Palomo, E.; González-Viñas, M. Aroma Characterization of Red Wines From cv. Bobal Grape Variety Grown in La Mancha Region. Food Research International 2011, 44(1), 61–70.
- Jiang, B.; Zhang, Z. Volatile Compounds of Young Wines from Cabernet Sauvignon, Cabernet Gernischet and Chardonnay Varieties Grown in the Loess Plateau Region of China. Molecules 2010, 15(12), 9184–9196.
- Gómez-Míguez, M.J.; Gómez-Míguez, M.; Vicario, I.M.; Heredia, F.J. Assessment of Colour and Aroma in White Wines Vinifications: Effects of Grape Maturity and Soil Type. Journal of Food Engineering 2007, 79(3), 758–764.
- Chira, K.; Pacella, N.; Jourdes, M.; Teissedre, P.L. Chemical and Sensory Evaluation of Bordeaux Wines (Cabernet-Sauvignon and Merlot) and Correlation with Wine Age. Food Chemistry 2011, 126(4), 1971–1977.
- Dennis, E.G.; Keyzers, R.A.; Kalua, C.M.; Maffei, S.M.; Nicholson, E.L.; Boss, P.K. Grape Contribution to Wine Aroma: Production of Hexyl Acetate, Octyl Acetate, and Benzyl Acetate During Yeast Fermentation is Dependent Upon Precursors in the Must. Journal of Agricultural and Food Chemistry 2012, 60(10), 2638–2646.
- Berli, F.J.; Fanzone, M.; Piccoli, P.; Bottini, R. Solar UV-B and ABA are Involved in Phenol Metabolism of Vitis Vinifera L. Increasing Biosynthesis of Berry Skin Polyphenols. Journal of Agricultural and Food Chemistry 2011, 59(9), 4874–4884.
- Tangolar, S.G.; Tangolar, S.; Blllr, H.; Ozdemir, G.; Sabir, A.; Cevlk, B. The Effects of Different Irrigation Levels on Yield and Quality of Some Early Grape Cultivars Grown in Greenhouse. Asian Journal of Plant Sciences 2007, 6(4), 643–647.
- Fanizza, G.; Ricciardi, L. The Effect of Vineyard Overhead Plastic Sheet Covering on Some Morphological and Physiological Characteristics in the Table Grape cv. Regina Dei Vigneti. Vitis Vinifera L. Agricoltura Mediterranea 1991, 121, 239–243.
- Júnior, M.J.P.; Hernandes, J.L.; Souza Rolim, G.D.; Blain, G.C. Microclimate and Yield Of’niagara Rosada’grapevine Grown on Vertical Upright Trellis and “Y” Shaped Under Permeable Plastic Cover Overhead. Revista Brasileira de Fruticultura 2011, 33(SPE1), 511–518.
- OIV. Codex Oenologique Internetional. 2006. Available online at http://www.oiv.int/oiv (accessed on19 May 2006).
- GB, GB/T15038. Analytical Methods of Wine and Fruit Wine; Beijing: China Standard Press, Beijing, China, 2006.
- Li, H. Wine Tasting; China Science Press: Beijing, China, 2006.
- Tao, Y.S.; Liu, Y.Q.; Li, H. Sensory Characters of Cabernet Sauvignon Dry Red Wine from Changli County (China). Food Chemistry 2009, 114(2), 565–569.
- Cai, J.; Zhu, B.Q.;Wang, Y.H.; Lu, L.; Lan, Y.B.; Reeves, M.J.; Duan, C.Q. Influence of Pre-Fermentation Cold Maceration Treatment on Aroma Compounds of Cabernet Sauvignon Wines Fermented in Different Industrial Scale Fermenters. Food Chemistry 2014, 154, 217–229.
- Wen, Y.Q.; He, F.; Zhu, B.Q.; Lan, Y.B.; Pan, Q.H.; Li, C.Y.; Reeves, M.J.; Wang, J. Free and Glycosidically Bound Aroma Compounds in Cherry (Prunus Avium L.). Food Chemistry 2014, 152, 29–36.
- Xu, X.Q.; Cheng, G.; Duan, L.L.; Jiang, R.; Pan, Q.H.; Duan, C.Q.; Wang, J. Effect of Training Systems on Fatty Acids and Their Derived Volatiles in Cabernet Sauvignon Grapes and Wines of the North Foot of Mt. Tianshan. Food Chemistry 2015, 181, 198–206.
- Hellin, P.; Manso, A.; Flores, P.; Fenoll, J. Evolution of Aroma and Phenolic Compounds During Ripening of “Superior Seedless” Grapes. Journal of Agricultural and Food Chemistry 2010, 58(10), 6334–6340.
- Yue, T.X.; Chi, M.; Song, C.Z.; Liu, M.Y.; Meng, J.F.; Zhang, Z.W.; Li, M.H. Aroma Characterization of Cabernet Sauvignon Wine from the Plateau of Yunnan (China) with Different Altitudes Using SPME-GC/MS. International Journal of Food Properties 2015, 18(7), 1584–1596.
- Noble, A.C.; Arnold, R.A.; Buechsenstein, J.; Leach, E.J.; Schmidt, J.; Stern, P.M. Modification of a Standardized System of Wine Aroma Terminology. American Journal of Enology and Viticulture 1987, 38(2), 143–146.
- Zea, L.; Moyano, L.; Moreno, J.A.; Medina, M. Aroma Series as Fingerprints for Biological Ageing in Fino Sherry-Type Wines. Journal of the Science of Food and Agriculture 2007, 87(12), 2319–2326.
- Peinado, R.A.; Mauricio, J.C.; Moreno, J. Aromatic Series in Sherry Wines with Gluconic Acid Subjected to Different Biological Aging Conditions by Saccharomyces Cerevisiae Var. Capensis. Food Chemistry 2006, 94(2), 232–239.
- Sánchez, P.E.; Gómez García, C.E.; Alonso, V.R.; González, V.M. Characterization of Aroma Compounds of Verdejo White Wines from the La Mancha Region by Odour Activity Values. Flavour and Fragrance Journal 2010, 25(6), 456–462.
- Wu, Y.; Zhu, B.; Tu, C.; Duan, C.; Pan, Q. Generation of Volatile Compounds in Litchi Wine During Winemaking and Short-Term Bottle Storage. Journal of Agricultural and Food Chemistry 2011, 59(9), 4923–4931.
- Noguerol-Pato, R.; González-Álvarez, M.; González-Barreiro, C.; Cancho-Grande, B.; Simal-Gándara, J. Aroma Profile of Garnacha Tintorera-Based Sweet Wines by Chromatographic and Sensorial Analyses. Food Chemistry 2012, 134(4), 2313–2325.
- Selli, S.; Cabaroglu, T.; Canbas, A.; Erten, H.; Nurgel, C.; Lepoutre, J.; Gunata, Z. Volatile Composition of Red Wine from Cv. Kalecik Karasι Grown in Central Anatolia. Food Chemistry 2004, 85(2), 207–213.
- Jiang, B.; Xi, Z.; Luo, M.; Zhang, Z. Comparison on Aroma Compounds in Cabernet Sauvignon and Merlot Wines from Four Wine Grape-Growing Regions in China. Food Research International 2013, 51(2), 482–489.
- Sumby, K.M.; Grbin, P.R.; Jiranek, V. Microbial Modulation of Aromatic Esters in Wine: Current Knowledge and Future Prospects. Food Chemistry 2010, 121(1), 1–16.
- Swiegers, J.H.; Pretorius, I.S. Yeast Modulation of Wine Flavor. Advances in Applied Microbiology 2005, 57, 131–175.
- Meng, J.F.; Xu, T.F.; Song, C.Z.; Yu, Y.; Hu, F.; Zhang, L.; Zhang, Z.W.; Xi, Z.M. Melatonin Treatment of Pre-Veraison Grape Berries to Increase Size and Synchronicity of Berries and Modify Wine Aroma Components. Food Chemistry 2015, 185, 127–134.
- Guth, H. Identification of Character Impact Odorants Oof Different White Wine Varieties. Journal of Agricultural and Food Chemistry 1997, 45(8), 3022–3026.
- Begala, M.; Corda, L.; Podda, G.; Fedrigo, M.A.; Traldi, P. Headspace Solid-Phase Microextraction Gas Chromatography/Mass Spectrometry in the Analysis of the Aroma Constituents of “Cannonau Of Jerzu” Wine. Rapid Communications in Mass Spectrometry 2002, 16(11), 1086–1091.
- Lu, K.; Qiao, H.; Xue, C.R.; Liu, S.W.; Xu, X.W. Aromatic Components Analysis in Litehi Brandy by GS-MS With Stir Bar Sportive Extraction. China Brewing 2014, 33(3), 137–140.
- Li, Z.; Zhang, P.; Huang, Y.; Cheng, M.; Zhu, Z.Q. Effect of Sulfur Dioxide Injury on Aroma Components of Postharvest Red Globe. Acta Botanica Boreali-Occidentalia Sinica 2011, 31(2), 385–392.
- Tominaga, T.; Murat, M.-L.; Dubourdieu, D. Development of a Method for Analyzing the Volatile Thiols Involved in the Characteristic Aroma of Wines Made from Vitis vinifera L. cv. Sauvignon Blanc. Journal of Agricultural and Food Chemistry 1998, 46(3), 1044–1048.
- Tominaga, T.; Blanchard, L.; Darriet, P.;Dubourdieu, D. A Powerful Aromatic Volatile Thiol, 2-Furanmethanethiol, Exhibiting Roast Coffee Aroma in Wines Made from Several Vitis v inifera Grape Varieties. Journal of Agricultural and Food Chemistry 2000, 48(5), 1799–1802.
- Randle, W.; Lancaster, J.; Rabinowitch, H.; Currah, L. Sulphur Compounds in Alliums in Relation to Flavour Quality. Allium Crop Science: Recent Advances 2002, 329–356.
- Etievant, P.X. Volatile Phenol Determination in Wine. Journal of Agricultural and Food Chemistry 1981, 29(1), 65–67.
- Li, X.X.; He, F.; Wang, J.; Li, Z.; Pan, Q.H. Simple Rain-Shelter Cultivation Prolongs Accumulation Period of Anthocyanins in Wine Grape Berries. Molecules 2014, 19(9), 14843–14861.
- Noguerol-Pato, R.; González-Barreiro, C.; Cancho-Grande, B.; Martínez, M.; Santiago, J.; Simal-Gándara, J. Floral, Spicy and Herbaceous Active Odorants in Gran Negro Grapes from Shoulders and Tips into the Cluster, and Comparison with Brancellao and Mouratón Varieties. Food Chemistry 2012, 135(4), 2771–2782.
- Reboredo-Rodríguez, P.; González-Barreiro, C.; Cancho-Grande, B.; Simal-Gándara, J. Effects of Sedimentation Plus Racking Process in the Extra Virgin Olive Oil Aroma Fingerprint Obtained by DHS–TD/GC–MS. Food and Bioprocess Technology 2013, 6(5), 1290–1301.
- Reboredo-Rodríguez, P.; González-Barreiro, C.; Cancho-Grande, B.; Simal-Gándara, J. Dynamic Headspace/GC–MS to Control the Aroma Fingerprint of Extra-Virgin Olive Oil from the Same and Different Olive Varieties. Food Control 2012, 25(2), 684–695.
- Noguerol-Pato, R.; González-Barreiro, C.; Simal-Gándara, J.; Martínez, M.; Santiago, J.; Cancho-Grande, B. Active Odorants in Mouratón Grapes from Shoulders and Tips into the Bunch. Food Chemistry 2012, 133(4), 1362–1372.
- Noguerol-Pato, R.; González-Barreiro, C.; Cancho-Grande, B.; Santiago, J.; Martínez, M.; Simal-Gándara, J. Aroma Potential of Brancellao Grapes from Different Cluster Positions. Food Chemistry 2012, 132(1), 112–124.
- Noguerol-Pato, R.; González-Álvarez, M.; González-Barreiro, C.; Cancho-Grande, B.; Simal-Gándara, J. Evolution of the Aromatic Profile in Garnacha Tintorera Grapes During Raisining and Comparison with That of the Naturally Sweet Wine Obtained. Food Chemistry 2013, 139(1), 1052–1061.
- Reboredo-Rodríguez, P.; González-Barreiro, C.; Rial-Otero, R.; Cancho-Grande, B.; Simal-Gándara, J. Effects of Sugar Concentration Processes in Grapes and Wine Aging on Aroma Compounds of Sweet Wines—A Review. Critical Reviews in Food Science and Nutrition 2015, 55(8), 1053–1073.
- González-Álvarez, M.; Noguerol-Pato, R.; González-Barreiro, C.; Cancho-Grande, B.; Simal-Gándara, J. Sensory Quality Control Of Young Versus Aged Sweet Wines Obtained by The Techniques of Both Postharvest Natural Grape Dehydration and Fortification with Spirits During Vinification. Food Analytical Methods 2013, 6(1), 289–300.
- Álvarez, M.G.; Noguerol-Pato, R.; González-Barreiro, C.; Cancho-Grande, B.; Simal-Gándara, J. Changes of the Sensorial Attributes of White Wines with the Application of New Anti-Mildew Fungicides Under Critical Agricultural Practices. Food Chemistry 2012, 130(1), 139–146.
- Noguerol-Pato, R.; Sieiro-Sampedro, T.; González-Barreiro, C.; Cancho-Grande, B.; Simal-Gándara, J. Evaluation of the Effect of Fenhexamid and Mepanipyrim in the Volatile Composition of Tempranillo and Graciano Wines. Food Research International 2015, 71, 108–117.
- González-Álvarez, M.; González-Barreiro, C.; Cancho-Grande, B.; Simal-Gándara, J. Impact of Phytosanitary Treatments with Fungicides (Cyazofamid, Famoxadone, Mandipropamid and Valifenalate) on Aroma Compounds of Godello White Wines. Food Chemistry 2012, 131(3), 826–836.
- Noguerol-Pato, R.; González-Rodríguez, R.; González-Barreiro, C.; Cancho-Grande, B.; Simal-Gándara, J. Influence of Tebuconazole Residues on the Aroma Composition of Mencía Red Wines. Food Chemistry 2011, 124(4), 1525–1532.
- González-Rodríguez, R.; Noguerol-Pato, R.; González-Barreiro, C.; Cancho-Grande, B.; Simal-Gándara, J. Application of New Fungicides Under Good Agricultural Practices and Their Effects on the Volatile Profile of White Wines. Food Research International 2011, 44(1), 397–403.
- Salgado, J.M.; González-Barreiro, C.; Rodríguez-Solana, R.; Simal-Gándara, J.; Domínguez, J.M.; Cortés, S. Study of the Volatile Compounds Produced by Debaryomyces Hansenii NRRL Y-7426 During the Fermentation of Detoxified Concentrated Distilled Grape Marc Hemicellulosic Hydrolysates. World Journal of Microbiology and Biotechnology 2012, 28(11), 3123–3134.
- Falcão, L.D.; de Revel, G.; Perello, M.C.; Moutsiou, A.; Zanus, M.C.; Bordignon-Luiz, M.T. A Survey of Seasonal Temperatures and Vineyard Altitude Influences on 2-Methoxy-3-Isobutylpyrazine, C13-Norisoprenoids, and the Sensory Profile of Brazilian Cabernet Sauvignon Wines. Journal of Agricultural and Food Chemistry 2007, 55(9), 3605–3612.
- Peinado, R.A.; Moreno, J.; Bueno, J.E.; Moreno, J.A.; Mauricio, J.C. Comparative Study of Aromatic Compounds in Two Young White Wines Subjected to Pre-Fermentative Cryomaceration. Food Chemistry 2004, 84(4), 585–590.
- Franco, M.; Peinado, R.A.; Medina, M.; Moreno, J. Off-Vine Grape Drying Effect on Volatile Compounds and Aromatic Series in Must from Pedro Ximénez Grape Variety. Journal of Agricultural and Food Chemistry 2004, 52(12), 3905–3910.
- Tao, Y.; Zhang, L. Intensity Prediction of Typical Aroma Characters of Cabernet Sauvignon Wine in Changli County (China). LWT–Food Science and Technology 2010, 43(10), 1550–1556.
- Ferreira, V.; López, R.; Cacho, J.F. Quantitative Determination of the Odorants of Young Red Wines from Different Grape Varieties. Journal of the Science of Food and Agriculture 2000, 80(11), 1659–1667.
- La Guerche, S.; Dauphin, B.; Pons, M.; Blancard, D.; Darriet, P. Characterization of Some Mushroom and Earthy off-Odors Microbially Induced by the Development of Rot on Grapes. Journal of Agricultural and Food Chemistry 2006, 54(24), 9193–9200.
- Pineau, B.; Barbe, J.C.; Van Leeuwen, C.; Dubourdieu, D. Which Impact for β-Damascenone on Red Wines Aroma. Journal of Agricultural and Food Chemistry 2007, 55(10), 4103–4108.
