ABSTRACT
Myristica fragrans derivatives are used as spices in cuisines and also serve as an important source of traditional medicine worldwide. The present study has evaluated the antioxidative potential of the hydro-methanolic extract of the fruit aril of M. fragrans, which is also referred to as mace. This study also includes a quantitative phytochemical assessment of the extract and focuses on the ability of the extract to inhibit xanthine oxidase, a molecular target involved in nucleic acid metabolism and the enzyme responsible for the development of gout disease. The extract was found to contain different classes of secondary metabolites, including flavonoids, phenolics, tannins, alkaloids, and saponins. Furthermore, the antioxidative potential of the extract was evaluated against the radicals 2,2-azino-bis(3-ethylbenzothiazoline- 6-sulfonic acid) diammonium salt and 2,2-diphenyl-1-pycrylhydrazyl, and there was a progressive reduction of the radicals with increasing doses of the extract. Moreover, a ferric reducing power assay also showed the antioxidant activity of the crude extract as compared to ascorbic acid, a well-known antioxidant. Another significant finding of the study is that the mace extract also considerably inhibits the enzyme xanthine oxidase in a concentration-dependent manner. Our results partially support the use of mace in dietary regimens as a nutraceutical that could serve as a prophylactic strategy or an adjuvant for the prevention and therapy of gout disease.
Introduction
The World Health Organization (WHO) has recognized traditional medicine as an accessible, affordable and culturally acceptable form of health care trusted by large numbers of people, which stands out as a way of coping with the relentless rise of chronic non-communicable diseases in the midst of soaring health-care costs and nearly universal austerity.[Citation1]
These observations provide an impetus to current efforts to rekindle the ancient renaissance, a belief in the practice of disease management through dietary and non-dietary agents found in plants. The relatively novel concepts of functional foods and nutraceuticals are fundamentally derivatives of the same philosophy, which was advocated by Hippocrates, the father of modern medicine and stated as “Let thy food be thy medicine, and thy medicine be thy food.”
In recent years, the potential of nutraceuticals/functional foods in mitigating a number of health-related issues has received wide scientific credence.[Citation2] The fruit seed (nutmeg) and aril (mace) of M. fragrans are regularly used as ingredients of spices to flavor various dishes worldwide.[Citation3] The derivatives of the plant also serve as constituents of traditional medicine in countries, such as India, China, Thailand, and England, to treat various ailments, such as inflammatory disorders and rheumatoid arthritis.[Citation4,Citation5] Most of the studies related to the pharmacological properties of M. fragrans have been carried out using nutmeg, but mace also seems to carry the potential for unexplored medicinal properties. Gout is the most common form of inflammatory arthritis and is caused by the chronic elevation of serum uric acid levels above the saturation point, leading to monosodium urate crystal formation. The global burden of gout is substantial and seems to have increased in many parts of the world over the past 50 years. Moreover, the incidence of gout increases with age in a linear fashion.[Citation6]
Allopurinol, a xanthine oxidase inhibitor, has been used for decades as a first-line treatment for hyperuricemia and gout.[Citation7] However, allopurinol is known to cause hypersensitivity reactions (allopurinol hypersensitivity syndrome), which manifest as a mild skin rash in approximately 2% of patients. Furthermore, with this treatment, approximately 0.4% of patients develop severe cutaneous adverse reactions (SCAR).[Citation8] SCAR includes a drug rash with eosinophilia and systemic symptoms, Stevens-Johnson syndrome, and toxic epidermal necrosis. Although rare, SCAR is life threatening and can result in multi-organ injuries, prolonged hospitalization, and an elevated risk of mortality.[Citation9]
Alternative options utilizing dietary molecules that are part of the routine human diet as preventive or adjuvant strategies are thought to be a safer and effective means to combat chronic diseases, such as cancer, diabetes, and gout. In this regard, a transition has been observed in the recent decade that may be called the “herbal renaissance,” which involves people consuming ethnic cuisines (derived from vegetable, fruits, and spices) based on their perceived health benefits.[Citation10] It has been reported that approximately 75% of U.S. households use dietary approaches to reduce their risk of diseases,[Citation10,Citation11] a philosophy that has been widely accepted and practiced for centuries in countries such as India and China.[Citation12]
In the present study, a popular plant, M. fragrans, which has been used as a spice in various cultures, was investigated for its health benefits as an antioxidative dietary factor. The experiments presented herein provide insight to the strong antioxidative activity of the spice, along with its putative mechanistic potential to impede the development of gout.
Materials and methods
Materials
Dried aril (mace) of M. fragrans was procured from the local market that supplies popular herbs and plants of traditional and medicinal value (Tabuk, Saudi Arabia). A plant taxonomist from the Department of Biology, Faculty of Science, University of Tabuk authenticated the dried aril. Methanol and ultrapure water were purchased from Thermo Fisher Scientific (Fremont, CA, USA). 2,2-azino-bis(3-ethylbenzothiazoline- 6-sulfonic acid) diammonium salt (ABTS) and 2,2-Diphenyl-1-pycrylhydrazyl (DPPH) were purchased from Fluka (St. Louis, MO, USA). Xanthine oxidase (E.C. 1.1.3.22), xanthine, allopurinol, gallic acid, ascorbic acid, and phosphate buffered saline (PBS) were purchased from Sigma-Aldrich Co. (St. Louis, MO, USA). All other chemicals and reagents were of analytical grade.
Extraction procedure
The extraction procedure was followed as described by Suarez and co-workers,[Citation13] with slight modifications. The mace (aril of M. fragrans) was rinsed with cold water and oven-dried at 45°C. The dried plant material was crushed into small pieces with a wooden mortar and pestle and further ground to fine dry powder using a blender. A quantity of 100 g of dry powder was then soaked in 1 L of 80% methanol in a conical flask placed in a water bath at 40°C for 24 h with constant shaking. The extracted mixture was then filtered through a double-layered, clean cheesecloth and subsequently through double-layered Whatman paper (Sigma-Aldrich Co., St. Louis, MO, USA). The filtrate was concentrated under reduced pressure at 35°C using a Buchi Rotavapor R-210 (Flawil, Switzerland). Finally, the concentrated extract was further placed under vacuum at –30°C for 3–4 days to yield a solid/thick paste. The residual material was found to weigh 12 g; thus, the total yield was 12% (w/w).
Analyses of the phytochemical constitution
The extract was analysed to determine the content of the major classes of phytochemicals, such as phenolics, flavonoids, tannins, alkaloids, and saponins. The quantitative determination of the total phenolic content was conducted as described earlier using pyrogallol as a standard compound,[Citation14] and the tannin content was determined using tannic acid as a reference.[Citation15] The alkaloid and saponin contents were determined by gravimetric estimation as described by Harborne,[Citation16] whereas the flavonoid content was determined as quercetin equivalents.[Citation17]
Antioxidant activity by an ABTS radical scavenging assay
Experiments were performed according to Re et al.[Citation18] with slight modifications. ABTS and potassium persulfate were dissolved in distilled water to final concentrations of 7 and 2.45 mM, respectively. These two solutions were mixed, and the mixture was allowed to stand in the dark at room temperature for 16 h before use to produce the ABTS radical (ABTS•+). For the study of the antioxidant capacity of the extract, the ABTS radical solution was diluted with distilled water to anabsorbance of 1.00 at 734 nm. The extract (5–100 μg/mL) or gallic acid standards (final concentration 0.5–2.5 μg/mL)were added to the diluted ABTS•+ solution (mixed) and allowed to stand at room temperature for 6 min. The absorbance was determined at 734 nm using a microplate reader (Versamax, Molecular Devices, Sunnyvale, CA, USA). The results were presented as % inhibition of the radicals (compared to the control without any test agent).
Antioxidant activity by an DPPH radical scavenging assay
DPPH is a purple, stable free radical, and its intensity is measured at 510 nm spectrophotometrically. Antioxidants reduce DPPH to 2, 2-diphenyl-1-picryl hydrazine, a colorless compound. A DPPH assay was carried out using the method of Vani et al.[Citation19] In brief, the total reaction mixture contained methanol, a positive control, various increasing concentrations of the standard compound or the test extract (10–250 μg/mL), and DPPH to a final concentration of 0.132 mM. The reaction mixture was incubated at 25°C for 20 min, and the absorbance was read at 510 nm using an ultraviolet (UV)-spectrophotometer. The control reaction was carried out without the test extract, which was replaced by appropriate volumes of the vehicle. Ascorbic acid was used as the standard compound. The results were presented as % inhibition of the radicals compared to the control without any test agent.
Reducing power assay
The Fe3+-reducing power of the extract was determined by a method described by Hazra et al.[Citation20] with slight modification.[Citation21] The assay involved (1) mixing different concentrations (10–250 μg/mL) of the mace extract or ascorbic acid as the standard in phosphate buffer (0.2 M, pH 6.6) with potassium hexacyanoferrate (0.1%), (2) incubating the mixture at 50°C for 20 min, (3) arresting the reaction with the addition of 10% tricarboxylic acid (TCA) and distilled water (2.5 mL), (4) adding an FeCl3 solution (0.01%) to the upper portion of the reaction mixture, (5) leaving the reaction mixture for 10 min at room temperature for color development and (6) measuring the absorbance at 700 nm. All tests were performed in triplicate. Ascorbic acid was used as a positive control. A higher absorbance of the reaction mixture indicated greater reducing power.
Xanthine oxidase inhibition assay
The in vitro xanthine oxidase inhibitory activity of the mace extract was assayed spectrophotometrically using xanthine as the substrate.[Citation22,Citation23] The assay mixture contained 1 mL of the extract dissolved in methanol (10–250 μg/mL), 2.9 mL of a phosphate buffer (pH 7.5) and 0.1 mL of the xanthine oxidase enzyme solution (0.1 units/mL in phosphate buffer, pH 7.5), which was prepared immediately before use. After pre-incubation at 25°C for 15 min, the reaction was initiated by the addition of 2 mL of the substrate solution (150 mM xanthine in the same buffer). The assay mixture was incubated at 25°C for 30 min. The reaction was then stopped by the addition of 1 mL of 1 N hydrochloric acid, and the absorbance was measured at 290 nm using an UV-spectrophotometer. Allopurinol, a known inhibitor of xanthine oxidase, was used as a positive control. The xanthine oxidase inhibitory activity was expressed as the percentage inhibition of xanthine oxidase in the above assay system and calculated using the formula:
where A represents the activity of the enzyme without the plant extract and B is the activity of the enzyme in the presence of the plant extract.
Statistical analysis
All experiments were performed in three different sets, with each set in triplicate. The data are expressed as the mean ± standard error of the mean (SEM). Statistical analysis was performed using an analysis of variance (ANOVA), followed by an F-test using SPSS version 11.5 (SPSS, Inc., Chicago, IL). Values of P that were ≤0.05 were considered significant.
Results and discussions
The phytochemical constitution of the mace extract displayed a spectrum of bioactive molecules
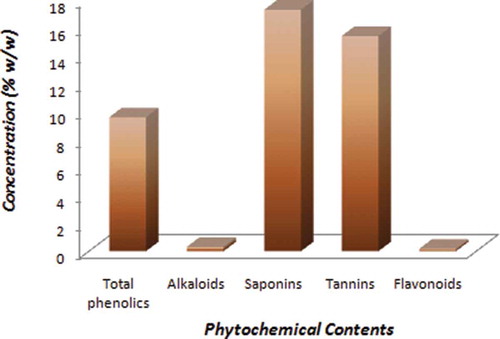
The quantitative analyses of the hydro-methanolic extract of mace showed the extract to contain the major classes of phytochemicals, such as phenolics, flavonoids, alkaloids, tannins, and saponins at various concentrations, as displayed in . These secondary metabolites have been documented to possess an array of pharmacological properties attributable to their ability to interfere with multiple signalling cascades that are critical to heterogeneous metabolic diseases.[Citation24] Plant-derived phytochemicals and plant extracts have been widely reported to have antioxidant, anti-inflammatory, anti-diabetic, and anti-cancer properties.[Citation12,Citation25,Citation26] The potential synergistic effects of the myriad components in a plant extract with varied chemical structures may account for the associated pharmacological significance. As shown in , the highest concentration was recorded for the saponins compared to the other classes of phytochemicals. In a recent study, saponins have been shown to possess anti-arthritic effects in vitro because of their ability to interfere with uric acid metabolism by inhibiting xanthine oxidase.[Citation27] Saponins derived from Chinese herbs have also been reported to reduce serum uric acid levels in hyperuricemic mouse model induced by potassium oxonate by mechanisms other than xanthine oxidase activity.[Citation28] The screening of potential xanthine oxidase inhibitors from 122 Chinese medicinal plants demonstrated that several medicinal plants, such as Glechomalongituba, Lycopuseruopaeus, Scutellariabarbata, and Cinnamomum cassia, exhibited strong inhibitory effects.[Citation29] Moreover, related phytochemical studies of these plants predicted that most of the natural xanthine oxidase inhibitors present in the active extracts might be polyphenolic compounds.[Citation30] Several studies have further demonstrated the anti-arthritic properties of these phytochemicals in preclinical models through mechanisms including decreased uric acid production, enhanced renal uric acid excretion, and reduced urate re-absorption to suppression of inflammation.[Citation30]
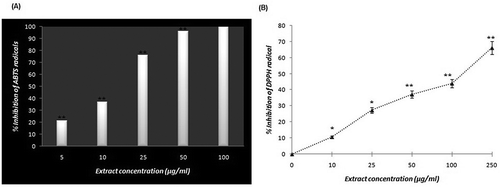
The antioxidative activities of the mace extract showed a dose-dependent inhibition of ABTS/DPPH radicals
Reactive oxygen species have emerged as key signalling molecules that play important roles in the progression of inflammatory disorders.[Citation31] Hence, for the most chronic diseases associated with oxidative stress, the major prophylactic strategy includes the regular consumption of fruits and vegetables rich in antioxidant phytochemicals. Mace is a part of various food cultures worldwide and primarily used as spice.[Citation3] In this study, the antioxidant capacity of mace extract was evaluated using an ABTS/DPPH radical scavenging assay. As shown in and , the mace extract caused the progressive scavenging of the ABTS•+ and DPPH radicals in a dose-dependent manner. The scavenging activity of the extract for the ABTS+ radical was found to be higher than that of the DPPH radical. This difference may be due to the presence of some potent molecules in the extract that are more capable of quenching the ABTS radical than the DPPH radical. Chronic or prolonged reactive oxygen species (ROS) production is considered central to the progression of inflammatory diseases.[Citation31] Inflammasomes (a component of innate immunity) are newly discovered multi-protein oligomer platforms that include the nod-like receptor protein (NLRP) 3 and 6, the NLR family CARD domain-containing protein (NLRC) 4 and the Absent in Melanoma-2 (AIM2) protein. These proteins play an important role in initiating and sustaining inflammation.[Citation32] ROS activates the inflammasome through mitogen-activated protein kinases (MAPK) and extracellular signal-regulated protein kinases 1 and 2 (ERK1/2). In addition, crystallizing compounds, such as monosodium urate, which is a characteristic of gout, also activate ROS that may result in inflammasome activation.[Citation33] Thus, it is imperative that any formulation used for the treatment of gout must also have an antioxidant potential to subdue the inflammatory component of the disease.
The in vitro reduction of Fe3+ by the mace extract exhibits its potential to participate in redox signaling
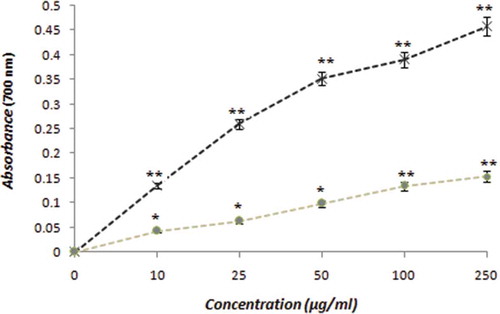
As displayed in , the results of our experiments demonstrated the ability of the extract to reduce Fe3+ in a fashion comparable to the well-known antioxidant ascorbic acid. In the reducing power assay, the presence of antioxidants in the extract result in the reduction of Fe3+ to Fe2+ by donating an electron. The free radical theory of ageing emphasizes oxidative stress resulting from cellular metabolism and the subsequent generation of ROS that leads to detrimental effects on cellular bimolecular structures constituted by proteins, lipids and DNA, the hallmarks of many chronic degenerative diseases.[Citation34] It is widely known that the incidence of gout increases in a linear fashion with the increase in age.[Citation6] Studies examining the antioxidative potential of phytochemicals have shown that antioxidant activities increase in direct proportion to the polyphenol content of the plant extracts used and this activity is thought to be mostly due to their redox properties,[Citation35,Citation36] which play an important role in neutralizing free radicals. Allopurinol, the first line treatment regimen for gout, has also been reported to improve endothelial function by mitigating oxidative stress in randomized human trials.[Citation37] Studies have also reported that iron mobilized from ferritin is able to convert superoxide and hydrogen peroxide, which are produced in large amounts in rheumatoid arthritis, leading to the extremely toxic hydroxyl radical.[Citation38] Thus, it is believed that oxidative-induced injuries to joints in arthritic conditions require a treatment or a prophylactic approach that includes bioactive formulations rich in antioxidants to enhance their intra- and extra-cellular redox capacities against oxidative stress.
Figure 3. The reducing power of the hydro-methanolic extract of mace (*) compared with the standard anti-oxidant ascorbic acid (x). The values reported are the mean ± SEM of three independent experiments. *p ≤ 0.05; **p ≤ 0.01: significant when compared with the control in the absence of the extract.
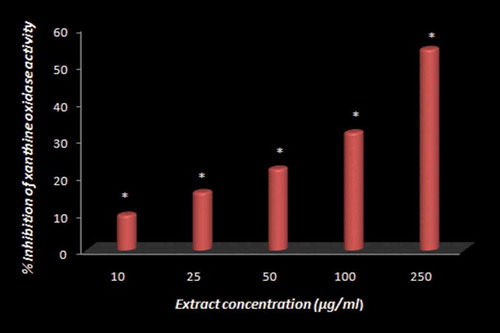
The inhibitory effect of mace extract on mammalian xanthine oxidase
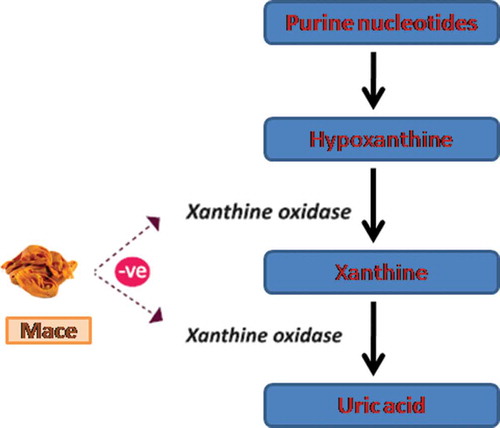
As presented in , the progressive inhibition of xanthine oxidase activity depended upon the concentration of the hydro-methanolic extract of mace. Elevated levels of uric acid in the blood are associated with the metabolic disorders gout and hyperuricemia, leading to the deposition of urate crystals in the joints and kidneys and the presence of gouty arthritis and uric acid nephrolithiasis.[Citation23] The enzyme xanthine oxidase catalyses the oxidation of hypoxanthine to xanthine and then to uric acid in the metabolism of purine bases[Citation39] (). The therapeutic approaches for the treatment or prevention of gout include either increasing the excretion of uric acid or reducing uric acid production. Xanthine oxidase inhibitors are of greater clinical value because they possess relatively milder side effects compared to uricosuric medications and anti-inflammatory agents. As mentioned earlier, allopurinol is the first choice treatment, as a xanthine oxidase inhibitor, among the clinical drugs prescribed for gout. However, the drug also causes side effects, such as hypersensitivity syndrome, Stevens-Johnson syndrome and renal toxicity,[Citation40] that are aggravated by age, which is commonly observed to be on the higher end in patients of gout. Recent understanding of utilizing dietary factors with pharmacological significance as prophylactic approaches for the primary prevention of various chronic disorders associated with poor lifestyle has enhanced the clinical use of dietary factors, which are nevertheless part of the human diet and constituents of traditional folk medicines. Unlike drugs, these components have multiple targets, thus offering a wide therapeutic window and a higher safety index. As mentioned above, the fruit seed (nutmeg) and aril (mace) of M. fragrans are regularly used as spices to flavor cuisines worldwide and also as constituents of traditional medicines in countries such as India, China, Thailand, and England to treat various ailments, such as inflammatory disorders and rheumatoid arthritis.
Conclusion
Our laboratory focuses on engaging dietary factors in the prevention of chronic diseases, including diabetes and cancer.[Citation12,Citation26,Citation41] This concept draws inspiration from our philosophy that suggests “Let’s go back to the roots,” which reflects the potential of the ancient practices of herbal medicine. Natural products obtained from dietary and non-dietary sources are an armamentarium of pharmacological agents that have the potential to make a difference in the way alternative therapies are used in disease prevention. Studies have reported the pharmacological significance and associated health benefits of a number of spices used in various ethnic cuisines and food cultures worldwide.[Citation42–Citation44] Modern medicine has revolutionized treatment strategies in the current period. However, in the light of emerging drug resistance in the cases of antibiotics and anti-cancer drugs, associated ill effects, organ toxicities, and expensive commercial interests leading to inaccessibility, it is believed to be judicious to have alternative strategies, such as prophylactic interventions, for most diseases associated with poor lifestyles, including stressful conditions, such as diabetes and gout. The results presented in this study support M. fragrans as an important source of bioactive formulations with a strong potential as an antioxidative agent. These findings also partially support the regular use of the spice for as a prophylactic or adjuvant therapy of gout. However, further studies are warranted to isolate the active ingredients of the plant and expand the realm of the current findings to animal studies and human trials.
Acknowledgments
The author acknowledges the expertise and support of Dr. Faisel Abu-Duhier and Dr. Showket H. Bhat.
References
- WHO. 2013. WHO Traditional Medicine Strategy, 2014–2023; WHO Press: Geneva, Switzerland.
- Ghosh, D.; Bagchi, D.; Konishi, T. Clinical Aspects of Functional Foods and Nutraceuticals: CRC Press/Taylor & Francis: Florida, USA, 2014; 1–445.
- Nagano, I. Myristicafragrans: An Exploration of the Spice. The Entheogen Review 2008, 16, 15–24.
- Akinboro, A.; Mohammad, A.B.; Asmawi, M.Z.; Sulaiman, S.F.; Sofiman, O.A. Antioxidants in Aqueous Extract of Myristicafragrans (Houtt.) Suppress Mitosis and Cyclophosphamide-Induced Chromosomal Aberrations in Allium Cepa L. Cells. Journal of Zhejiang University–ScienceB 2011, 12, 915–922.
- Somani, R.; Karve, S.; Jain, D.; Jain, K.; Singhai, A.K. Phytochemical and Pharmacological Potential of Myristicafragranshoutt: A Comprehensive Review. Pharmacognosy Reviews 2008, 2, 68–76.
- Kuo, C.F.; Grainge, M.J.; Zhang, W.; Doherty, M. Global Epidemiology of Gout: Prevalence, Incidence and Risk Factors. Nature Review Rheumatology 2015, 11, 649–662.
- Nuki, G. An appraisal of the 2012 American College of Rheumatology Guidelines for the Management of Gout. Current Opinion in Rheumatology 2014, 26, 152–161.
- Arellano, F.; Sacristan, J.A. Allopurinol Hypersensitivity Syndrome: A Review. Annals of Pharmacotherapy 1993, 27, 337–343.
- Gomes, E.R.; Demoly, P. Epidemiology of Hypersensitivity Drug Reactions. Current Opinion in Allergy & Clinical Immunology 2005, 5, 309–316.
- Christine, M.K.; Milner, J.A. Herbs and Spices in Cancer Prevention and Treatment. In Herbal Medicine: Biomolecular and Clinical Aspects; Benzei, I.F.F.; Wachtel-Galor, S.; Eds.; 2nd Ed., CRC Press: Boca Raton, FL, 2011.
- Sloan, A.E. Top 10 Global Food Trends. Food Technology 2005, 59, 20–32.
- Ullah, M.F.; Bhat, S.H.; Hussain, E.; Abu-Duhier, F.; Hadi, S.M.; Ahmad, A.; Sarkar, F.H. Cancer Chemopreventive Pharmacology of Phytochemicals Derived from Plants of Dietary and Non-Dietary Origin: Implication for Alternative and Complementary Approaches. Phytochemistry Reviews 2014, 13, 811–833.
- Suarez, B.; Alvarez, A.I.; Garcia, D.; Barrio, G.D.; Lobo, A.P.; Parra, F. Phenolic Profiles, Antioxidant Activity and in Vitro Antiviral Properties of Apple Pomace. Food Chemistry 2010, 120, 339–342.
- Blainski, A.; Lopes, G.C.; Palazzo De Mello, J.C. Application and Analysis of the Folin–Ciocalteu Method for the Determination of the Total Phenolic Content from Limoniumbrasiliense L. Molecules 2013, 18, 6852–6865.
- Makkar, H.P.S. Quantification of Tannins in Tree Foliage: A Laboratory Manual, FAO/IAEA Working Document, Joint FAO/IAEA Division of Nuclear Techniques in Food and Agriculture, Vienna, Austria, 2000.
- Harborne, J.B. Phytochemical Methods: A Guide to Modern Techniques of Plant Analysis; Chapman and Hall: London, UK, 1976; 99–100, 109–110, 144–147, 185–188.
- Miliauskas, G.; Venskutonis, P.R.; Van Beck, T.A. Screening of Radical Scavenging of Some Medicinal and Aromatic Plant Extracts. Food Chemistry 2004, 85, 231–237.
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant Activity Applying an Improved ABTS Radical Cation Decolorization Assay. Free Radical Biology and Medicine 1999, 26, 1231–1237.
- Vani, T.; Rajani, M.; Sarkar, S.; Shishoo, C.J. Antioxidant Properties of the Ayurvedic Formulation—Antioxidant Properties of the Ayurvedic Formulation—Triphala and Its Constituents. International Journal of Pharmaceutics 1997, 5, 303–317.
- Hazra, B.; Biswas, S.; Mandal, N. Antioxidant and Free Radical Scavenging Activity of Spondiaspinnata. BMC Complementary and Alternative Medicine 2008, 8, 63.
- Mukherjee, S.; Pawar, N.; Kulkarni, O.; Nagarkar, B.; Thopte, S.; Bhujbal, A.; Pawar, P. Evaluation of Free-Radical Quenching Properties of Standard Ayurvedic Formulation Vayasthapana Rasayana. BMC Complementary and Alternative Medicine 2011, 11, 38.
- Owen, P.L.; Johns, T. Xanthine Oxidase Inhibitory Activity of Northeastern North American Plant Remedies Used for Gout. Journal of Ethnophamacology 1999, 64, 149–160.
- Abdullahi, A.; Hamzah, R.U.; Jigam, A.A.; Kabiru, A.Y.; Muhammad, H.; Sakpe, S.; Adefolalu, F.S.; Isah, M.C.; Kolo, M.Z. Inhibitory Activity of Xanthine Oxidase by Fractions of Cratevaadansonii. Journal of Acute Disease 2012, 1, 126–129.
- Ramawat, K.G.; Dass, S.; Mathur, M. The Chemical Diversity of Bioactive Molecules and Therapeutic Potential of Medicinal Plants. In Herbal Drugs: Ethnomedicine to Modern Medicine; Ramawat, K.G.; Ed.; Springer-Verlag: Berlin, Heidelberg, Germany, 2009; 7–32.
- Ullah, M.F.; Bhat, S.H.; Hussain, E., Abu-Duhier, F.; Hadi, S.M.; Ahmad, A.; Sarkar, F.H. Pharmacological Intervention Through Dietary Nutraceuticals in Gastrointestinal Neoplasia. Critical Reviews in Food Science & Nutrition 2016, 56, 1501–1518.
- Ullah, M.F. Sulforaphane (SFN): An Isothiocyanate in a Cancer Chemoprevention Paradigm. Medicines 2015, 2, 141–156.
- Xu, F.; Zhao, X.; Yang, L.; Wang, X.; Zhao, J. A New Cycloartane-Type Triterpenoidsaponin Xanthine Oxidase Inhibitor from Homonoiariparia Lour. Molecules 2014, 19, 13422–13431.
- Wu, X.H.; Ruan, J.L.; Zhang, J.; Wang, S.Q.; Zhang, Y.W. Pallidifloside D, a Saponin Glycoside Constituent from Smilax Riparia, Resist to Hyperuricemia Based on URAT1 and GLUT9 in Hyperuricemic Mice. Journal of Ethnopharmacology 2014, 18, 201–205.
- Kong, L.; Cai, Y.; Huang, W.; Cheng, C.H.; Tan, R. Inhibition of Xanthine Oxidase by Some Chinese Medicinal Plants Used to Treat Gout. Journal of Ethnopharmacology 2000, 73, 199–207.
- Bochu, W.; Ling, X. A Review of Phytotherapy of Gout: Perspective of New Pharmacological Treatments. Pharmazie 2014, 69, 243–256.
- Griffith, B.; Pendyala, S.; Hecker, L.; Lee, P.J.; Natarajan, V.; Thannickal, V.J. NOX Enzymes and Pulmonary Disease. Antioxidants and Redox Signaling 2009, 11, 2505–2516.
- Wu, J.; Fernandes-Alnemri, T.; Alnemri, E.S. Involvement of the AIM2, NLRC4, and NLRP3 Inflammasomes in Caspase-1 Activation by Listeriamonocyto Genes. Journal of Clinical Immunology 2010, 30, 693–702.
- Harijith, A.; Ebenezer, D.L.; Natarajan, V. Reactive Oxygen Species at the Cross Roads of Inflammasome and Inflammation. Frontiers in Physiology 2014, 5(352), 1–11.
- Bhaumik, U.K.; Kumar, A.D.; Selvan, V.T.; Saha, P.; Gupta, M.; Mazumder, U.K. Antioxidant and Free Radical Scavenging Property of Methanol Extract of Blumealanceolaria Leaf in Different in Vitro Models. Pharmacology Online 2008, 2, 74–89.
- Zheng, W.; Wang, S.Y. Antioxidant Activity and Phenolic Compounds in Selected Herbs. Journal of Agriculture and Food Chemistry 2001, 49, 5165–5170.
- Adedapo, A.A.; Jimoh, F.O.; Afolayan, A.J.; Masika, P.J. Antioxidant Activities and Phenolic Contents of the Methanol Extracts of the Stems of Acokantheraoppositifolia and Adeniagummifera. BMC Complementary and Alternative Medicine 2008, 8, 54–60.
- George, J.; Carr, E.; Davies, J.; Belch, J.J.H.; Struthers, A. High-Dose Allopurinol Improves Endothelial Function by Profoundly Reducing Vascular Oxidative Stress and Not by Lowering Uric Acid. Circulation 2006, 114, 2508–2516.
- Biemond, P.; Swaak, A.J.G.; Van Eijk, H.G.; Koster, J.F. Intra-Articular Ferritin-Bound Iron in Rheumatoid Arthritis: A Factor That Increases Oxygen Free Radical-Induced Tissue Destruction. Arthritis & Rheumatism 2005, 29, 1187–1193.
- Borges, F.; Feranandes, E.; Roleira, F. Progress Towards the Discovery of Xanthine Oxidase Inhibitors. Current Medicinal Chemistry 2002, 9, 195–217.
- Burke, A.; Smyth, E.; FitzGerald, G.A. Analgesic-Antipyretic Agents: Pharmacotherapy of Gout. In Goodman & Gilman’s the Pharmacological Basis of Therapeutics; Brunton, L.L; Lazo, J.S; Parker, K.L; Eds.; McGraw-Hill Medical Publishing Division: New York, NY, 2006; 706–710.
- Ullah, M.F.; Bhat, S.H.; Abu-Duhier, F. Anti-Diabetic Potential of Hydro-Alcoholic Extract of Moringaperegrina Leaves: Implication as Functional Food for Prophylactic Intervention in Prediabetic Stage. Journal of Food Biochemistry 2015, 39, 349–490.
- Opara, E.I.; Chohan, M. Culinary Herbs and Spices: Their Bioactive Properties, the Contribution of Polyphenols and the Challenges in Deducing Their True Health Benefits. International Journal of Molecular Sciences 2014, 15, 19183–19202.
- Younsi, F.; Trimech, R.; Boulila, A.; Ezzine, O.; Dhahri, S.; Boussaid, M.; Messaoud, C. Essential Oil and Phenolic Compounds of Artemisia Herba-Alba (Asso.): Composition, Antioxidant, Antiacetylcholinesterase, and Antibacterial Activities. International Journal of Food Properties 2016, 19, 1425–1438.
- Szabo, M.R.; Radu, D.; Gavrilas, S.; Chambre, D.; Iditoiu, C. Antioxidant and Antimicrobial Properties of Selected Spice Extracts. International Journal of Food Properties 2010, 13, 535–545.