ABSTRACT
The rheological and physical characteristics of non-fat set yogurt produced using co-cultures of ropy EPS-producing Streptococcus thermophilus S3.3 and Lactobacillus delbrueckii subsp. bulgaricus LTM and of Streptococcus thermophilus S3.3 and Lactobacillus delbrueckii subsp. bulgaricus H+-ATPase-defective mutant L6 under different conditions were studied. Yogurts produced with mutant L6 had a higher pH and water-holding capacity and better textural properties compared to those produced with the LTM strain. The highest storage modulus (G′) and thixotropic loops were found for yogurt made with L6 at 42°C on day 21 of cold storage. This yogurt contained a network of thick, continuous protein aggregates and large void spaces.
Introduction
Yogurt has long been recognized as a dietary product with many desirable effects.[Citation1] Traditional yogurt production involves the fermentation of milk using Lactobacillus delbrueckii ssp. bulgaricus (L. bulgaricus) and Streptococcus thermophilus (S. thermophilus).[Citation2] Non-fat yogurts are becoming increasingly popular due to their nutritional and potentially therapeutic characteristics.[Citation3,Citation4] The rheological and physical characteristics of non-fat set yogurt are key parameters of its quality because reducing the fat content of yogurt alters its mechanical and sensory characteristics.[Citation3] Two major defects of set yogurt are low firmness and a high degree of syneresis, or whey separation, on the surface.[Citation5]
Bacterial cells may interact with milk proteins through the production of exopolysaccharide (EPS) which may remain attached to the cells and/or interact with proteins.[Citation6] Many studies have further investigated EPS-producing bacterial cultures to assess their potential for the manufacture of reduced-fat or low-fat yogurts with desirable textural properties.[Citation4,Citation7–Citation10] This interest is due to the water-binding ability and texture-promoting properties of EPS.[Citation11] EPS-producing starter cultures can be categorized into two groups: capsular EPS-producing starter cultures and ropy EPS-producing starter cultures.[Citation12] Capsular EPS- and ropy EPS-producing starter cultures reportedly affect the viscoelastic properties of set yogurt differently.[Citation13] Hassan et al.[Citation14] and Hassan et al.[Citation15] observed that set yogurt prepared using EPS-producing starter was less firm than the control sample. In addition, L. bulgaricus produces lactic acid throughout refrigerated storage, a process that is known as “post-acidification.”[Citation16] The survival of S. thermophilus in yogurt is quite low because the low pH of the yogurt (4.1–4.5) reduces the viability of this probiotic bacteria. The change in pH may affect EPS synthesis by S. thermophilus and weaken the interactions between EPSs and proteins, reducing the desired properties of the yogurt. Moreover, it is essential that products sold with better physical characteristics and health claims meet the criterion of a minimum of 106 Colony-Forming Units (CFU)/mL of probiotic bacteria all the way through to expiration date.[Citation17] The best approach to limit post-acidification while still producing satisfactory yogurt flavor is to regulate the growth and maintenance of L. bulgaricus by controlling its energy metabolism.[Citation18] One of the key enzymes involved in the energy metabolism of lactic acid bacteria (LAB) is the H+-ATPase.[Citation19]
The temperature of fermentation may directly affect the viscosity of yogurt[Citation20] or may affect it indirectly via bacterial EPS production.[Citation21] A higher fermentation temperature causes a higher gelation pH and subsequently reduces the solubilization of colloidal calcium phosphate, leading to a gel with a weak structure. EPS production also appears to be temperature-dependent,[Citation20,Citation22] although the viscosity of yogurt is not always positively correlated with EPS concentration.[Citation21,Citation23] The effect of EPSs, strains and other factors on the rheological and physical properties of non-fat set yogurt vary, suggesting that the nature of the EPS-protein interactions that affect its texture are complex. This study aimed to investigate the rheological, textural and microstructural differences in non-fat yogurts prepared using co-cultures of EPS-producing S. thermophilus and L. bulgaricus or the L. bulgaricus H+-ATPase-defective mutant at different fermentation temperatures (30, 37, and 42°C) and throughout storage (at 1, 7, and 21 days).
Materials and methods
Materials
S. thermophilus S3.3, L. bulgaricus LTM and L. bulgaricus H+-ATPase-defective mutant L6 (mutant derived from L. bulgaricus LTM) were used as the three EPS- producing strains in this study. These bacteria were obtained from the Key Lab of Dairy Science of the Ministry of Education (Harbin, China). The stock cultures were maintained at –80°C in 12% (w/v) sterile reconstituted skim milk (RSM) containing 40% (v/v) sterile glycerol. The microorganisms were activated in 12% (w/v) sterile RSM at 37°C for 18 h. This process was repeated three times prior to yogurt manufacture.
Preparation of L. bulgaricus H+-ATPase-defective mutant L6
The mutant was prepared according to the method of Ongol et al.[Citation19] Cells of L. bulgaricus LTM at the stationary growth phase were harvested by centrifugation at 3000 × g, for 10 min at 4°C. The cell pellet was washed twice in 0.85% (w/v) sterile NaCl solution and suspended in NaCl solution to an OD of 0.680 ± 0.014. Suspended cells (0.1 mL) were inoculated into Man, Rogose and Sharpe broth (MRS) agar (HKM, Guangdong, China) plates containing 50 μg/mL neomycin sulfate and incubated at 37°C for 72 h. Single colonies were picked and streaked on fresh MRS agar plates containing neomycin and incubated as previously described. Three repeats of the above procedure were performed to purify the colonies. The growth of the mutants was checked, and only L6 mutants that had a peak OD of less than 0.7 and did not decrease the pH of MRS broth (HKM, Guangdong, China) below 4.8 after 48 h of cultivation were selected and kept as glycerol stocks at –80°C.
Yogurt batch production
Low-heat skim milk powder (34% [w/w] total protein, Nordmilch AG, Bremen, Germany) was reconstituted to 12% (w/v) with distilled water and pasteurized at 90°C for 8 min in a water bath. The milk was inoculated with 2% (v/v) S. thermophilus and L. bulgaricus (1:1, v/v). The inoculated milk was poured aseptically into sterile 200-mL glass containers, which were then placed in an incubator at 30, 37, or 42°C. Fermentation was terminated until the pH reached 4.5 and then yogurts were immediately stored in a cold room (4°C) for 21 days. The rheological and physical properties of the yogurt batches were assessed on day 1 (approximately 12 h post-fermentation) and after 7 and 21 days of cold storage.
Water-holding capacity (WHC)
To determine the WHC, a modified version of the centrifugation method of Parnell-clunies et al.[Citation24] was used. Samples (30 g) from each batch were weighed in centrifuge tubes. The tubes were centrifuged at 4000 × g for 20 min at 4°C. The whey was separated from the samples, and the samples were then reweighed. The WHC was expressed as weight of residual sediment per 100 g yogurt.
Measurement of pH
The pH was determined using a Delta 320 pH meter (Mettler-Toledo, Switzerland). The pH meter was calibrated at 25°C, and all of the measurements were compensated for temperature.
Instrumental textural analysis
A textural profile analysis (TPA) was conducted using the method of Rawson et al.[Citation25] The TPA tests were conducted using a TA.XT plus texture analyzer (version 2.0.7.0., Stable Micro Systems, Godalming, UK) equipped with a 5-kg load cell (USA). A 35-mm-diameter probe was used to determine the textural profile of a set yogurt sample at 15 ± 0.5°C. The samples were compressed by 30% of their original depth. Each samples had a diameter of 40 mm and was 30 mm high. The speed of the probe was fixed at 1 mm s−1 during the pre-testing, testing, and post-testing of the samples. The data presented are the averages of three replications. The “peak” or maximum force was used as a measurement of firmness—the higher the value, the firmer the sample. The area of the curve up to this point was used as a measure of consistency—the higher the value, the thicker the consistency of the sample. The maximum negative force was used as a measure of the cohesiveness of the sample—the more negative the value, the more “cohesive” the sample.
Rheological properties
A Malvern Gemini II rheometer (Malvern, UK) was used to perform the rheological measurements. The modified method of Purohit et al.[Citation26] and Purwandari et al.[Citation27] was employed as follows: yogurts were brought to 20°C for 1 h and stirred 20 times using a spoon to obtain homogeneous samples for the rheological analyses. One-gram samples were used and parallel plate geometry (60-mm diameter) with a gap of 0.5 mm was used to perform the dynamic oscillatory experiments at 20 ± 1°C. The elastic (G′) and viscous (G″) moduli were recorded as a function of stress. To evaluate the rheological properties of the yogurt, frequency sweeps were performed at a constant strain of 5% at 20 ± 1°C. The frequency was increased from 0.1 to 10 Hz in 100 linear increments. Viscoelastic moduli were recorded as a function of frequency. To obtain flow curves, the rate modus (r = 1–100, 100–1 s−1, 25°C) was controlled. The thixotropy was calculated by measuring the area of the hysteresis curve (the loop area between the upward and downward flow curves). The shear rate was increased from 0 to 100 s−1 in 50 linear steps over 60 s and then decreased from 100 to 0 s−1 in 50 linear steps over 60 s while the temperature was maintained at 20 °C.
Microstructure of yogurt
The microstructure of the set yogurts was observed using confocal laser scanning microscopy (CSLM). The CSLM inverted microscope (Leica TCS SP2, Germany) was equipped with an Ar/Kr visible light laser and a 40 × (oil) objective. The fluorescent dye rhodamine B (excitation and emission wavelengths of 543 and 625 nm, respectively) was used to stain the protein network.[Citation28] The resolution of the acquired digital images was 1024 × 1024 pixels. Thirty microliters of a 2 mg/mL of an aqueous solution of rhodamine B was mixed with 5 mL of yogurt, and a few drops of the mixture were then transferred to a slide and covered with a cover slip.
Statistical analysis
The experiment shown in was independently replicated three times. The data were analyzed using Duncan’s multiple range test using a statistical software package (SPSS 19). Differences were considered significant at p < 0.05.
Table 1. The pH, WHC, and textural properties of low-fat yogurt batches fermented by ropy EPS-producing S. thermophilus S3.3 and L. bulgaricus LTM or the L. bulgaricus H+-ATPase-defective mutant L6 at 30, 37, or 42°C and stored for 21 days at 4°C.
Results and discussion
pH, WHC, and textural properties of non-fat set yogurts
shows the pH, WHC, and textural properties of the non-fat set yogurt prepared with EPS-producing S. thermophilus and L. bulgaricus LTM or with EPS-producing S. thermophilus and L. bulgaricus mutant L6 at 30, 37, and 42°C after 1, 7, and 21 day of storage. Both strains had a significant (p < 0.05) effect on the pH. The pH of the L. bulgaricus L6 mutant yogurt was significantly (p < 0.05) higher than that of the L. bulgaricus LTM yogurt () after 7 and 21 days of storage at different temperatures. The fermentation time to reach the ultimate pH was obviously different between the strains, with the L. bulgaricus mutant L6 yogurt requiring a long time period to reach pH 4.5 (data not shown). During storage, the L6 strain slowly produces acid after day 7 at different temperatures, whereas the LTM strain continued producing acid until the end of 21 days storage. Several studies[Citation22,Citation27] obtained similar results when investigating ropy strains and capsular strains during yogurt fermentation. Reduction in the H+-ATPase activity of L. bulgaricus might reduce growth and solute transport,[Citation23] and this result would be very important for EPS-producing LAB during fermentation and subsequent refrigerated storage.
The WHC during cold storage was also significantly (p < 0.05) affected by the tested strain, fermentation temperature, and storage period. The WHC of the L6 (mutant) yogurts tended to be significantly (p < 0.05) higher than those of the LTM yogurts. Fermentation at 30°C resulted in products with a greater (p < 0.05) WHC than fermentation at other temperatures due to the formation of smaller pores at lower fermentation temperatures.[Citation20,Citation29] Prolonged storage slightly affected the WHC (p > 0.05), as demonstrated by comparing the WHC of samples that were fermented under the same conditions after day 7 storage, and while WHC changed was further found at day 21 storage for LTM at 37°C and L6 at 30°C. Under higher pH conditions, the survival of EPS-producing S. thermophilus is enhanced and its ropy EPSs result in more thixotropic loops due to EPS-EPS interactions and EPS-protein interactions.[Citation14]
The effects of the tested strains on the textural characteristics of the yogurts are shown in . The firmness of the yogurts fermented with L6 at different temperatures were significantly increased (p < 0.05), but the yogurts with L6 were significantly increased (p < 0.05) at 30°C during storage and at 37°C at 1 day storage. The consistency of the yogurts fermented with L6 were significantly increased (p < 0.05) at 30°C for 7 days storage and 37°C after 7 days and were significantly decreased (p < 0.05) at 30 and 42°C at 1 day, while other samples did not difference. The cohesiveness of the yogurts fermented with L6 were significantly increased (p < 0.05) at different temperatures during storage. The difference in the textural properties of the yogurts may be attributed to structural phenomena. Ropy EPSs enhance the viscosity of yogurt due to EPS-protein crosslinks that relax faster than protein–protein bonds, although under certain conditions, the protein network is a greater determining factor for the structure than the EPSs.[Citation25] Some researchers also found that EPS-producing strain improved yield and textural properties of low-fat cheese[Citation30] and ropy EPS bound to the protein matrix of the cheese can produce a dense network that helped to increase water and fat retention and leading to a more open structure of the cheese that gave a more cohesive and less elastic product.[Citation31]
Rheological properties
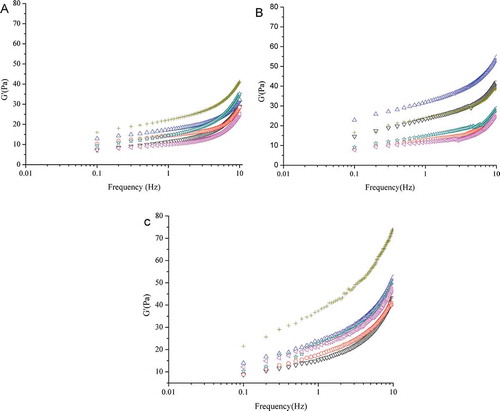
The storage modulus (G′) of the set yogurt batches was significantly (P < 0.05) affected by the strain, fermentation temperature and storage period. These results differ slightly from those of Purwandari et al.[Citation27] The G′ increased toward the end of the storage period (), whereas only slight differences (p > 0.05) among the yogurt batches were observed at day 7, However, firmness, consistency and cohesiveness significantly (p < 0.05) increased after day 7 (). The highest values for the textural and rheological properties were reached at day 21; the yogurt fermented using the L6 mutant at 42°C showed the highest G′ (74.15 Pa) on day 21 of cold storage. For both the LTM and L6 strains, the lower fermentation temperatures (30 and 37°C) resulted in yogurt batches with a lower G′ compared to those produced attained using the higher fermentation temperature (42°C). Temperature had the greatest effect on textural properties; yogurts produced at 30°C exhibited lower firmness, consistency, and cohesiveness. This result ( and ) contradicts the findings of Kristo et al.,[Citation32] who reported that a higher fermentation temperature produced less firm yogurt. Ropy yogurt has been shown to have a low G′,[Citation14] whereas our results showed that yogurt prepared with ropy EPS-producing S. thermophilus and the L. bulgaricus H+-ATPase-defective mutant at 42°C had a higher G′ on day 21 of storage than after shorter storage periods. The tendency of yogurt to become more solid during storage may be affected by the degree of EPS-protein crosslinking that occurs at specific temperatures.
Figure 1. Storage modulus (G′) of non-fat set yogurt batches as a function of the oscillatory frequency. Yogurt samples were produced using S. thermophilus S3.3 with L. bulgaricus LTM or mutant L6 at 30, 37, or 42°C (▽, ○, and △ for LTM and ☆, ◁, and ╂ for L6) and stored for 21 days at 4°C. Samples were taken on A: day 1, B: day 7, and C: day 21 of storage.
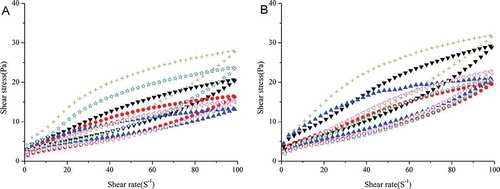
The thixotropic loops of the non-fat set yogurt batches were also significantly affected (p < 0.05) by the strain and storage period but not by the fermentation temperature (p > 0.05; ). The largest loop was observed for yogurt prepared with L6 at 42°C on day 21 of storage. Thixotropic loops, which are frequently observed when conducting the shear rate sweep of viscoelastic materials, are considered to reflect the difference between the energy required for structural degradation and rebuilding.[Citation33] Greater viscosity was strongly correlated with a greater loop area; the yogurt prepared with L6 at 42°C and stored for 21 days had a more satisfactory viscosity. In addition, on day 21, all of the yogurt batches, except the yogurt prepared with L6 at 30°C, tended to have better structural reversibility than they had on day 1.
Microstructure
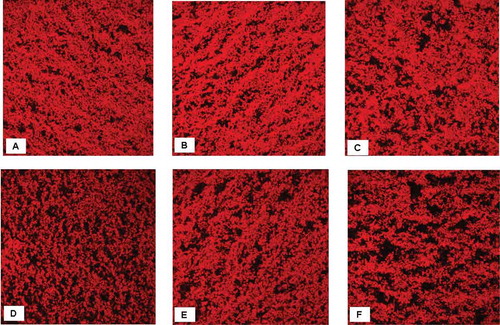
Micrographs of the set yogurt prepared with S. thermophilus S3.3 and L. bulgaricus LTM or with S. thermophilus S3.3 and the L6 mutant are shown in . The protein network, which was stained with rhodamine B, appears red in the images. The set yogurt prepared with the ropy EPS-producing strains exhibited a network of thick, continuous protein aggregates and large void spaces, with EPS located in the serum pores[Citation34] Some of the samples (C, E, and F) contained thick, dense protein strands, consistent with the results of Hassan et al.,[Citation13] whereas yogurt prepared with the L6 mutant at 30°C exhibited a network of fine protein strands and small pores. These results are consistent with the results shown in and . Thus, yogurt prepared with ropy EPS-producing S. thermophilus and the L. bulgaricus L6 mutant rather than the LTM strain at certain fermentation temperatures exhibited better microstructure. This result may be unrelated to the considerable amount of EPSs because the excess EPSs would be trapped in pockets between the casein clusters and constrained around bacterial cells, reducing the probability of interactions between the casein clusters and EPSs and intensifying the mutual interactions of casein particles in regions with highly concentrated EPSs.[Citation34]
Figure 3. Confocal laser scanning micrographs of non-fat set yogurts prepared using S. thermophilus S3.3 with L. bulgaricus LTM (A, B, and C) or mutant L6 (D, E, and F) at 30, 37, or 42°C, respectively, and then stored for 21 days at 4°C.
In this study, the rheological and physical characteristics of non-fat set yogurts with ropy EPS-producing S. thermophilus S3.3 and L. bulgaricus LTM and L. bulgaricus mutant L6 were researched under different fermentation temperatures and storage time. Amatayakul et al.[Citation8] considered that the firmness between samples with capsular EPS- or ropy EPS-producing starter cultures was not different, and they speculated ropy EPS can affect on permeability of serum through skim milk gel and increase the viscosity of serum. In our study, pH of the yogurts with L6 were lower, and most texture properties were better than samples with LTM. Yerlikaya et al. also found that the slight decrease of pH affected the quality of set-type yogurts and led to the increasing viscosity in samples.[Citation35] Some researchers improved the quality of yogurts by adding milk protein-based ingredient rather than improvement of strains, Unal and Akalin[Citation36] improved the textural, microstructural, and some sensorial characteristics of probiotic yogurt by different rate of sodium-calcium caseinate (SCC) or whey protein concentrate (WPC) in milk. They found yogurts with SCC showed much higher values of firmness and viscosities and resulted in a coarse, smooth, and more compact protein network, while yogurt with WPC caused higher WHC and a finer and bunched structure. The use of SCC or WPC increased the texture scores during refrigerated storage period of 28 days.[Citation36,Citation37]
Although the mutant strain used in this study was shown to significantly alter the textural properties of the set yogurts, some of the results cannot be definitively explained; thus, the effect of higher fermentation temperatures on the rheological and physical characteristics of set yogurts made with ropy EPS-producing S. thermophilus and the L. bulgaricus mutant strain should be assessed in future work.
Conclusion
Yogurt prepared with ropy EPS-producing S. thermophilus S3.3 and L. bulgaricus LTM or with ropy EPS-producing S. thermophilus S3.3 and L. bulgaricus H+-ATPase-defective mutant L6 at different fermentation temperatures and then stored for different periods at 4°C had different rheological and physical properties. The duration of storage of the non-fat set yogurts had an obvious effect on the viscoelastic and flow behavioral parameters. Yogurt prepared with the L6 mutant at suitable fermentation temperature conditions showed better properties than yogurt prepared with the L. bulgaricus LTM and the microstructure of this yogurt was characterized by large, dense protein clusters, and large pores. Therefore, there is a conditional correlation between the mutant strain and the rheological characteristics of the yogurt. The yogurts made with the mutant strain were more solid and had a greater WHC.
Funding
This work was supported by National Natural Science Funds (31301519) and the Natural Science Foundation of Heilongjiang Province of China (C201134).
Additional information
Funding
References
- Miklavec, K.; Pravst, I.; Grunert, K.G.; Klopčič, M.; Pohar, J. The Influence of Health Claims and Nutritional Composition on Consumers’ Yoghurt Preferences. Food Quality and Preference 2015, 43, 26–33.
- Michael, M.; Phebus, R.K.; Schmidt, K.A. Impact of a Plant Extract on the Viability of Lactobacillus Delbrueckii ssp. Bulgaricus and Streptococcus Thermophilus in Nonfat Yogurt. International Dairy Journal 2010, 20, 665–672.
- Sandoval-Castilla, O.; Lobato-Calleros, C.; Aguirre-Mandujano, E.; Vernon-Carter E.J. Microstructure and Texture of Yogurt as Influenced by Fat Replacers. International Dairy Journal 2004, 14, 151–159.
- Ramchandran, L.; Shah, N.P. Effect of Exopolysaccharides on the Proteolytic and Angiotensin-I Converting Enzyme-Inhibitory Activities and Textural And Rheological Properties of Low-Fat Yogurt During Refrigerated Storage. Journal of Dairy Science 2009, 92, 895–906.
- Walstra, P.; Geurts, T.J.; Noomen A. Dairy Technology: Principles of Milk Properties and Processes; Marcel Dekker: New York, NY, 1999; 533–535 pp.
- Vlahopoulou, I.; Bell, A.E.; Wilbey, R.A. Effects of Starter Culture and Its Exopolysaccharides on the Gelation of Glucono-δ-Lactone-Acidified Bovine and Caprine Milk. International Journal of Dairy Technology 2001, 54, 135–140.
- Folkenberg, D.M.; Dejmek, P.; Skriver, A.; Guldager, H.S.; Ipsen R. Sensory and Rheological Screening of Exopolysaccharide Producing Strains of Bacterial Yoghurt Cultures. International Dairy Journal 2006, 16, 111–118.
- Amatayakul, T.; Sherkatb, F.; Shah, N.P. Physical Characteristics of Set Yoghurt Made with Altered Casein to Whey Protein Ratios and EPS-Producing Starter Cultures at 9 and 14% Total Solids. Food Hydrocolloids 2006, 20, 314–324.
- Robitaille, G.; Tremblay, A.; Moineau, S.; St-Gelais, D.; Vadeboncoeur, C.; Britten, M. Fat-Free Yogurt Made Using a Galactose-Positive Exopolysaccharide-Producing Recombinant Strain of Streptococcus Thermophilus. Journal of Dairy Science 2009, 92, 477–482.
- De Vuyst, L.; Weckx, S.; Ravyts, F.; Herman, L.; Leroy, F. New Insights into the Exopolysaccharide Production of Streptococcus Thermophilus. International Dairy Journal 2011, 21, 586–591.
- Broadbent, J.R.; McMahon, D.J.; Welker, D.L.; Oberg, C.J.; Moineau, S. Biochemistry, Genetics, and Applications of Exopolysaccharide Production in Streptococcus Thermophilus: A Review. Journal of Dairy Science 2003, 86, 407–423.
- Cerning, J. Exocellular Polysaccharides Produced by Lactic Acid Bacteria. FEMS Microbiology Letters 1990, 87, 113–130.
- Hassan, A.N.; Corredig, M.; Frank, J.F. Capsule Formation by Nonropy Starter Cultures Affects the Viscoelastic Properties of Yogurt During Structure Formation. Journal of Dairy Science 2002, 85, 716–720.
- Hassan, A.N.; Frank, J.F.; Schmid, K.A.; Shalabi, S.I. Textural Properties of Yogurt Made with Encapsulated Non-Ropy Lactic Cultures. Journal of Dairy Science 1996, 79, 2098–2103.
- Hassan, A.N.; Frank, J.F. Modification of Microstructure and Texture of Rennet Curd by Using a Capsule-Forming Non-Ropy Lactic Cultures. International Journal of Dairy Technology 1997, 64, 115–121.
- Shah, N.P. Probiotic Bacteria: Selective Enumeration and Survival in Dairy Foods. Journal of Dairy Science 2000, 83, 894–907.
- Kurman, J.A.; Rasic, J.L. The Health Potential of Products Containing Bifidobacteria. In Therapeutic Properties of Fermented Milks; Robinson, R.K.; Ed.; Elsevier Science Publishers Ltd.: London, 1991; 117–158 pp.
- Mollet, B. Genetically Improved Starter Strains: Opportunities for the Dairy Industry. International Dairy Journal 1999, 9, 11–15.
- Ongol, M.P.; Sawatari, Y.; Ebina, Y.; Sone, T.; Tanaka, M.; Tomita, F.; Yokota, A.; Asano, K. Yoghurt Fermented by Lactobacillus Delbrueckii subsp. Bulgaricus H+-ATPase-Defective Mutant Exhibits Enhanced Viability of Bifidobacterium Breve During Storage. International Journal of Food Microbiology 2007, 116, 358–366.
- Lee, W.J.; Lucey, J.A. Structure and Physical Properties of Yoghurt Gels: Effect of Inoculation Rate and Incubation Temperature. Journal of Dairy Science 2004, 87, 3153–3164.
- Ruas-Madiedo, P.; Tuinier, R.; Kanning, M.; Zoon, P. Role of Exopolysaccharides Produced by Lactococcus Lactis subsp. Cremoris on the Viscosity of Fermented Milks. International Dairy Journal 2002, 12, 689–695.
- Haque, A.; Richardson, R.K.; Morris, E.R. Effect of Fermentation Temperature on the Rheology of Set and Stirred Yoghurt. Food Hydrocolloids 2001, 15, 593–602.
- De Vuyst, L.; Zamfir, M.; Mozzi, F.; Adriany, T.; Marshall, V.; Degeest, B.; Vaningelgem, F. Exopolysaccharide-Producing Streptococcus Thermophilus Strains as Functional Starter Cultures in the Production of Fermented Milks. International Dairy Journal 2003, 13, 707–717.
- Parnell-Clunies, E.M.; Kakuda, Y.; Deman, J.M. Influence of Heat Treatment of Milk on The Flow Properties of Yoghurt. Journal of Food Science 1986, 51, 1459–1462.
- Rawson, H.L.; Marshall, V.M. Effect of Ropy Strains of Lactobacillus Delbrueckii subsp. Bulgaricus and Streptococcus Thermophilus on Rheology of Stirred Yogurt. International Journal of Food Science and Technology 1997, 32, 213–220.
- Purohit, D.H.; Hassan, A.N.; Bhatia, E.; Zhang, X.; Dwivedi, C. Rheological, Sensorial, And Chemopreventive Properties of Milk Fermented with Exopolysaccharide-Producing Lactic Cultures. Journal of Dairy Science 2009, 92, 847–856.
- Purwandari, U.; Shah, N.P.; Vasiljevic, T. Effects of Exopolysaccharide-Producing Strains of Streptococcus Thermophilus on Technological and Rheological Properties of Set-Type Yoghurt. International Dairy Journal 2007, 17, 1344–1352.
- Girard, M.; Schaffer, L.C. Gelation and Resistance to Shearing of Fermented Milk: Role of Exopolysaccharides. International Dairy Journal 2007, 17, 666–673.
- Ruas-Madiedo, P.; Zoon, P. Effect of Exopolysaccharide Producing Lactococcus Lactis Strains and Temperature on the Permeability of Skim Milk Gels. Colloids and Surfaces A: Physicochemical and Engineering Aspects 2003, 213, 245–253.
- Zhang, L.; Li, X.; Ren, H.; Liu, L.; Ma, L.; Li, M.; Bi, W. Impact of Using Exopolysaccharides (EPS)-Producing Strain on Qualities of Half-Fat Cheddar Cheese. International Journal of Food Properties 2015, 18, 1546–1559.
- Lluis-Arroyo, D.; Flores-Nájera, A.; Cruz-Guerrero, A.; Gallardo-Escamilla, F.; Lobato-Calleros, C.; Jiménez-Guzmán, J.; García-Garibay, M. Effect of an Exopolysaccharide-Producing Strain of Streptococcus Thermophilus on the Yield and Texture of Mexican Manchego-Type Cheese. International Journal of Food Properties 2014, 17, 1680–1963.
- Kristo, E.; Biliaderis, C.G.; Tzanetakis, N. Modelling of Rheological, Microbiological and Acidification Properties of a Fermented Milk Product Containing a Probiotic Strain of Lactobacillus Paracasei. International Dairy Journal 2003, 13, 517–528.
- Holdsworth, S.D. Rheological Models Used for the Prediction of Flow Properties of Food Products. Transactions Institution Chemical Engineering Part C 1993, 71, 139–179.
- Kristo, E.; Miao, Z.T.; Corredig, M. The Role of Exopolysaccharide Produced by Lactococcus Lactis subsp. Cremoris in Structure Formation and Recovery of Acid Milk Gels. International Dairy Journal 2011, 21, 656–662.
- Yerlikaya, O.; Akpinar, A.; Kilicp, S. Physico-Chemical, Microbiological, Rheological and Sensorial Properties of Set-Type Yoghurt Produced with Different Origin Wild Lactobacillus Delbrueckii subsp. Bulgaricus and Streptococcus Thermophilus. Italian Journal of Food Science 2013, 25, 412–420.
- Unal, G.; Sibel Akalin, A.S. Influence of Fortification with Sodium-Calcium Caseinate and Whey Protein Concentrate on Microbiological, Textural, and Sensory Properties of Set-Type Yoghurt. International Journal of Dairy Technology 2013, 66, 264–272.
- Unal, G.; Dinkci, N.; Hayaloglu, A.A. Microstructural, Textural and Sensory Characteristics of Probiotic Yoghurts Fortified with Sodium Calcium Caseinate or Whey Protein Concentrate. Journal of Dairy Science 2012, 95, 3617–3628.