ABSTRACT
A novel starch was isolated from litchi kernel and compared with mango kernel starch. The properties studies were physicochemical, morphological, pasting, rheological, X-ray diffraction, and morphological. Litchi kernel starch had significantly (p < 0.05) higher amylose content and solubility, whereas its swelling power and paste clarity were lower than mango kernel starch. Peak viscosity and breakdown viscosity of litchi kernel starch were lower than mango kernel starch; however, it showed higher setback viscosity. The scanning electron microscopy revealed the presence of starch granules varying in size and shape from small to large and oval to elliptical, respectively, in litchi kernel starch. The mean length of small and large litchi starch granules was 9.52 and 50 µm, respectively, while the mean breadth of small and large litchi starch granules was 7.14 and 35.7 µm, respectively. X-ray diffractions of both litchi and mango kernel starches showed typical A-type patterns. During heating, litchi kernel starch showed higher peak G’ (storage modulus), G’’ (loss modulus), and breakdown in G’ in comparison to mango kernel starch. The peak tan δ of litchi and mango kernel starch was <1, which indicated that both litchi and mango kernel starches were more elastic than viscous.
Introduction
Litchi (Litchi chinensis) belongs to the Sapindaceae family, and it is native to subtropical areas of Southern China. It is grown commercially in many subtropical areas, such as Israel, Australia, Thailand, Taiwan, India, Vietnam, parts of Africa, and at higher elevations in Mexico and Central and South America. The top five world litchi producing countries are China, India, Taiwan, Thailand, and Vietnam. According to Indian Horticulture Database,[Citation1] There were 5.85 lakh tones of litchi produced annually in India. The productivity of litchi in India is relatively higher compared to other growing regions, averaging about 7 metric tons per hectare. The various cultivars of litchi vary widely not only in texture, size, shape, and color due to cultivar differences, but also in the levels of biochemical composition.[Citation2] The fruit is small, round, oval, and borne in loose clusters.
Mango (Mangifera indica) is an important fruit crop cultivated in tropical regions. Among fruits, mango occupies top position in India, covering an area of roughly 2.5 million hectares.[Citation3] The total mango production in world was about 4,33,00,069 tons and India had the highest share (1,80,02,000 tons).[Citation3] Depending on the variety, mango kernels contains about 77% carbohydrate on the dry weight basis;[Citation4] while Garg and Tandon[Citation5] reported 58% starch, 2.9% reducing sugars, 0.8% pectin, and 1.1% tannins in mango kernels.
Starch is the most abundant polysaccharide after cellulose and is deposited in partially crystalline granules varying in morphology and molecular structure between and within plant species.[Citation6] It is the only qualitatively important digestible polysaccharide and has been regarded as nutritionally superior to low molecular weight carbohydrates or sugars. The use of both native and modified starches have rapidly increased for manufacturing of various fabricated foods.[Citation7]
Different starches have specific physicochemical and functional properties due to their different botanical sources. The physical properties of starch granules have been determined by the fine structure of the polysaccharide and the percentage distributions of amylose and amylopectin.[Citation8] Various researchers have reported the structural and functional properties of starches from different sources such as seeds corn,[Citation9] wheat,[Citation10] rice,[Citation11] and pigeon peas.[Citation12] Thus, extensive research has been carried out on starch obtained from commercial important sources such as cereals, legumes, and tubers; however, limited work is reported on non-commercial starch sources particularly from kernels of fruits. Recently some work on characterization[Citation13] and modification[Citation14] of tamarind kernel starch has been reported.
After industrial processing, considerable amounts of mango and litchi kernels (seeds) are discarded as waste and creates problem for both industry and environment, therefore, if utilized as starch source it will minimize industrial waste and adds in profit. Therefore, the present study was carried out to isolate starch from litchi kernels as no information is available on its physico-chemical and functional properties. Starch from litchi and mango kernel were isolated and compared for their physicochemical, morphological, X-ray, and rheological properties.
Materials and methods
Materials
Litchi and mango were procured from a local market of Sirsa, India. All chemical and reagents used in study were of analytical grade. Various chemicals such as sodium hydrogen sulphite, potassium iodide, potassium hydroxide, were procured from Sigma-Aldrich (St. Louis, MO, USA). Resublimed iodine and hydrochloric acid were procured from Fisher Scientific (Mumbai, India). Ethyl alcohol was procured from Jiangsu Huaxi International (China).
Starch isolation
Starches were isolated using the method described by Kaur et al.[Citation15] Litchi and mangos were washed, peeled, and the seeds were separated from the pulp using a pulper. Seeds were washed to remove any traces of adhering pulp and were cut into small pieces and steeped in distilled water containing 0.16% sodium hydrogen sulphite for 12 h at 50oC. The steep water was drained off and the seeds were ground in a laboratory blender. The ground slurry was screened through nylon cloth (100 mesh). The material left on the nylon cloth was washed thoroughly with distilled water. The filtrate slurry was allowed to stand for 1 h. The supernatant was removed by suction and the settled starch layer was resuspended in distilled water and centrifuged (Remi R-247, Mumbai, India) in wide-mouthed cups at 10,800 × g for 5 min. The upper non-white layer was scraped off. The white layer was resuspended in distilled water and centrifuged three to four times. The starch was then collected and air dried at 40oC in hot air oven (NSW-144, New Delhi, India).
Physicochemical properties of starches
The amylose content, swelling power (SP), solubility, and paste clarity of litchi and mango kernel starch were determined, using the method of Williams et al.,[Citation16] Schoch,[Citation17] and Bello-Perez et al.,[Citation18] respectively.
Pasting properties
Pasting properties of starches were evaluated with a specially designed starch cell of Modular Compact Rheometer-52 (Anton Paar Co. Ltd., Austria). Viscosity profiles of starches were recorded using starch suspensions (8%, w/w; 15 g total weight). A programed heating and cooling cycle was used where the samples were held at 50oC for 1 min, then heated to 95oC at a rate of 6oC/min and held at 95oC for 2.7 min, then the sample were cooled from 95 to 50oC at a rate of 6oC/min followed by holding at 50oC for 2 min. Parameters recorded were pasting temperature (PT), peak viscosity (PV), trough viscosity (TV; minimum viscosity at 95oC), final viscosity (FV; viscosity at 50oC), breakdown viscosity (BV; [BV = PV – TV]), and setback viscosity (SV; [SV = FV – TV]).
Morphological properties
Scanning electron micrographs were taken by a Jeol JSM-6100 scanning electron microscope (Jeol Ltd., Tokyo, Japan). Litchi kernel starch was suspended in ethanol to obtain a 1% suspension. One drop of the starch–ethanol solution was applied to an aluminium stub using double-sided adhesive tape and the starch was coated with gold-palladium (60:40). An accelerating potential of 10 kV was used during micrography.
X-ray diffraction analysis
X-ray diffraction analysis was performed using as X-ray diffractometer (Philips, X’pert MPD high resolution XRD, Almelo, Netherlands) operated at 40 kV and 40 mA. Diffractograms were obtained from 4o (2θ) to 30o (2θ) using a scanning speed of 4o/min.
Rheological properties
A small amplitude oscillatory rheological measurement was made for litchi and mango kernel starch with Modular Compact Rheometer-52 (Anton Paar, Austria), equipped with parallel plate system (4 cm diameter). The small amplitude measurements were performed to determine the effect of temperature on rheological behavior of starch suspensions. The gap size was set at 1000 µm. The strain and angular frequency were set at 2% and 10 rad/s, respectively. The dynamic rheological properties, such as storage modulus (G’), loss modulus (G’’), and loss factor (tan δ) were determined. Starch suspensions of 15% (w/w) concentration were loaded onto the ram of the rheometer and covered with a thin layer of low-density silicon oil. The starch samples were subjected to temperature sweep test and were heated from 45 to 95oC and cooled from 95 to 45oC at a rate of 2oC/min. The effect of storage at 10oC for 10 h on the rheological behavior of starch pastes was also studied. The starch slurry (15%, w/w) was stirred for 30 min at 25oC on the magnetic stirrer and then heated at 95oC for 15 min in a water bath with mild agitation. Immediately after heating, the pastes were poured on the ram of rheometer preheated at 95oC and the excess material was wiped off with spatula. The exposed sample was covered with a thin layer of silicon oil to prevent evaporation during heating. The strain, angular frequency, and heating rate of 2%, 10 rad/s and 2oC/min, respectively, were used. The measurements were taken during cooling of paste from 95 to 10oC, held for 10 h at 10oC and subsequently heating from 10 to 95oC at a rate of 2oC/min.
Statistical analysis
Means (n = 3), standard deviation (SD), linear regression analysis and 95% confidence intervals were calculated using Microsoft Excel 2007 (Microsoft Corp., Redmond, WA). Data was subjected to a single way analysis of variance (ANOVA).
Results and discussion
Physicochemical properties of starches
Litchi kernel starch showed significantly (p < 0.05) higher amylose content in comparison to mango kernel starch. The amylose content of litchi and mango kernel starches was 19.2 and 16.9%, respectively (). Similar values of amylose content were reported earlier for mango kernel starches by Kaur et al.,[Citation15] however, no information about litchi kernel starch is available in the literature. The amylose content of the starch granules varies with the botanical source of the starch and is affected by the climatic conditions and soil type during growth.[Citation19] Litchi kernel starch showed significantly (p < 0.05) lower SP in comparison to mango kernel starch. The SP of the litchi and mango kernel starch at 90oC was 17.13 and 21.73 (g/g), respectively (). The SP observed for mango kernel starch was higher than that reported earlier by Kaur et al.[Citation15] (18-19.7 g/g). Litchi kernel starch showed significantly (p < 0.05) higher solubility in comparison to mango kernel starch. The solubility of litchi and mango kernel starch at 90oC was 14.17 and 11.90%, respectively. The solubility of mango kernel starch observed in our study was lower than those reported earlier by Kaur et al.[Citation15] (14.1–14.9%). SP and solubility can be used to assess the extent of interaction between starch chains, within the amorphous and crystalline domains of the starch granule.[Citation20] Starch swelling occurs concomitantly with loss of birefringence and precedes solubilization.[Citation21] Litchi kernel starch showed significantly (p < 0.05) lower paste clarity in comparison to mango kernel starch. The values of paste clarity of litchi and mango kernel starches decreased from 5.66 to 0.46% and 8.96 to 1.16%, respectively, during storage at 4oC after 120 h (). Paste clarity provides the information on the behavior of starch paste when the light passes through it and depends on the swollen and non-swollen granule remnants.[Citation9]
Table 1. Amylose content, swelling power, solubility, and paste clarity of litchi and mango kernel starches.
Pasting properties of starches
Starch can be widely applied in the food industry partially due to viscosity change of starch paste during heating and cooling.[Citation22] The pasting properties of litchi and mango kernel starches are shown in and . Mango kernel starch showed a significantly (p < 0.05) higher peak and BV in comparison to litchi kernel starch. The PV of litchi and mango kernel starches was 1833 and 2092 mPa.s, respectively. The increase in viscosity with temperature may be attributed to the removal of water from the exuded amylose by the granules as they swell.[Citation23] Litchi kernel starch showed significantly (p < 0.05) higher trough and the lower BV in comparison to mango kernel starch. TV of litchi and mango kernel starch was 1753 and 1646 mPa.s while BV of litchi and mango kernel starch was 78.6 and 443.3 mPa.s, respectively. Litchi kernel starch showed significantly (p < 0.05) higher FV in comparison to mango kernel starch. FV of litchi and mango kernel starch was 3165 and 2383 mPa.s, respectively. The increase in FV might be due to the aggregation of the amylose molecules.[Citation24] Litchi kernel starch showed significantly (p < 0.05) higher SV in comparison to mango kernel starch. SV of litchi and mango kernel starch was 1409 and 734 mPa.s, respectively. The high setback values shown by litchi kernel starch make them unsuitable for food applications where low syneresis rate is required such as in frozen and refrigerated food.[Citation12] A non-significant (p < 0.05) difference was observed for PTs of both the starches. The PT of litchi and mango kernel starch was 82.4 and 83.2oC, respectively. The high PT of both the starches indicates their higher resistance toward swelling. The pasting properties of starch were considered to be affected by its amylose content and chain-length distribution of amylopectin, with a larger proportion of long chains resulting in a lower PV if the starches had similar amylose content.[Citation25]
Table 2. Pasting properties of starches isolated from litchi and mango kernel starches.
Morphological properties of starch
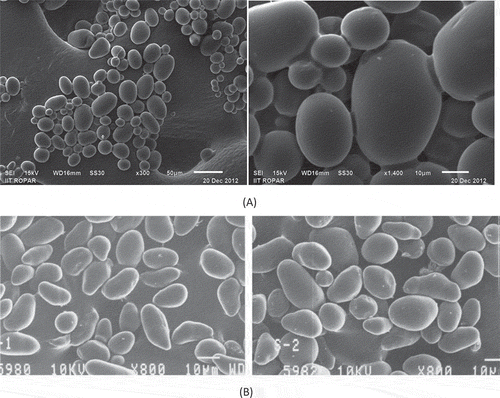
The scanning electron microscopy of litchi kernel starch shows the presence of starch granules varying in size and shape from small to large and oval to elliptical, respectively (). The figure shows that mean length of small and large litchi starch granules was 9.52 and 50 µm, respectively, while the mean breadth of small and large litchi starch granules was 7.14 and 35.7 µm, respectively. Kaur et al.[Citation15] reported that size of starch granules vary with plant species and variety. The surfaces of the majority of granules were smooth with no evidence of fissures when viewed at 300 and 1400× magnification. The morphology of starch granules depends on the biochemistry of the chloroplast or amyloplast, as well as physiology of the plant.[Citation26] The membranes and the physical characteristics of the plastids may also be responsible for providing a particular shape or morphology to starch granules during granule development.[Citation27,Citation28]
X-ray diffraction
X-ray diffraction is also known as fingerprint of the crystal structure within starch grains. On the basis of X-ray diffraction crystal structure of starch is of four types, i.e., A, B, C, and V.[Citation29] The X-ray diffractogram for litchi kernel starch is shown in . The X-ray diffractogram of litchi kernel starch shows A-type X-ray diffraction pattern, which is typically found in many cereal starches. Typically, X-ray diffraction of A-type starch shows first, second, and third peak at 15, 18, and 23o, respectively. Litchi kernel starch showed strong reflections at 15 and 23o (2θ) and an unresolved doublet at 17 and 18o (2θ) which shows that this starch is of A-type starch. Sandhu and Lim[Citation30] had earlier reported A-type X-ray diffraction for starch granules from mango kernels. The amylopectin of A-type starch is thought to contain a higher proportion of shorter branched chains[Citation31] and a higher number of chains per cluster[Citation32] than B-type starch.
Figure 3. X-ray diffractograms of starches from A: litchi kernel and B: mango kernel starch.[Citation30]
![Figure 3. X-ray diffractograms of starches from A: litchi kernel and B: mango kernel starch.[Citation30]](/cms/asset/c8bfdb03-a9e2-4ce2-aa9c-274d9bbff2c8/ljfp_a_1188403_f0003_b.gif)
Rheological properties of starches
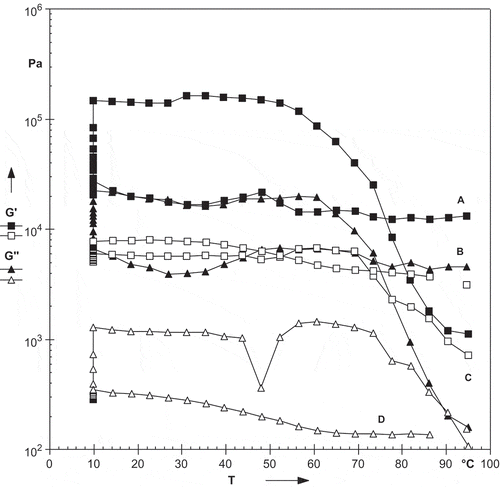
The rheological properties of starches are dependent on several factors, such as type of starch, amylose/amylopectin ratio, starch concentration, pH, and the presence and concentration of other compounds.[Citation33] The rheological properties of starches isolated from litchi kernel and mango kernel during temperature sweep testing are shown in . During heating, litchi kernel starch showed significantly (p < 0.05) higher peak G’ (storage modulus) and G’’ (loss modulus) in comparison to mango kernel starch (). The peak G’ of litchi and mango kernel starch was 2303 and 1396 Pa, respectively. While, peak G’’ of litchi and mango kernel starch was 268 and 162 Pa, respectively. During the heating cycle, the G’ and G’’ of litchi kernel starch was the highest at 82.6oC and then decreased with increase in temperature. The initial increase in G’ could be attributed to the degree of granular swelling which fill the entire available volume of the system.[Citation34] Prolonged heating destroys the gel structure which resulted in decrease of G’.[Citation35] Litchi kernel starch showed significantly (p < 0.05) higher breakdown in comparison to mango kernel starch. The breakdown in G’ is the difference between peak G’ at TG’ and minimum G’ at 90oC. The breakdown in G’ for litchi and mango kernel starch was 545 and 322 Pa, respectively. A non-significant (p < 0.05) difference was observed for peak tan δ and TG’ for both starches. The TG’ for litchi and mango kernel starch was 82.2 and 82.4oC, respectively. The peak tan δ of litchi and mango kernel starch was <1, which indicates that litchi and mango kernel starches were more elastic than viscous. The loss factor, tan δ (G’’/G’) is the ratio of energy lost to energy stored during a cycle.[Citation36] During cooling, litchi kernel starch showed significantly (p < 0.05) higher peak G’ (storage modulus) and G’’ (loss modulus) in comparison to mango kernel starch (). Both the starches had higher peak G’ and G’’ during cooling as compared to heating cycle which may be due to formation of hydrogen bonds. Hesarinejad et al.[Citation37] reported that greater increase in G’ could be attributed to the formation of a three-dimensional network structure due to the strong interactions.
Table 3. Rheological properties of starches from litchi and mango kernel starches.
Figure 4. Effect of heating on rheological properties of starches isolated from litchi kernel (A = G′, B = G″) and mango kernel (C = G′, D = G″).
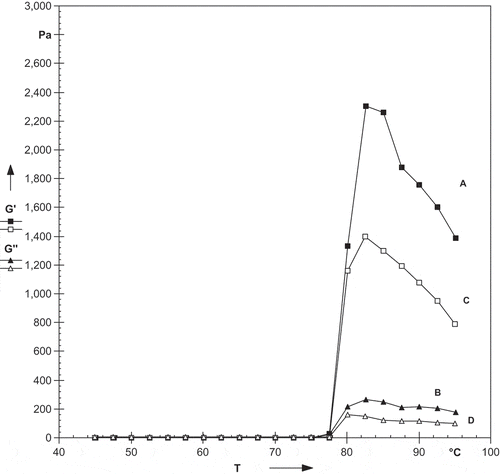
Figure 5. Effect of cooling on rheological properties of starches isolated from litchi kernel (A = G′, B = G″) and mango kernel (C = G′, D = G″).
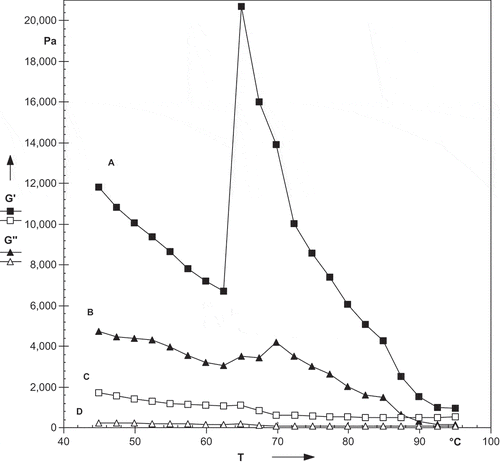
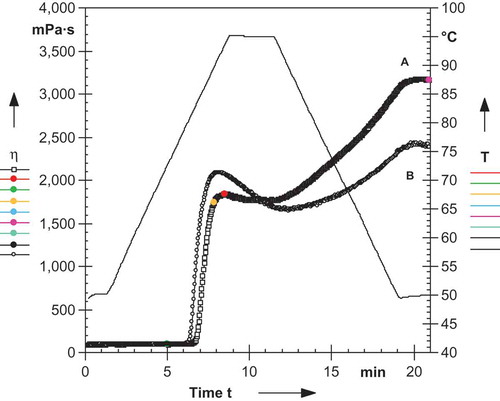
shows the effect of cooling of litchi and mango kernel starch paste from 95 to 10oC, holding at 10oC for 10 h and reheating from 10 to 95oC. During cooling from 95 to 10oC the G’ increases with decreased in temperature. The increase in viscosity during the cooling period indicates a tendency of various constituents present in the hot paste (swollen granules, fragments of swollen granules, colloidally, and molecularly dispersed starch molecules) to associate or retrograde as the temperature of the paste decreased.[Citation38] The peak G’ for litchi and mango kernel starch was 164,115 and 7695 Pa, respectively, while peak G’’ for litchi and mango kernel starch was 22,440 and 1434 Pa, respectively. During holding at 10oC for 10 h the G’ and G’’ decreased with time. For litchi kernel starch G’ decreased from 146,481 to 20,808 Pa while G’’ decreased from 22,440 to 5645 Pa while for mango kernel starch G’ decreased from 7747 to 5083 Pa while G’’ decreased from 1288 to 290 Pa. During reheating the slight increase was noticed. This shows that the structures once formed on cooling did not disintegrate by reheating at the same scan rate.
Conclusions
Starches extracted from litchi and mango kernel showed significant differences in physicochemical, pasting, and rheological properties. Litchi kernel starch showed the higher values for amylose content and solubility, whereas SP and paste clarity were found lower as compared to mango kernel starch. PV was observed lower for litchi kernel starch. Litchi and mango kernel starches showed oval to elliptical shaped granules. The X-ray diffractogram for both litchi and mango kernel starches showed A type X-ray diffraction pattern which resembles cereal starches. Litchi kernel starch showed higher values for peak G’ and G’’ during heating and cooling in comparison to mango kernel starch which shows that litchi kernel starch have good gel strength and elasticity. Utilization of litchi kernels for starch properties would minimize waste and adds value to fruit processing industries. The present investigation enlightens the important properties of litchi kernel starch; therefore, it may help in proper utilization of this starch. Further work should be carried out on modifications of this starch and evaluation of its properties for their food and non-food utilization.
Acknowledgments
The authors thank Dr. Narinder Singh, IIT Ropar, India for conducting the scanning electron microscopy.
References
- Indian Horticulture Database. http://nhb.gov.in/area-pro/NHB_Database_2015.pdf (2014) (accessed on 12 November 2015).
- Bhushan, B.; Pal, A.; Narwal, R.; Meena, V.S.; Sharma, P.C.; Singh, J. Combinatorial Approaches for Controlling Pericarp Browning in Litchi (Litchi Chinensis) Fruit. Journal of Food Science and Technology 2015. DOI:10.1007/s13197-015-1712-8
- FAO–Food and Agriculture Organization of United Nations. FAOSTAT Statistics Database–Agriculture, Rome, Italy. www.fao.org (2013) (accessed on 25 October 2015).
- Zein, R.E.; El-Bagoury, A.A.; Kassab, H.E. Chemical and Nutritional Studies on Mango Seed Kernels. Journal of Agricultural Science 2005, 30, 3285–3299.
- Garg, N.; Tandon, D.K. Amylase Activity of A. Oryzae Grown on Mango Kernel After Certain Pretreatments and Aeration. Indian Food Packer 1997, 51(5), 26–29.
- Blazek, J.; Copeland, L. Pasting and Swelling Properties of Wheat Flour and Starch in Relation to Amylose Content. Carbohydrate Polymers 2008, 71, 380–387.
- Yavuz, M.; Tilki, T.; Karabacak, C.; Erol, O.; Ibrahim Unal, H.; Uluturk, M.; Cabuk, M. Electrorheological Behavior of Biodegradable Modified Corn Starch/Corn Oil Suspensions. Carbohydrate Polymers 2010, 79(2), 318–324.
- Boyer, C.D.; Shannon, J.C. Carbohydrates of the Kernel. In Corn: Chemistry and Technology; Watson, S.A.; Ramstad, P.E.; Eds.; American Association of Cereal Chemist: St Paul, MN, 1987; 253–272.
- Sandhu, K.S.; Singh N. Some Properties of Corn Starches II: Physicochemical, Gelatinization, Retrogradation, Pasting and Gel Textural Properties. Food Chemistry 2007, 101, 1499–1507.
- Majzoobi, M.; Rowe, A.J.; Connock, M.; Hill, S.E.; Harding, S.E. Partial Fractionation of Wheat Starch Amylose and Amylopectin Using Zonal Ultracentrifugation. Carbohydrate Polymers 2003, 52, 269–274.
- Singh, N.; Kaur, L.; Sandhu, K.S.; Kaur, J.; Nishinari, K. Relationships Between Physicochemical, Morphological, Thermal, Rheological Properties of Rice Starches. Food Hydrocolloids 2006, 20, 532–542.
- Kaur, M.; Sandhu, K.S. In Vitro Digestibility, Structural and Functional Properties of Starch from Pigeon Pea (Cajanus Cajan) Cultivars Grown in India. Food Research International 2010, 43, 263–268.
- Kaur, M.; Singh, S. Physicochemical, Morphological, Pasting and Rheological Properties of Tamarind (Tamarindus Indica L.) Kernel Starch. International Journal of Food Properties 2015. DOI:10.1080/10942912.2015.1121495
- Kaur, M.; Bhullar, G.K. Partial Characterization of Tamarind (Tamarindus Indica L.) Kernel Starch Oxidized at Different Levels of Sodium Hypochlorite. International Journal of Food Properties 2016, 19, 605–617.
- Kaur, M.; Singh, N.; Sandhu, K.S.; Guraya, H.S. Physicochemical, Morphological, Termal and Rheological Properties of Starches Separated from Kernels of Some Indian Mango Cultivars. Food Chemistry 2004, 85, 131–140.
- Williams, P.C.; Kuzina, F.D.; Hlynka, I. A Rapid Calorimetric Procedure for Estimating the Amylose Content of Starches and Flours. Cereal Chemistry 1970, 47, 411–420.
- Schoch, T.J. Swelling Power and Solubility of Granular Starches. In Method in Carbohydrate Chemistry, Vol. 4; Whistler, R.L.; Ed.; Academic Press, Inc.: New York, NY, 1964.
- Bello-Perez, L.A.; Roger, P.; Baud, B.; Colonna, P. Macromolecular Features of Starches Determined by Aqueous High-Performance Size Exclusion Chromatography. Journal of Cereal Science 1998, 27, 267–278.
- Morrison, W.R.; Azudin, M.N. Variation in Amylose and Lipid Contents and Some Physical Properties of Rice Starches. Journal of Cereal Science 1987, 5, 35–37.
- Ratnayake, W.S.; Hoover, R.; Warkentin, T. Pea Starch: Composition, Structure and Properties—A Review. Starch/Starke 2002, 54, 217–234.
- Singh, N.; Sandhu, K.S.; Kaur, M. Characterization of Starches Separated from Indian Chickpea (Cicer Arietinum L.) Cultivars. Journal of Food Engineering 2004, 63(4), 441–449.
- Choi, H.; Kim, W.; Shin, M. Properties of Korean Amaranth Starch Compared to Waxy Millet and Waxy Sorghum Starches. Starch/Starke 2004, 56(10), 469–477.
- Ghiasi, K.; Varriano-Marston, K.; Hoseney, R.C. Gelatinization of Wheat Starch. II. Starch–Surfactant Interaction. Cereal Chemistry 1982, 59, 86.
- Miles, M.J.; Morris, V.J.; Orford, P.D.; Ring, S.G. The Role of Amylose and Amylopectin in the Gelation and Retrogradation of Starch. Carbohydrate Research 1985, 135, 271–281.
- Sandhu, K.S.; Singh, N.; Lim, S.T. A Comparison of Native and Acid Thinned Normal and Waxy Corn Starches: Physicochemical, Thermal, Morphological and Pasting Properties. LWT–Food Science and Technology 2007, 40, 1527–1536.
- Badenhuizen, N.P. The Biogenesis of Starch Granules in Higher Plants; Appleton Crofts: New York, NY, 1969.
- Jane, J.L.; Kasemsuwan, T.; Leas, S.; Ia, A.; Zobel, H.; Robyt, J.F. Anthology of Starch Granule Morphology by Scanning Electron Microscopy. Starch 1994, 46, 121–129.
- Lindebooma, N.; Chang, P.R.; Tylera, R.T. Analytical, Biochemical and Physicochemical Aspects of Starch Granule Size, with Emphasis on Small Granules Starches: A Review. Starch 2004, 56, 89–99.
- Zeng, J.; Li, G.; Gao, H.; Ru, Z. Comparison of A and B Starch Granules from Three Wheat Varieties. Molecules (Basel, Switzerland) 2011, 16(12), 10570–10591.
- Sandhu, K.S.; Lim, S.-T. Structural Characteristics and in Vitro Digestibility of Mango Kernel Starches (Mangifera Indica L.). Food Chemistry 2008, 107(1), 92–97.
- Hizukuri, S.; Kneko, T.; Takeda, Y. Measurement of the Chain Length of Amylopectin and Its Relevance to the Origin of Crystalline Polymorphism of Starch Granules. Biochemica et Biophysica Acta 1983, 760, 188–191.
- Takeda, Y.; Shibahara, S.; Hanashiro, I. Examination of the Structure of Amylopectin Molecules by Fluorescent Labeling. Carbohydrate Research 2003, 338, 471–475.
- Choi, H.-M.; Yoo, B. Rheology of Mixed Systems of Sweet Potato Starch and Galactomannans. Starch/Stärke 2008, 60(5), 263–269.
- Eliasson, A.C. Viscoelastic Behavior During the Gelatinization of Starch I. Comparison of Wheat, Maize, Potato and Waxy-Barley Starches. Journal of Texture Studies 1986, 17, 253–265.
- Tsai, M.L.; Li, C.F.; Lii, C.Y. Effects of Granular Structure on the Pasting Behavior of Starches. Cereal Chemistry 1997, 714, 750–757.
- Ferry, J.D. Illustrations of Viscoelastic Behavior of Polymeric Systems. In Viscoelastic Properties of Polymers; Ferry, J.D.; Ed.; J. Wiley & Sons, New York, NY, 1970; 34–58.
- Hesarinejad, M.A.; Koocheki, A.; Razavi, S.M.A. Dynamic Rheological Properties of Lepidium Perfoliatum Seed Gum: Effect of Concentration, Temperature and Heating/Cooling Rate. Food Hydrocolloids 2014, 35, 583–589.
- Singh, N.; Singh, J.; Kaur, L.; Singh, S.N.; Singh, G.B. Morphological, Thermal and Rheological Properties of Starches form Different Botanical Sources. Food Chemistry 2003, 81, 219–231.