ABSTRACT
This work was designed to elucidate selected physicochemical, functional, and structural properties of native and modified yam (Dioscorea rotundata) starch. The isolated starch was chemically modified using 5, 10, 15, 20, and 25% phosphoric acid solution at 50°C for 1 h, and yield, swelling power, gelation, water holding capacity, paste clarity, blue value, and amylose and amylopectin content of the native and modified yam starch were determined. Structural changes in the native and starch modified with 25% phosphoric acid were evaluated using Fourier transform infrared spectroscopy and optical microscopy. The result showed that the yield, swelling power, water holding capacity, paste clarity, blue value and amylose and amylopectin content of native yam starch was 33.38% (217 g), 3.84 g/g, 1.0 v/g, 10%, 0.52 and 25.96, respectively, whereas gelation study of the native and modified starch indicated that native starch was viscous and modified starch firm. However, yield, swelling power, water holding capacity, paste clarity, blue value, and amylose content of modified yam starch reduced in a dose dependent manner with phosphoric acid. The reduction in the values of the various functional properties could be associated with the effect of phosphoric acid on the starch granular structure. The result of Fourier transform infrared spectroscopy and optical microscopy revealed that the yam starch was modified by phosphoric acid with changes in functional groups spectra such as –OH stretch (3177 cm−1), H2O absorbed (1644 cm−1) (amorphous region), C-H stretch (2923 cm−1), CH2O (1253 cm−1), and C-O-C (1078 cm−1) when compared to native starch. The morphology of native and modified yam starch granules ranged from oval to eliptical. However, modified starch granules were rough in surface. In conclusion, the characterized physicochemical and functional properties and structure exhibited by native and modified yam starch indicated that, yam could be a cheap and valuable source of starch for industrial application.
Introduction
Yam (Dioscorea spp.) is a set of annual or perennial climbing plants with underground tubers that are suitable for human consumption. They are of immense economic importance and nourishment to the people of Africa, the Caribbean, and Asia.[Citation1] There are about 600 species[Citation2] of yam; however, the most widely accepted ones for staple food are six and these include; Dioscorea rotundata (white yam), D. alata (water yam), D. cayenensis (yellow yam), D. esculenta (Chinese yam), D. bulbifera (aerial yam), and D. dumetorum (trifoliate yam).[Citation3] Among the six species commonly cultivated in Nigeria (West Africa), D. rotundata is the most widely grown and generally considered to be the best in terms of food quality and thus, of highest market value.[Citation4]
Nigeria is one of the largest producers of yam in the world; however, due to high α-amylase level in yam, which is usually triggered by heat in the environment, its starch is easily degraded into sugar and consequently there is weight loss, sprouting, rotting, and insect infestation. As a result of this physiological and biochemical responses in yam tuber, a substantial amount is lost during storage. In order to forestall this loss, there is a need to expand utilization of yam through industrial processing to minimize postharvest losses. Reduction in postharvest losses of yam tuber will lead to higher economic growth as increased earnings from this crop will be achieved. The starch proportion of yam tubers presents a prospect for processing it into powdered starches. Updated data indicated that, yams are not recognized among the most common sources of industrial starch, which is principally provided by corn, potato, wheat, cassava, and rice.[Citation5,Citation6]
Starch forms the main component of yam and provides a large proportion of daily caloric intake for its ardent consumers. The value of yam as a basic food has been attributed to the high digestibility of its starch, which is dependent on its granular morphology and size.[Citation7] Yam tubers contain about 70–82% starch,[Citation8] the domestic and industrial processing of yams, the eating and storage quality of yam-containing products and perhaps the physiological effectiveness of the bioactive ingredients depend greatly on its starch functional properties.[Citation9]
Starch is a major raw material for a number of industries including textiles, paper, adhesives, pharmaceuticals, and food. The demand for both native and modified starches (MSs) will continually be on the increase as industrialization rises. In a developing country like Nigeria, industrial starch is usually met through importation. However, if locally sourced starch is well modified and characterized, could suitable for both local and international industrial applications.
Important to starches application in industries is the amount and quality of starch obtained from crops as well as the functional properties of native and MSs. Lack of meaningful information on the functional properties of the starches is one of the limiting factors for industrial application of non-conventional starch such as yams.[Citation10] On one hand, yam potential of being processed into value-added products is influenced by its physicochemical and functional and structural properties. On the other hand, modification of starch with phosphoric acid could simultaneously achieve three effects, which are; esterification, crosslinking and hydrolysis. These modification effects of phosphoric acid among others, had been shown to enhance stability of starch granules to swelling, high temperature and shear.[Citation11] It also reduces water sensitivity of starch and improves barrier properties especially in starch-based edible biofilms.[Citation12,Citation13] Thus, such a phosphoric acid MS could be used to improve texture in soups and sauces, and in bakery and dairy products.[Citation14] This study was, therefore, designed to evaluate the effect of phosphoric acid modification on the physicochemical, functional, and structural properties of yam starch.
Material and methods
Production of yam (Dioscorea rotundata) starch
The back of yam was peeled to expose the inner white layer which was sliced into several smaller pieces, thoroughly washed and crushed with a grater. The paste (650 g) was mixed with appropriate volume of water and sieved with muslin cloth and allowed to stand for 2 h. Thereafter, the supernatant was decanted leaving the starch slurry that settled at the bottom of the beaker. The paste slurry was air-dried for 48 h at room temperature after which it was ground to a fine powder using a mortar and pestle and sieved (100 μm) and stored in an airtight container for further analysis.
Modification and yield of starch
A modified method of Sathe and Salunkhe,[Citation15] was adopted for starch modification. Starch (20 g) was dispersed in 50 mL of 5–25% solution of phosphoric acid and stirred with magnetic stirrer (BioCote-Stuart US 152 model) for 1 h at 50°C. The MS slurries were then cooled to room temperature and centrifuge at 2000 rpm for 5 min to separate the MS. The MS was oven-dried at 50°C and stored for subsequent analysis. The percentage yield of MS was calculated thus;
The MS (25%) was subjected to Fourier transform infrared (FTIR) spectroscopy and optical microscopy.
Functional characterization of native and modified yam starch
Determination of swelling power
The swelling power was evaluated using the method of Leach et al.[Citation16] A sample (0.1 g) of native or MS sample each was weighed and quantitatively transferred into a clean dried test tube and weighed (w1). The starch was then dispersed in 10 mL of distilled water. The resultant slurry was heated at 90°C for 30 min in a thermostatic water bath (±2°C). The mixture was cooled and centrifuged at 2000 rpm for 5 min. The residue obtained from the above experiment (after centrifugation) with water it retained was quantitatively transferred to a clean, dried test tube used earlier and weighed (w2).
Gelation studies
A solution (2 g/15 mL) of native and modified yam starch were prepared in test tubes. The starch suspensions were thoroughly vortexed for 5 min and were heated for 30 min at 80°C in a water bath, followed by rapid cooling under running cold tap water for 1 h. The gelation of native MS was determined when the sample from the inverted test tube did not fall down or slip.
Water holding capacity (WHC)
WHC was determined according to the method described by Medcalf and Gilles[Citation17] with a few modifications. WHC was determined by centrifugation. Native and MS samples (1 g) were suspended in 10 mL of distilled water in pre-weighed centrifuge tubes, slurry was vortexed vigorously for 5 min and allow to stand for 1 h. The starch slurry was centrifuged at 4000 × g for 5 min. The supernatant free water was removed carefully from and the volume determined with measuring cylinder (25 ± 0.2 mL). WHC was calculated as the ratio of volume of water absorbed to mass of dry starch.
Paste clarity
The clarity transmittance of native and modified yam starches was carried out according to Ceballos et al.[Citation21] This was determined by measuring percentage transmittance of 1% starch solution heated in boiling water for 30 min at 600 nm wavelengths against water blank on a colorimeter.
Physicochemical characterization of native and modified yam starch
Determination of blue value and amylose content of native and modified yam starch
The samples were prepared according to the method of Gilbert and Spragg.[Citation18] The native or MS (0.1 g) was weighed into a test tube; 10 mL of distilled water was added and heated in a boiling thermostatic water bath for 30 min to solubilize the starch. The starch solution was brought down, cooled and 5 mL transferred into a 100 mL standard volumetric flask, 2 mL of stock iodine solution (0.2 g) I2/2.0 g KI/100 mL) was added and the volume made up to mark with distilled water. The resulting colour was left for 60 s to fully develop before the absorbance was read at 600 nm with a colorimeter (JENWAY 6051 model).
The blue value was calculated according to the formula below:
Determination of amylose and amylopectin content of native and modified yam starch
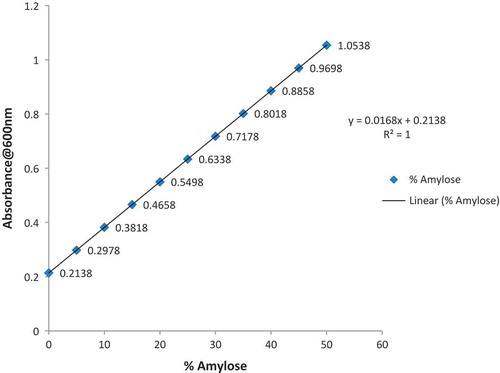
The amylose content of native and phosphoric acid modified yam starch was determined using the standard curve designed by Saeed et al.[Citation19] Amylopectin content was calculated by the difference of total starch minus amylose content:[Citation20]
where Y = absorbance at 600 nm and X = %amylose.
Optical microscopy
The native or phosphoric acid (25%) MS sample solution was placed on a glass slide and stain with 0.2% iodine solution. Observation and imaging were taken at 100× and 200× magnification lens of light microscope connected to a computer.
Structural characterization of native and modified yam starch
FTIR spectroscopy
The FTIR spectrum of native or modified (25%) yam starch was acquired on a PerkinElmer FTIR spectrophotometer (PerkinElmer, Inc., MA, USA) using potassium bromide (KBr) discs prepared from powdered samples mixed with dry KBr. Spectrum was recorded (16 scans) in the transparent mode from 4000 to 400 cm−Citation1, at 4 cm−Citation1 resolution.
Result and discussion
Isolation of yam starch
The result showed that, yam (Dioscorea rotundata) yielded 33.38% (217 g) native starch. According to Addy et al.,[Citation22] some varieties of Dioscorea rotundata contained 12.0–25% starch. The starch content of yam used in this present study was higher, perhaps because the yam had stayed for about 4 months after harvest and the water level had reduced appreciably. The starch content of yam is very comparable to other sources of starch such as cassava, cocoyam, and potatoes. Authors[Citation23–Citation25] reported that cassava, cocoyam, and potatoes contain 33.5, 24.5, and 20% yield of starch, respectively. Therefore, the starch content of yam in this present study is high and could be a good and cheap source of starch for industrial application.
Swelling power and yield
The results of the swelling power of native and MS and the yield of starch after modification are presented in . The result showed that, swelling and yield decreased with increase in phosphoric acid concentration. Swelling power of phosphoric acid-treated yam starches was different from native starch because native starch showed higher swelling power than acid-MSs. Swelling power of native yam starch was 3.84 g/g, which decreased to 0.96 g/g over 25% phosphoric acid treatment. Tester and Karkalas[Citation26] stated that when starch is heated in the presence of excess amounts of water, hydrogen bonds stabilizing the structure of the double helices in crystallize get broken and replaced by hydrogen bonds of water molecules, as a result, the starch granule swells and the volume increases.
Table 1. Swelling behavior and yield of native and modified starch.
Morrison et al.[Citation27] reported that acid-MSs usually experience hydrolysis of some glycosidic bonds, which occurs first in the amorphous regions of the starch containing branch points (amylopectin). Since the extent of starch swelling depends largely on the molecular weight and shape of amylopectin molecule, hydrolysis of branching point, α-1, 6 glycosidic bonds, which constitute amylopectin molecule, will invariably limit swelling of starch. Therefore, the swelling power phosphoric acid, MS experienced a gradual decline with an increase in the phosphoric acid concentration[Citation28] in this study.
Swelling power provides evidence of the level of interaction between starch amylose and amylopectin within the amorphous and crystalline domains. Hoover[Citation29] reported that the extent of this interaction is influenced by the amylose/amylopectin ratio and by the characteristics of amylose and amylopectin in terms of molecular weight/distribution, degree and length of branching, and conformation. Swelling power of native starch is primarily a property of the amylopectin content while amylose acts as an inhibitor of swelling. It, therefore, shows that, “the more the amylose the less the swelling power of starch.”
Treating starch with acidic reagent like phosphoric acid started when the need for starch granules that are tough enough to resist disintegration on cooking arose. Such starch derivatives form a covalent network and are capable of tolerating extreme pH and high shear processes commonly found in food and drug manufacturing industries.[Citation30] This property of MS as expressed in yam starch in the present study rendered it fit for industrial food and drug producing industries.
The yields of yam starch modified with 5–25% phosphoric acid were very good, which ranged from 85.60 to 93.05% (). Significant recovery of modified yam starch achieved in this study is in agreement with previous findings by Lin, Lee, and Chang.[Citation28] According to their work, the 91–100% yield was attained for potato and corn starches treated with acidified alcohol. You and Izydorczyk[Citation31] also observed very high yields (91.5–99.8%) of acid–alcohol treated barley starches. They attributed higher yields to very little degradation of barley starches during acid–alcohol hydrolysis. Therefore the relatively high yield recorded in this study could be associated with the reduced effect of phosphoric acid on the branching point of α-D bonds (1-6) glycosidic bond. Amylopectin chain of starch possesses higher molecular weight than amylose. Thus, the gradual reduction in yield as treatment increased with increased phosphoric acid concentration is an indication of α-1, 6 glycosidic bond hydrolysis.
Gelation
The result of gelation studies is shown in . The result indicated that, native starch was viscous, whereas MS remain firm to the bottom of the test tube. When starch is heated in excess water, the starch granules experiences an order–disorder phase transition. This phase transition is a non-equilibrium process attributed to diffusion of water into the granule leading to hydration and swelling, loss of double helical structure, amylose leaching, and total disruption of granules resulting in starch paste.[Citation29] Acid modification decreased the gelation potential of the MS. The effect of acid hydrolysis on α-1,6 glycosidic bond in the amylopectin amorphous region might have resulted in reduced hindrance of helical structure of starch, thus reducing its gelling property is reduced and need more concentration for proper gellification. Notably, gel formation in starches involves swelling and hydration of starch granules, which occur predominantly in the amorphous region of starches.
WHC
The result of WHC is presented in . The result showed that, native starch had higher WHC than the MS. WHC refers to the total amount of water held by a starch gel under a defined state of conditions.[Citation32] This functional property of starch is a function of the crystallinity properties of starch; starches with low crystallinity possess high WHC in comparison to those with high crystalinity and hence, it is dependent on the amylose content of starch.[Citation33] The inter- and intra granular forces in starch molecule are also important factors; the weaker the forces the higher the WHC.[Citation34] Modification of starch with procedures which involves phosphoric acid reduce the ability of starch to absorb water and, therefore, the WHC of the acid modified starch reduced with respect to the concentration of acid applied. This low WHC of acid treated starch could be attributed to the reduction of the armorphous region in the starch granule. This reduces the number of available binding sites for water in starch granules.[Citation35]
Blue value and amylose content of native and MS
The result of blue value and amylose content of native and MS is presented in . The result showed that, the blue–black color developed and amylose content decreased as the concentration of phosphoric acid increased. Usually acid treatment attack α-1,6 glycosidic linkages in starch and thereby reducing amylopectin to amylose, and hence the higher amount of amylose. However, the formation of amylose during acid hydrolysis depends greatly on the concentration of the acid and duration of treatment. Wurzburg[Citation36] observed that starches are usually degraded into dextrins on prolonged treatment with acid, as a result of hydrolyzing α-D (1, 4) glycosidic linkages. Phosphorus based reagent had been identified as a good starch crosslinking reagent forming mono, di-type of the ester bond.[Citation37] The increased substitution of phosphate (PO−4) functional group caused the starch to be covalently crosslinked, and that might be another good reason for the reduction in amylose values.
Table 2. Blue value, amylose, and amylopectin content of native and modified starch.
Thus, the reduction in blue value and amylose content of the phosphoric acid modified yam starch could be associated to an increased formation of dextrins and introduction of phosphate groups in the course of acid treatment. Therefore, the gradual reduction in blue value and amylose content of MS further confirmed the structural changes which had occurred in starch during phosphoric acid treatment. However, further treatment of the starch with acid resulted into significant disappearance of blue colour and appearance of amylopectin characteristic violet–red when stained with iodine solution.[Citation38]
Paste clarity
The result of paste clarity is presented in . From the result, clarity of starch has a linear relationship with the concentration of phosphoric acid treatment. Clarity characteristics of starches are dependent on the botanical source of starch and the overall purity of the starch in case of cereal starches. However, paste clarity of starch is also influenced by the amylose content especially in cereal starches.[Citation39] To improve paste clarity of starch, chemical modification is usually a solution according to Zhang et al.[Citation40] In the present study, starch clarity was achieved with phosphoric acid modification and there was corresponding rise in clarity as treatment increased.
Optical microscopy
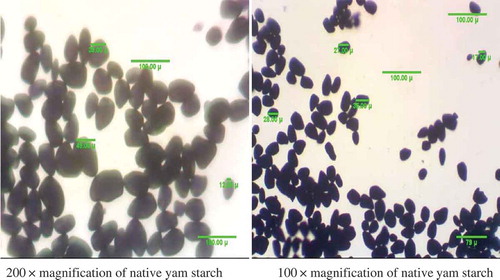
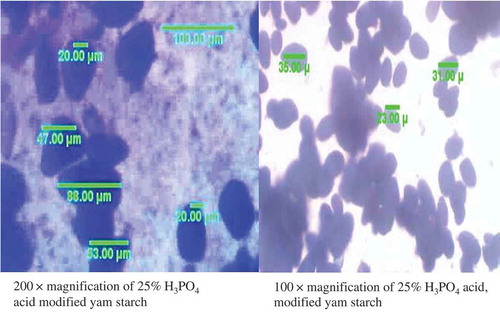
The result of the optical microscopy of the native and modified yam starch is presented in and . The photomicrograph of the morphology of starch obtained from the native yam (Dioscorea rotundata) and phosphoric acid MS showed that, the granular shapes of native starch are distinct and larger than the MS. The phosphoric acid-MS showed surface roughness compared to the native starch. However, the starch granules are more or less oval to elliptical shaped with the smaller granules appearing spherical. From the result, an average granular size range from 8.32 to 59.65 µm. Granular size and size distribution of the yam starches range from 9.88 to 56.81 µm.[Citation22] Lin et al.[Citation28] had also presented surface roughness of maize and potato starch granules upon 15 days of acid–alcohol modification. They also reported partial protuberances on potato starch granules after modification.
Granule shape and size have been identified as an important feature for the starch extraction industry because they define a mesh size for application and purification sieves.[Citation41] The granule size and shape affect the functional characteristics of starches and may influence their industrial uses.[Citation1] Starch granule shape and size contributes to the rate at which starch gelatinizes its gelatinization temperature, swelling power, and viscosity.[Citation42,Citation43] Smaller granules have high molecular bonding which leads to lower swelling.
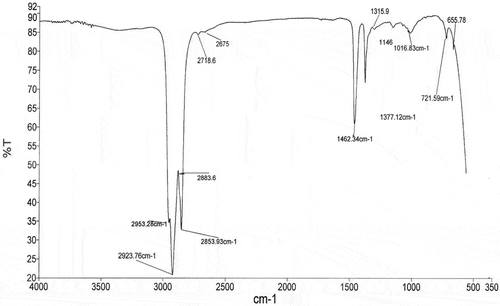
FTIR spectroscopy
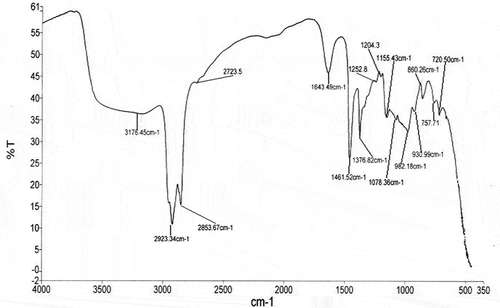
The result of FTIR spectroscopy of the native and modified yam starch is presented in and , whereas assignments to bands are summarized in . The analysis of the FTIR spectra was performed in order to identify the major functional groups of the native and MS ( and ). The spectrum of the native yam starch presents a broad, strong band at 3177 cm−Citation1 due to the stretching vibration of the O−H bond. A small peak in the region of 2923 and 2854 cm −Citation1 is assigned to the stretching vibration of C−H bond from glucose. An important peak at 1644 cm−Citation1, is assigned to scissors vibrations of O−H coming from water of hydration. Peaks in the region of 1462 and 1377 cm−Citation1 are related to the folding of C−H bonds, whereas 1253 cm−Citation1 represents −CH2OH. The absorptions at 1155, 1078, and 994 cm−Citation1 are typical of the system C−O, C−C, C−O−H, C−O−C, and C−O−H skeletal mode vibration of α-(1→4) glycosidic linkage, which characterize the polysaccharides. The free anomeric carbon C(1)−H and −CH2 deformation occurred at 860 cm−Citation1 while 700 cm−Citation1 represents the skeletal mode of pyranose ring form of polysaccharides.[Citation44]
Table 3. Effect of modification on absorption bands of yam starch.
The FTIR spectrum of the modified yam starch () showed that, O−H broad band was completely modified when compared to the native starch (3177 cm−Citation1). The peaks at 2953–2884 cm−Citation1 represent C−H stretch, whereas 1462 and 1377 cm−Citation1 is related to bending of −CH bond. The absorption at 1146 cm−Citation1 is assigned to vibration mode of C−O, C−C and 1062 cm−Citation1 represents C−O−H bending. The bands at 656 cm− Citation1 represent a skeletal mode of pyranose ring. There was no absorption band for O−H stretch, H2O absorbed (cm−Citation1; amorphous region) and the C−O−C (cm−Citation1) which represents the skeletal mode vibration of α-(1→4) glycosidic linkage. From the result, there was an indication of modification effect of phosphoric acid on the yam starch.
The phosphoric acid MS derivative when compared to native starch indicated a total shift of –OH stretch (cm−Citation1), H2O (cm−Citation1) absorbed at the amorphous domain, −CH2OH (cm−Citation1) and C−O−C (cm−Citation1) functional groups. According to Rutenberg and Solarek,[Citation45] native starches can be crosslinked when mixed in an aqueous system of reagents capable of reacting with at least two of the hydroxyl groups of neighboring molecules. Although the type of reagent used and crosslinking conditions determine the ratio of mono and di-type bonds (esters with phosphorous based agents). Starch phosphates are esters derived from phosphoric acid. The starch phosphate class is the crosslinked type which contains mono-, di-, and tri-ester starch phosphate.[Citation46] From the foregoing, at least two points (−OH and −CH2OH) of attachment were created for incoming phosphate groups and filled upon starch reaction with phosphoric acid.
The disappearance of −H2O (cm−Citation1) at the amorphous domain showed that, the mechanism of hydrolysis of glycosidic bond in starch’ granules utilized the water molecule inside the granules. From the result, phosphoric acid preferentially degraded the amorphous regions of the starch during the modification with the occurrence of cracks inside the granules and this explains the disappearance of the band representing water in the amorphous regions of the starch at 1644cm−Citation1. It is, therefore, evident that phosphoric acid modified yam starch and there was phosphorylation at –OH stretch (cm−Citation1) and –CH2OH (cm−Citation1).
Conclusion
The present study revealed significant changes in physicochemical, functional properties as well as structure of the native and MS. The phosphoric acid effect on physicochemical, and functional properties of yam starch was observed. FTIR spectroscopy and optical microscopy analyses also showed that there are substantial structural differences between the native and modified yam starch. Modification of the yam starch with phosphoric acid resulted into esterification, crosslinking and hydrolysis with concomitant effect on functional and structural properties like swelling, amylose content and some functional groups, and surface roughness of starch granules. From the foregoing, yam starch could be a cheap and good source of starch for industrial application. Thus, industrial processing and application of yam starch should be encouraged to prevent the physiological deterioration affecting yam during storage, improve agricultural practice and productivity and food security.
References
- Baah, F.D.; Maziya-Dixon, B.; Asiedu, R.; Oduro I.; Ellis W.O. Nutritional and Biochemical Composition of D. Alata (Dioscorea spp.) Tubers. Journal of Food, Agriculture and Environment 2009, 7(2), 373–378.
- Amani, N.G.; Buléon, A.; Kamenan, A.; Colonna, P. Variability In Starch Physicochemical and Functional Properties of Yam (Dioscorea spp.) Cultivated in Ivory Coast. Journal of the Science of Food and Agriculture 2004, 84, 2085–2096.
- Otegbayo, B.O.; Achidi, A.U.; Asiedu, R.; Bokanga, M. Food Quality Attributes of Pona Yams, Proceedings of the Eighth Triennial Symposium of the International Society for Tropical Root Crops, Ibadan, Nigeria, 2001, 12–16 November, 2011.
- Markson, A.A.; Omosun, G.; Madunagu, B.E.; Amadioha, A.C.; Wokocha, R. Physicochemical Alteration of Tissues of White Yam (Dioscorea Rotundata Poir) Tubers Incited by Botryoiplodia Theobromae Pat. International Journal of Current Research 2010, 4, 055–061.
- Alexander, R.J. Starch in Plastics. Cereal Foods World 1996, 41, 426–427.
- Ostertag, C. World Production and Marketing of Starch. In Cassava Flour and Starch: Progress in Research and Development, O’Brien, G.M., Best, R., Eds., CIAT-CIRAD: Columbia, CA, 1996; 105–120.
- Sebio, L.; Chang, Y.K. Effects of Selected Process Parameters in Extrusion of Yam Flour (Dioscorearotundata) on Physicochemical Properties of the Extrudates. Nahrung 2000, 44(2), 96–101.
- Gallant, D.J; Bewa, H.; Buy, Q.H.; Bouchet, B.; Szylit O.; Sealy, L. Ultrastructural and Nutritional Aspects of Some Tropical Tuber Starches. Starch/Stärke 1982, 34, 255–262.
- Wang, W.-M.; Lai, V.M.-F.; Chang, K.-F.; Lu, S.; Ho, H.-H. Effect of Amylopectin Structure on the Gelatinization and Pasting Properties of Selected Yam (Dioscorea spp.) Starches. Starch/Stärke 2006, 58, 572–579.
- Riley, C.K.; Wheatley, A.O.; Asemota, H.N. Isolation and Characterization of Starches from Eight Dioscorea Alata Cultivars Grown in Jamaica. African Journal of Biotechnology 2006, 5(17), 1528–1536.
- Wurzburg, O.B. Cross-Linked Starches. (Modified Starch Properties and Uses). CRC Press, Inc.: Boca Raton, FL, 1986.
- Olsson, E.; Hedenqvist, M.S.; Johansson, C.; Järnström, L. Influence of Citric Acid and Curing on Water Sorption, Diffusion and Permeability of starch Films. Carbohydrate Polymers 2013, 94(2), 765–772.
- Ghanbarzadeh, B.; Almasi, H.; Entezami, A.A. Improving the Barrier and Mechanical Properties of Corn Starch-Based Edible Films: Effect of Citric Acid and Carboxymethyl Cellulose. Industrial Crops and Products 2011, 33(1), 229–235.
- Hirsch, J.B.; Kokini, J.L. Understanding the Mechanism of CrossLinking Agents (POCl3, STMP, and EPI) Through Swelling Behavior and Pasting Properties of Cross-Linked Waxy Maize Starches. Cereal Chemistry Journal 2002, 79(1), 102–107.
- Sathe, S.K.; Salunkhe, D.K.J. Functional properties of the great Northern bean (Phaseolus vulgaris L.) Proteins: Emulsion, Foaming, Viscosity, and Gelation Properties. Journal of Food Science 1981, 46, 71–75.
- Leach, H.W.; McCowen, L.D.; Scoch, T.J. Structure of the starch granule I. Swelling and solubility patterns of various starches. Cereal Chemistry 1959, 36, 534–543.
- Medcalf, G.; Gilles, A. Wheat Starches: Comparison of Physicochemical Properties. Cereal Chemistry 1965, 42, 558–568.
- Gilbert, G.A.; Spragg, S.P. Iodometric Determination of Amylase. In Methods in Carbohydrate Chemistry; Whistler, R.L.; Ed.; Academic Press: Orlando, FL, 1964; 168–169 pp.
- Taghvaei-Ganjali, S.; Motiee, F.; Shakeri, E.; Abbasian, A. Effect of Amylose/Amylopectin Ratio on Physico-Mechanical Properties of Rubber Compounds Filled by Starch. Journal of Applied Chemical Researches 2010, 4(14).
- Segura-Campos, M.R.; López-Sánchez, S.M.; Castellanos-Ruelas, A.; Betancur-Ancona, D.; Chel-Guerrero, L. Physicochemical and Functional Characterization of Mucuna Pruries Depigmented Starch for Potential Industrial Applications. International Journal of Organic Chemistry 2015, 5, 1–10.
- Ceballos, H.; Sánchez, T.; Morante, N.; Fregene, M.; Dufour, D.; Smith, A.M.; Denyer, K.; Pérez, J.C.; Calle, F.; Mestres, C. Discovery of an Amylose-Free Starch Mutant in Cassava (Manihot Esculenta Crantz). Journal of Agricultural and Food Chemistry 2007, 55(18), 7469–7476.
- Addy, R.N.A.; Wireko-Manu, F.D.; Oduro, I. Physicochemical and Pasting Properties of Starch Extracted from Four Yam Varieties. Journal of Food and Nutrition Sciences 2014, 2(6), 262–269.
- Hoover, R.; Hadziyev, D. Characterization of Potato Starch and Its Monoglyceride Complexes. Starch 1981, 33, 290–300.
- Bradbury, J.H. Chemical Composition of Tropical Root Crops. ASEAN Food Journal 1988, 4, 3–13.
- Salwa, M.; Hanan, M.A.; Neressrien, M.N. Physicochemical Properties of Starch Extracted from Different Source and Theor Application in Pudding and White Sauce. World Journal of Dairy and Food Science 2010, 5(2), 173–182.
- Tester, R.F.; Karkalas, J. Swelling and Gelatinization of Oat Starches. Cereal Chemistry 1996, 73, 271–273.
- Morrison, W.R.; Tester, R.F.; Snape, C.E.; Law, R.; Gidley, M.J. Swelling and Gelatinization of Cereal Starches. IV. Some Effects of Lipid-Complexed Amylose and Free Amylose in Waxy and Normal Barley Starches. Cereal Chemistry 1993, 70(4), 305–391.
- Lin, J.H.; Lee, S.Y.; Chang, Y.H. Effect of Acid-Alcohol Treatment on the Molecular Structure and Physicochemical Properties of Maize and Potato Starches. Carbohydrate Polymer 2003, 53, 475–482.
- Hoover, R. Composition, Molecular Structure, and Physicochemical Properties of Tuber and Root Starches: A Review. Carbohydrate Polymers 2001, 45, 253–267.
- Hirsch, J.B.; Kokini, J.L. Understanding the Mechanism of Cross-Linking Agents (POCl3, STMP, and EPI) Through Swelling Behavior and Pasting Properties of Cross-Linked Waxy Maize Starches. Cereal Chemistry 2002, 79(1), 102–107.
- You, S.; Izydorczyk, M.S. Comparison of the Physicochemical Properties of Barley Starches after Partiala-Amylolysis and Acid/Alcohol Hydrolysis. Carbohydrate Polymers 2007, 69, 489–502.
- Pinnavaia, G.; Pizzirani, S. Evaluation of the Degree of Gelatinization of Starchy Products by Water Holding Capacity. Starch/Stärke 1998, 50(2–3), S64–67.
- Agama-Acevedoa, E.; Rosab, A.; Méndez-Montealvoa, G.; Bello-Péreza, L. Physicochemical and Biochemical Characterization of Starch Granules Isolated of Pigmented Maize Hybrids. Starch/Stärke 2008, 60, 433–441.
- Aryee, F.; Oduro, I.; Ellis, W.; Afuakwa, J. The Physicochemical Properties of Flour Samples from the Roots of 31 Varieties of Cassava. Food Control 2006, 17, 916–922.
- Lawal, O.S. Composition, Physicochemical Properties and Retrogradation Characteristics of Native, Oxidized, Acetylated and Acid-Thinned New Cocoyam (Xanthosoma Sagittifolium) Starch. Food Chemistry 2004, 87, 205–218.
- Wurzburg, O.B. Modified Starches: Properties and Uses. CRC Press: Boca Raton, FL, 1989; 277 p.
- Koch, V.H.; Bommer, H.D.; Koppers, J. Analytical Investigations on Phosphate Cross-Linked Starches. Starch 1982, 34, 16–21.
- Ogunmolasuyi, A. Martin. Modification of Starch Extracted from Cassava with Acidified Ethanol. British Journal of Pharmaceutical Research 2015, 8(4), 1–7.
- Davis, J.; Supatcharee, N.; Khandelwal, R.; Chibbara, R. Synthesis of Novel Starches in Planta: Opportunities and Challenges. Starch/Stärke 2003, 55, 107–120.
- Zhang P, Whistler R, BeMiller J, Hamaker B. Banana starch: production, Physicochemical properties, and digestibility-a review. Carbohy. Poly. 2005, 59: 443–458
- Leonel, M.; Sarmento, S.B.S.; Cereda, M.P. New Starches for the Food Industry: Curcuma Longa and Curcuma Zedoaria. Carbohydrate Polymers 2003, 54, 385–388.
- Tsakama, M.; Mwangwela, A.M.; Manani, T.A.; Mahungu, N.M. Physicochemical and Pasting Properties of Starch Extracted from Eleven Sweet Potato Varieties. African Journal of Food Science and Technology 2010, 1(4), 090–098.
- Singh, N.; Singh, J.; Kaur, L.; Sodhi, N.S.; Gill, S.B. Morphological, Thermal and Rheological Properties of Starches from Different Botanical Sources. Food Chemistry 2003, 81, 219–231.
- Pavia, D.L.; Lampman, G.M.; Kriz, G.S.; Vyvyan, J.R. Introduc¸ ão à Espectroscopia [Introduction to spectroscopy]; Rolland-Sabaté, A.; Sanchéz, T.; Eds.; Cengage Learning: Buléon, São Paulo, 2010.
- Rutenberg, M.W.; Solarek, D. Starch Derivatives: Production and Uses. Chapter X. In Starch: Chemistry and Technology. Academic Press: Orlando, FL, 1984.
- Hamilton, R.M.; Paschall, E.F. Production and Uses of Starch Phosphates. In Starch Chemistry and Technology, Vol. 2., Whistler, R.L.; Paschall, E.F.; Eds.; Academic Press: New York, NY, 1967.