ABSTRACT
Resistant starches were isolated from four banana cultivars: Musa AAA Cavendish, Musa ABB Bluggoe, Musa ABB Pisan Awak, and Musa AA Pisang mas. The structural and physicochemical properties of banana resistant starches were studied. Results showed that the particle size and shape of four banana resistant starches were different. Cavendish and Bluggoe banana resistant starch had a C-type crystalline structure, whereas Pisan Awak and Pisang mas had a B-type. The water-holding capacity of Pisang mas was the maximum. The solubility of Pisan Awak and Pisang mas was higher as compared to Cavendish and Bluggoe. The transparency of Cavendish banana resistant starch was the highest. More amylose was observed in Bluggoe and Pisan Awak banana resistant starch, whereas more amylopectin was observed in Cavendish and Pisang mas banana resistant starch. The initial pasting temperatures of Cavendish and Bluggoe banana resistant starches were higher as compared to Pisan Awak and Pisang mas banana resistant starch. The peak viscosity of Cavendish banana resistant starch was the highest in these four samples. The heat stability of Bluggoe banana resistant starch was the best one in the four banana resistant starches. The retrogradation was hard in the case of Bluggoe and Pisan Awak banana resistant starch. In conclusion, the properties of the four banana resistant starch samples were not the same, indicating that these could be used in different food products.
Introduction
The banana is an important fruit in tropical and subtropical zones. It has an abundance of nutritional ingredients and is effective at relieving constipation.[Citation1] An unripe banana, with a green peel, has plenty of resistant starch (RS), which comprises approximately 70% (dry base) of the peeled banana fruit. RS, which aids in body weight control, blood sugar and blood lipid regulation, and intestinal disease prevention, is a valuable food material.[Citation2] Therefore, investigations on the structural and physicochemical properties of banana resistant starch (BRS) are useful for future utilizations.
Research on BRS primarily concerns its digestibility in vitro and in vivo,[Citation3,Citation4] and the use of banana starch to produce RS.[Citation5,Citation6] Wang et al. reported on the properties of BRS and its laxative effects. They found that BRS was effective in accelerating the movement of the small intestine and in shortening the start time of defecation, but did not impact body weight and food intake.[Citation1] Olvera-Hernández et al. investigated the effect of BRS (Musa cavendish AAA) on metabolic control in Wistar rats with a high-sucrose diet and indicated that providing native BRS resulted in beneficial changes in glucose homeostasis and levels of serum lipids in rats fed a high sucrose diet.[Citation7] Agama-Acevedoa et al. studied the physicochemical, digestibility, and structural characteristics of starch isolated from banana cultivars.[Citation8] Nasrin et al. compared the physico-chemical characterization of culled plantain pulp starch, peel starch, and flour.[Citation9] Sarawong et al. investigated the effect of extrusion cooking on the physicochemical properties, RS, phenolic content, and antioxidant capacities of green banana flour.[Citation10] In terms of BRS, Wang et al. reported on the changes in RS of two banana cultivars (Musa AAA group, Cavendish subgroup and Musa ABB group, Pisang Awak subgroup) during postharvest storage.[Citation11] Vatanasuchart et al. studied the RS content, in vitro starch digestibility and physico-chemical properties of flour and starch prepared from six Thai bananas. And they found that an effect of the BB genotype on the resistance of banana starch granules to enzymatic digestion due to amylose molecules and the crystallinity of amylopectin.[Citation12] The banana has many cultivars. Musa AAA Cavendish, Musa ABB Bluggoe, Musa ABB Pisan Awak, and Musa AA Pisang mas are extensively planted in Guangdong Province, China. In our research, RS was isolated from these four banana cultivars. Then, the structural and physicochemical characteristics of the BRS were investigated and compared to accumulate fundamental research data for the development and utilization of BRS. And this research will be the base for the functional investigations of BRS in the future.
Materials and methods
Materials
Four banana cultivars were studied in this research: Musa AAA Cavendish, Musa ABB Bluggoe, Musa ABB Pisan Awak, and Musa AA Pisang mas. These cultivars were planted and consumed in Guangdong Province, China. The bananas used here were unripe, with completely green peels and were hard in texture and angular. All chemicals used in this study were of analytical grade.
Isolation and determination of BRS content
Using the method reported by Cheng et al.[Citation13] the bananas were peeled, pulped, and digested with pectinase and amylase to remove pectin, cellulose, protein, and digestible starch. The digested banana pulp was centrifuged at 3000 r/min for 15 min; the resulting precipitate was dehydrated at 50°C, ground, and stored at 5°C. The RS content was determined by the method reported by Goni, Garcia-Diz, Manas, and Saura-Calixto.[Citation14] Briefly, the method consisted of the removal of protein and digestible starch, the solubilization and enzymatic hydrolysis of RS, and the quantification of RS. Human gastric and intestinal conditions (pH and transit time) were simulated. The RS content of the four samples was 80%.
Structural observations of BRS
Light microscopy and polarizing microscopy
BRS samples were dissolved in glycerol (50% concentration) and observed under a microscope (Vanox BHS-2, Olympus Corporation, Japan) using both natural and polarized light.
Scanning electron microscopy (SEM)
Particles of BRS powder were scanned using a S3700N scanning electron microscope (Hitachi, Japan). The samples were fixed on an objective table coated with platinum (10–20 nm thickness).
Wide angle x-ray diffraction (XRD)
Cu-Kα radiation was used to scan BRS samples over the 2θ=4–60° range, at a step interval of 0.04°, a scanning rate of 17.7 s per step, a voltage of 40 kV, and a current of 40 mA. The D8 ADVANCE x-ray diffractometer from Bruker Corporation (Germany) was used for the XRD analyses.
Infrared (IR) spectroscopy
BRS samples were pressed in KBr. An IR spectrometer (VECTOR33, Bruker Corporation, Germany) was used to scan the samples from 4000 to 400 cm−Citation1 of the IR region.
Physicochemical properties of BRS
Water-holding capacity (WHC)
WHC was measured by the method reported by Toyokawa et al.[Citation15] Briefly, 20 mL of starch suspension (5 g/100 mL) was transferred to centrifuge tubes and heated in a water bath for 15 min at 50, 70, or 90°C. The tubes were centrifuged at 3000 r/min for 15 min. The supernatant was discarded and the tubes containing sediment were placed at a 45° angle for 10 min to permit water drainage. After drainage and weighing, the WHC was calculated by Eq. (1).
where m0 is the weight of the starch sample, m1 is the weight of the centrifuge tube, and m2 is the weight of the starch sample and centrifuge tube after water drainage.
Solubility (S) and swelling power (SP)
S and SP were determined using the method reported by Aparicio-Saguilána et al.[Citation16] In this experiment, 20 mL of starch suspension (5 g/100 mL) was transferred to centrifuge tubes and heated in a water bath for 30 min at 50, 70, or 90°C. After cooling to room temperature, the tubes were centrifuged at 3000 r/min for 15 min. The sediment and supernatant were separated and the sediment was dried and weighed. S and SP were calculated using Eqs. (2) and (3), respectively.
where A is the weight of dry dissolved solids in the supernatant, W is the weight of the sample, and D is the weight of the sediment.
Transparency
An aqueous starch solution was prepared by mixing 1.0 g of starch with 99.0 g of water. This solution was water-bathed in a boiling water for 15 min under continuous stirring. After bath, and cooling to room temperature, the resulted was detected at 620 nm (UV-1800 spectrophotometer, Shimadzu Co., Japan) for transparency. Distilled water was used as a blank control, which was considered to have a transparency of 100%.
Starch–iodine absorption spectra
Spectra of iodine-bound starch samples were determined using the method reported by Klucinec and Thompson.[Citation17] The BRS (50 mg) was dispersed into 10.0 mL DMSO containing 10% of 6.0 M urea. Subsequently, 2.0 mL of the dispersed solution, 25 mL of distilled water, and 1.0 mL of I2-KI (2.0 mg I2/mL and 20.0 mg KI/mL) were pipetted into a 50-mL volumetric flask and mixed respectively. The mixed solution was fixed to 50 mL with distilled water. Control solutions were prepared without BRS. A ultraviolet (UV)-visible spectrophotometer (UV-1800, Shimadzu Co., Japan) Scanning on each sample was performed from 500 to 800 nm with UV-visible spectrophotometer (UV-1800, Shimadzu Co., Japan); λmax for each sample was defined as the wavelength that resulted in the highest absorbance value.
Pasting properties
A micro visco-amylo-graph (Visgraph-E, Brabender Instruments, Inc., Germany) was used to determine the viscosity profiles (in Brabender units, [BUs]) of the starch samples. Briefly, dispersions of the BRS (6%, dry basis) were transferred to the micro visco-amylo-graph and subjected to thorough agitation. The dispersion was brought to an initial temperature of 30°C and then to 95°C at a rate of 1.5°C/min. The temperature of the dispersion was maintained at 95°C for 30 min; subsequently, the dispersion was cooled to 50°C at a rate of 1.5°C/min and maintained at 50°C for 30 min.[Citation16]
Statistical analyses
Data were analyzed by the SPSS statistical software package, v. 19.0 (IBMcompany) and were expressed as the mean ± standard deviation. One-way analysis of variance (ANOVA) was used to compare the different BRS samples; Levene’s test was used to assess homogeneity of variances; and Bonferroni’s test was used for multiple comparisons. The statistical significance was set at p < 0.05.
Results and discussion
Structural characteristics
Light microscopy and polarizing microscopy
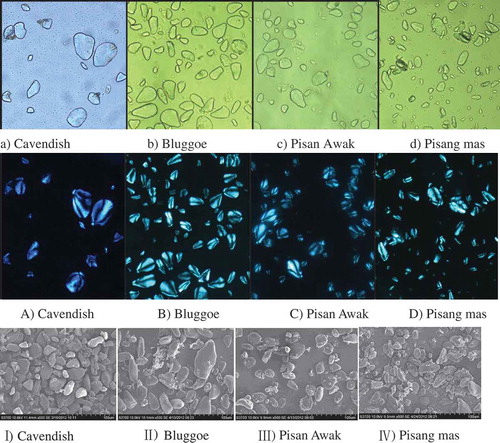
As shown in –, some of the four BRS particles showed as sunflower seed shape and others elliptic. Cavendish BRS were bigger and Pisang mas BRS were smaller. Bluggoe BRS showed much slender and Pisan Awak BRS showed round. All four BRS had annular striation, and the starch omphalion was located at the thin end of the starch particles. Annular striation is formed by alternate arrangements of crystalline region and amorphous region. It was observed by polarizing microscopy that the Maltese crosses were very evident in the four BRS particles (–), exhibited by X-shaped patterns. The Maltese crosses divided the starch particles into four white areas. And the points of these crosses were at the thin tip of the starch particles.
SEM
, I–IV shows the SEM of the samples, in which the forms of the starch particles are clearly observed. The surfaces of the four BRS were smooth and the granules were unbroken. Some Cavendish BRSs were big and the others were small. A majority of the Cavendish BRS was elliptic but a small amount triangular or irregular in shape. The diameter of the particles was approximately10–60 μm. Bluggoe BRS was thin and long, differently in size with diameter from approximately 10–90 μm. Pisan Awak BRS showed round, with size from 10–40 μm. A majority of the Pisang mas BRS was feeble and small with sizes from approximately 5–50 μm. Soares et al. found that small and round starch particles are easy to hydrolyze with an enzyme.[Citation18] Therefore, Pisang mas BRS may be more easily degraded by enzymes than the three other BRS.
Wide angle XRD
The crystalline properties of starch can be determined by XRD. Starch particle structure mainly consists of alternating crystalline region and non-crystalline region (amorphous region). In addition, there is a microcrystalline structure between the crystalline and non-crystalline region. The crystalline area is structurally tight and orderly; thus, it is hard to erode by acid and enzyme. However, the non-crystalline area is easily eroded by acid and enzyme.[Citation19,Citation20] The crystalline characteristics and degree of crystallinity directly impact the application performance of the starch. Thus, the crystalline properties of BRS were studied to benefit BRS product development.
As shown in , the XRD spectra of Cavendish and Bluggoe were similar, except for the difference in intensity of the first peak. The XRD spectra of Pisan Awak and Pisang mas were similar, therein only the intensity of the first peak was different. From and (double diffraction angles 2θ data), there are apparent diffraction peaks of the four BRS at approximately 5.6°, 15.0°, 17.0°, and 23.0º. The strongest diffraction peak was at approximately 17.0° for the four BRS samples. Bluggoe and Pisan Awak BRS had strong diffraction peaks at 5.6º, whereas Cavendish and Pisang mas BRS had weak peaks at this point. Cavendish and Bluggoe BRS had a single peak at approximately 23.0º, thus they belonged to a C-type crystalline structure. Pisan Awak and Pisang mas BRS had double peaks at approximately 23.0º, thus they were a B-type crystalline structure. The crystalline structures of the banana starch about A type,[Citation21] B type,[Citation8] and C type[Citation22] were reported. However, in our research, the RS of Musa AAA Cavendish, Musa ABB Bluggoe, Musa ABB Pisan Awak, and Musa AA Pisang mas are proven to belong to B and C type, but not A type.
Table 1. Analysis of x-ray diffraction spectra of resistant starch samples isolated from four banana cultivars.
Figure 2. X-ray diffraction spectra of resistant starch samples isolated from four banana cultivars.
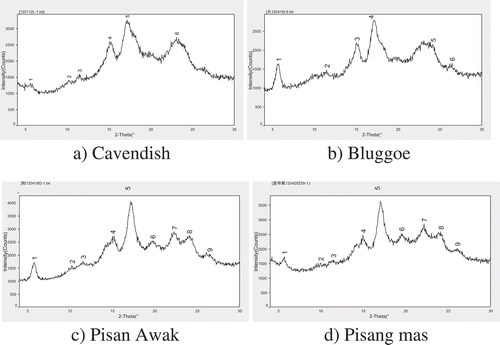
The absolute degrees of crystallinity are defined as the ratio of the area of the crystalline region (including the crystalline and microcrystalline region) to the total area (including the crystalline, microcrystalline, and non-crystalline region). Absolute crystallinity reflects the order of degrees of the molecular arrangement. The relative degrees of crystallinity are defined as the ratio of the area of the crystalline region to the area of the crystalline region plus the microcrystalline region. The relative crystallinity reflects the proportion of the compact crystalline region. It is inferred from that the sequence of absolute crystallinity was Pisan Awak > Pisang mas > Cavendish > Bluggoe; and relative crystallinity was Pisan Awak > Bluggoe > Cavendish > Pisang mas. The crystalline region is difficult to hydrolyze by enzyme. Therefore, the relative crystallinity can be used to measure the resistance of starch. The higher the relative crystallinity is, the stronger the resistance to enzyme of the starch has. Consequently, the resistance of Pisan Awak BRS has the strongest of the four BRS in this study.
IR spectroscopy
The IR spectra were similar for the four BRS samples, which indicated that the functional groups of the four BRS were the same. Based on the report by Kacurakova,[Citation23] the strong peak at 3000–3600 cm−Citation1 was caused by a stretching vibration of the OH groups. The peak at 2932 cm−Citation1 was generated by a stretching vibration of the saturation of CH, CH2. The absorption peak of the C=O bond was at 1650 cm−Citation1. The absorption peaks of the C-O-C and C-O bonds were at 1160 and 1085 cm−Citation1. The stretching vibration of C-O in the pyranose ring alcohol was at 993 cm−Citation1. The absorption bands of D-glucopyranose were at 930, 860, and 766 cm−Citation1.
Physicochemical properties of BRS
WHC, S, SP, and transparency
shows that the WHC of the four BRS increased as the temperature increased. Except for Bluggoe, the data of the three other banana cultivars at different temperatures had significant differences in WHC. At the same temperature, only the WHC of Pisang mas BRS was significantly larger than the others. This might be resulted from the small Pisang mas BRS particles with a larger specific surface area. The WHC of Pisan Awak BRS was significantly different from the other cultivars at 70 and 90ºC.
Table 2. The water-holding capacity, solubility, swelling power, and transparency of resistant starch samples isolated from four banana cultivars*.
The S of the four BRS increased as the temperature increased (). Only the data of Cavendish was significantly different at the three temperatures. At 50 and 70ºC, the sequence of S was Pisang mas > Pisan Awak > Cavendish > Bluggoe. Pisang mas and Pisan Awak BRS S were significantly different from the two other cultivars. At 90ºC the sequence of S was Pisan Awak > Pisang mas > Cavendish > Bluggoe. There was a significant difference among cultivars.
The same banana cultivar showed the similar SP at different temperatures. At the same temperature, the SP of Pisan Awak and Bluggoe was larger, but the differences were not significant. It can be inferred that different BRSs can be chosen according to their S and SP during product exploitation. The sequence of transparency was Cavendish > Pisan Awak > Bluggoe > Pisang mas. There were significant differences among cultivars (). The transparency property can serve as a reference in the application of a RS for drinks.
Starch–iodine absorption spectra
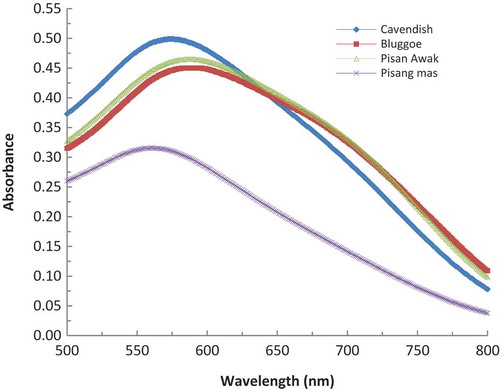
The maximum absorption wavelengths are different between amylose and amylopectin. The maximum absorption wavelengths of amylose are larger than that of amylopectin. Klucinec and Thompson reported that the maximum absorption wavelengths of amylose for high-amylose maize starches are 643–655 nm, whereas those of amylopectin are 559–583 nm.[Citation17] BRS contains both amylose and amylopectin[Citation11] and as illustrated in , the maximum absorption wavelengths of samples of Bluggoe, Pisan Awak, Cavendish, and Pisang mas were 592–596, 584–591, 576–572, and 560–561 nm, respectively. Therefore, Bluggoe and Pisan Awak BRS contained more amylose, whereas Cavendish and Pisang mas BRS contained more amylopectin.
Pasting properties
The gelatinization temperature, viscosity, and stability information of the samples of Bluggoe, Pisan Awak, Cavendish and Pisang mas were obtained from Brabender curves. In , A is the initial gelatinization temperature, namely the temperature at which the starch paste begins to increase in viscosity. The sequence based on the initial gelatinization temperature is Cavendish > Bluggoe > Pisang mas > Pisan Awak. B is the maximum gelatinization, also is referred to the peak value viscosity, which indicates the viscosity and corresponding temperature when the curve reaches the highest point for the first time. C is the viscosity of the heating terminal, namely the viscosity at 95ºC. The B and C parameters of the four BRS showed as the sequence Cavendish > Pisang mas > Bluggoe > Pisan Awak. D is the viscosity of the cooling starting point, namely the viscosity kept at 95ºC for 30 min. E is the viscosity of the cooling terminal, namely the viscosity at 50ºC. F is the viscosity kept at 50ºC for 30 min. In terms of D, E, and F parameters of the four BRS, only Cavendish were significantly larger than the other three. Bluggoe, Pisan Awak, and Pisang mas showed no significant differences.
Table 3. Characteristic parameters of the viscosity of resistant starch samples isolated from four banana cultivars.
C–D is the change in viscosity during a continuing high temperature stage, which reflects the tolerance of the gelatinized starch to high temperatures and shearing action. The value of C–D is smaller, indicating the heat stability is better. As seen in , the heat stability of Bluggoe was the best, and Pisang mas was the worst among the four BRS samples. The heat stability of Pisan Awak was good, Cavendish was moderate among the four BRS samples. E–F is the viscosity stability at a cool temperature. The value of E–F is larger, indicating the cool stability is worse. The cool stability of Cavendish BRS was the lowest. The heat and cool viscosity stability characteristics can provide the basis for the design and calculation for processing starch products. E–D is the setback. The starch would be subject to facilitate retrogradation if the E–D value is larger. Thus, the Cavendish BRS was the most likely to undergo retrogradation, followed by the Pisang mas BRS. Comparatively, Bluggoe and Pisan Awak BRS were not likely to undergo retrogradation.
Conclusion
Cavendish BRS particles were larger and Pisang mas BRS were smaller. Bluggoe BRS were more slender, and Pisan Awak BRS were rounder. The Maltese crosses of four BRS displayed X-shaped patterns. The omphalion of the four BRS were located at the thin end of the starch particles. The diameters of the four BRS were from 5–90 μm.
Cavendish and Bluggoe BRS belonged to C-type crystalline structure, whereas Pisan Awak and Pisang mas BRS belonged to B-type crystalline structure. The resistance to enzymolysis by Pisan Awak BRS was the strongest. At the same temperature, only the WHC of Pisang mas BRS was always significantly larger than the other three cultivars; the S of Pisang mas and Pisan Awak were significantly larger than the other two BRS. The order of transparency was Cavendish > Pisan Awak > Bluggoe > Pisang mas. Bluggoe and Pisan Awak BRS contained more amylose, whereas Cavendish and Pisang mas BRS contained more amylopectin. Brabender Micro Visco-Amylo-Graph analysis showed that the initial gelatinization temperatures of Cavendish and Bluggoe BRS were higher than that of Pisan Awak and Pisang mas. For the Cavendish BRS, the peak viscosity was the highest and the cool viscosity stability was the lowest. Bluggoe BRS was the best in heat viscosity stability. Cavendish BRS was the most likely to retrograde, whereas Bluggoe and Pisan Awak BRS were difficult to retrograde.
Funding
This research was supported by The National Natural Science Foundation of China (Grant No. 31301530); the Fundamental Research Funds for the Central Universities of China (Grant No. 2015ZZ122, 2014ZZ0058).
Additional information
Funding
References
- Wang, J.; Huang, J.H.; Cheng, Y.F.; Yang, G.M. Banana Resistant Starch and Its Effects on Constipation Model Mice. Journal of Medicinal Food 2014a, 17, 902–907.
- Niba, L.L. Resistant Starch: A Potential Functional Food Ingredient. Nutrition and Food Science 2002, 2, 62–67.
- Faisanta, N.; Buléona, A.; Colonnaa, P.; Molisa, C.; Lartiguea, S.; Galmichea, J.P.; Champa, M. Digestion of Raw Banana Starch in the Small Intestine of Healthy Humans: Structural Features of Resistant Starch. British Journal of Nutrition 1995, 73, 111–123.
- Langkilde, A.M.; Champ, M.; Andersson, H. Effects of High-Resistant-Starch Banana Flour (RS2) on in Vitro Fermentation and the Small-Bowel Excretion of Energy, Nutrients, and Sterols: An Ileostomy Study. American Journal of Clinical Nutrition 2002, 75, 104–111.
- González-Sotoa, R.A.; Mora-Escobedob, R.; Hernández-Sánchezb, H.; Sánchez-Riveraa, M.; Bello-Péreza, L.A. The Influence of Time and Storage Temperature on Resistant Starch Formation from Autoclaved Debranched Banana Starch. Food Research International 2007, 40, 304–310.
- Aparicio-Saguilán, A.; Gutiérrez-Meraz, F.; García-Suárez, F.J.; Tovar, J.; Bello-Péreza, L.A. Physicochemical and Functional Properties of Cross-Linked Banana Resistant Starch. Effect of Pressure Cooking. Starch-Stärke 2008, 60, 286–291.
- Olvera-Hernández, V.; Aparicio-Trápala, M.A.; Ble-Castillo, J.L.; Muñozcano, J.; Rodríguezblanco, L. Effect of Banana Resistant Starch (Musa Cavendish AAA) on Metabolic Control in Wistar Rats with a High-Sucrose Diet. Universidad y Ciencia 2012, 28, 51–56.
- Agama-Acevedoa, E.; Nuñez-Santiagoa, M.C.; Alvarez-Ramirezb, J.; Bello-Péreza, L.A. Physicochemical, Digestibility and Structural Characteristics of Starch Isolated from Banana Cultivars. Carbohydrate Polymers 2015, 124, 17–24.
- Nasrin, T.A.A.; Noomhorm, A.; Anal, A.K. Physico-Chemical Characterization of Culled Plantain Pulp Starch, Peel Starch, and Flour. International Journal of Food Properties 2015, 18, 165–177.
- Sarawong, C.; Schoenlechner, R.; Sekiguchi, K.; Berghofer, E.; Perry K.W. Ng. Effect of Extrusion Cooking on the Physicochemical Properties, Resistant Starch, Phenolic Content and Antioxidant Capacities of Green Banana Flour. Food Chemistry 2014, 143, 33–39.
- Wang, J.; Tang, X.J.; Chen, P.S.; Huang, H.H. Changes in Resistant Starch from Two Banana Cultivars During Postharvest Storage. Food Chemistry 2014b, 156, 319–325.
- Vatanasuchart, N.; Niyomwit, B.; Wongkrajang, K. Resistant Starch Content, in Vitro Starch Digestibility and Physico-Chemical Properties of Flour and Starch from Thai Bananas. Maejo International Journal of Science and Technology 2012, 6, 259–271.
- Cheng, Y.F.; Yang, G.M.; Wang, J.; Du, B.; Bao, J.Y.; Wen, S.N.; Ding, W.R. Effects of Spray Drying Technologies on the Retention Rate of Banana Resistant Starch. Transactions of the Chinese Society of Agricultural Engineering 2008, 24, 282–286.
- Goni, I.; Garcia-Diz, L.; Manas, E.; Saura-Calixto, F. Analysis of Resistant Starch: A Method for Foods and Food Products. Food Chemistry 1996, 56, 445–449.
- Toyokawa, H.; Rubenthaler, G.L.; Powers, J.R.; Schanus, E.G. Japanese Noodle Qualities.II. Starch Components. Cereal Chemistry 1989, 66, 387–391.
- Aparicio-Saguilána, A.; Flores-Huicochea, E.; Tovar, J.; García-Suárez, F.; Gutiérrez-Meraz, F.; Bello-Pérez, L.A. Resistant Starch-Rich Powders Prepared by Autoclaving of Native and Lintnerized Banana Starch: Partial Characterization. Starch-Starke 2005, 57, 405–412.
- Klucinec, J.D.; Thompson, D.B. Fractionation of High-Amylose Maize Starches by Differential Alcohol Precipitation and Chromatography of the Fractions. Cereal Chemistry 1998, 75, 887–896.
- Soares, C.A.; Peroni-Okita, F.H.; Cardoso, M.B.; Shitakubo, R.; Lajolo, F.M.; Cordenunsi, B.R. Plantain and Banana Starches: Granule Structural Characteristics Explain the Differences in Their Starch Degradation Patterns. Journal of Agricultural And Food Chemistry 2011, 59, 6672–6681.
- Mahanta, C.L.; Ali, S.Z.; Bhattacharya, K.R.; Mukherjee, P.S. Nature of Starch Crystallinity in Parboiled Rice. Starch-Stärke 1989, 41, 171–176.
- Yang, J.F.; Luo, Z.G.; Luo, F.X. To Review the Starch Crystallinity. Science and Technology of Food Industry 2007, 7, 240–243.
- Bello-Perez, L.A.; Agama-Acevedo, E.; Sayago-Ayerdi, S.; Moreno-Damian, E.; Figueroa, J.D.C. Some Structural, Physicochemical and Functional Studies of Banana Starches from Two Varieties Growing in Guerrero, Mexico. Starch-Stärke 2000, 52, 68–73.
- Waliszewski, K.N.; Aparicio, M.A.; Bello, L.A.; Monroy, J.A. Changes of Banana Starch by Chemical and Physical Modification. Carbohydrate Polymers 2003, 52, 237–242.
- Kacurakova, M.; Wilson, R.H. Developments in Mid-Infrared FT-IR Spectroscopy of Selected Carbohydrates. Carbohydrate Polymers 2001, 44, 291–303.