ABSTRACT
In this study the structural features, antioxidant, and tyrosinase inhibitory activities of proanthocyanidins in leaves of two tea cultivars, namely Huangjingui and Qilan, were investigated. The matrix-assisted laser desorption/ionization time-of-flight mass spectrometry and thiolysis-high performance liquid chromatography-electrospray ionization mass spectrometry analyses confirmed that these proanthocyanidins were built up a mixture of procyanidins, propelargonidins, and prodelphinidins, with the predominance of procyanidins. The proanthocyanidins in leaves of Huangjingui and Qilan cultivars exhibited potent antioxidant activities with IC50 values of 87.36 ± 0.83 and 97.33 ± 0.61 µg/mL in 2,2-diphenyl-1-picrylhydrazyl radical scavenging assay, 43.57 ± 0.25 and 55.01 ± 0.22 µg/mL in 2,2’-azinobis(3-ethylbenzothiazoline-6-sulfonic acid) radical scavenging assay, and with ferric reducing antioxidant power values of 4.83 ± 0.03 and 3.97 ± 0.02 mmol ascorbic acid equivalent/g in ferric reducing antioxidant power assay. It was also found that these proanthocyanidins had strong inhibition on tyrosinase activity with IC50 values of 54.8 ± 1.27 and 20.0 ± 0.89 µg/mL for Huangjingui and Qilan cultivars, respectively. The results indicated that the proanthocyanidins in leaves of two tea cultivars could be considered as natural antioxidants and tyrosinase inhibitors applied in pharmaceutical and cosmetic industries in the future.
Introduction
Tea (Camellia sinensis L.), with an annual production of over three million tons,[Citation1] is one of the most popular non-alcoholic beverages in the world.[Citation2] It is believed that consumption of tea is associated with a lower incidence of certain chronic disease[Citation3,Citation4] due to its abundant active components polyphenols.[Citation5] According to the records of Ho et al.,[Citation6] tea polyphenols account for up to 30% of the dry weight of the fresh tea leaves.
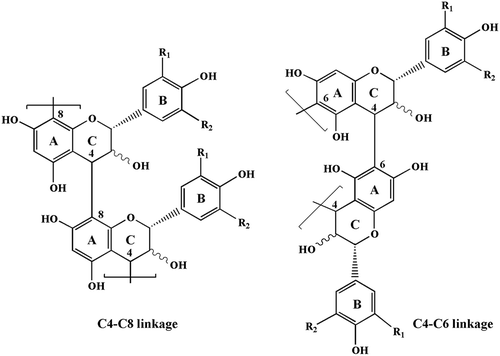
Proanthocyanidins also termed as condensed tannins, are a structurally complex subclass of polyphenolic compounds that are composed of flavan-3-ol subunits linked mainly through C4-C8 or C4-C6 bonds. The most widely distributed proanthocyanidins in plants are propelargonidins, procyanidins and prodelphinidins (), consisting of (epi)afzelechin, (epi)catechin, and (epi)gallocatechin units, respectively. In recent years, proanthocyanidins have received considerable attention arising from their potential health benefits, including antitumor effects, antioxidant, and tyrosinase inhibitory activities.[Citation7–Citation11] Based on the report by Robertson,[Citation12] proanthocyanidins make up about 2–3% of the dry weight of tea leaves. Although the predominant flavan-3-ol monomers constituting the proanthocyanidins in tea leaves have been identified to be epigallocatechin gallate, epicatechin gallate, epigallocatechin, and epicatechin,[Citation13] detailed information on the proanthocyanidin profiles including polymer chain length, chemical constitution of individual chains, and the sequential succession of monomer units in individual chains, have not been well resolved.
Figure 1. Chemical structure of proanthocyanidin polymers: R1, R2 = H, propelargonidins; R1 = OH, R2 = H, procyanidins; R1, R2 = OH, prodelphinidins.
In the present study, two tea cultivars, namely Huangjingui and Qilan commonly cultivated in Fujian province, were chosen for investigation. The proanthocyanidins in freshly harvested leaves of them were isolated through a Sephadex LH-20 column. Their structural features were characterized using matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF-MS) and thiolysis coupled with reversed-phase high-performance liquid chromatography-electrospray ionization mass spectrometry (thiolysis-HPLC-ESI-MS) analyses. Moreover, the free radical scavenging capacity, ferric reducing antioxidant power, and tyrosinase inhibitory activity of these proanthocyanidins were also evaluated.
Materials and methods
Chemicals and reagents
2,2-Diphenyl-1-picrylhydrazyl (DPPH), 2,2’-azinobis(3-ethylbenzothiazoline-6-sulfonic acid) (ABTS), 2,4,6-tripyridyl-S-triazine (TPTZ), butylated hydroxyanisole (BHA), ascorbic acid, 2,5-dihydroxybenzoic acid, cesium chloride, benzyl mercaptan, mushroom tyrosinase, 3,4-dihydroxyphenylalanine, Sephadex LH-20 and HPLC standards were purchased from Sigma-Aldrich (St. Louis, MO, USA). Water was purified in the laboratory by a Millipore Milli-Q apparatus (TGI Pure Water Systems, USA). Acetonitrile and trifluoroacetic acid (TFA) for HPLC analysis were of HPLC grade. All other reagents and solvents used were of analytical grade.
Plant materials
The first three to five of developmentally matured leaves of Huangjingui and Qilan cultivars were hand-harvested randomly in May 6, 2012 in Anxi, Fujian province, Southeast China. Leaves damaged by insects or mechanical factors were avoided. The harvested leaves were washed and immediately freeze dried using a desktop freeze-dryer at –56°C for 72 h, then ground finely and stored at –20°C prior to extraction. The two tea cultivars are identified by Professor Zhen-Ji Li (School of Life Sciences, Xiamen University).
Extraction and purification of the proanthocyanidins
Freeze-dried leaf powders (10 g of each) of Huangjingui and Qilan cultivars were extracted with 200 mL of 70% aqueous acetone and kept for 24 h at the room temperature. The extracts were then centrifuged at 3500 × g for 15 min and the supernatant was collected. The same procedure was repeated three times. The acetone extracts were pooled and rotary-evaporated under vacuum at 38°C to remove acetone. The aqueous phase was recovered and partitioned successively with hexane and dichloromethane in order to remove pigments, lipids, and nonpolar materials. The remaining aqueous fractions were then loaded onto a Sephadex LH-20 column (30 × 2.5 cm i.d.), which was eluted successively with 50% aqueous methanol and 70% aqueous acetone. The fractions of 70% aqueous acetone were retained. The acetone was removed under reduced pressure, and the remaining aqueous fractions were lyophilized to obtain the respective purified proanthocyanidins in leaves of two tea cultivars.
MALDI-TOF-MS analysis
The MALDI-TOF-MS spectra were recorded on a Bruker Reflex III instrument (Bruker, Germany). The method described by Wei et al.[Citation14] was applied to analyze the proanthocyanidins in leaves of Huangjingui and Qilan cultivars. The irradiation source was a pulsed nitrogen laser with a wavelength of 337 nm, and the duration of the laser pulse was 3 ns. In the positive reflectron mode, an accelerating voltage of 20.0 kV and a reflectron voltage of 23.0 kV were used.
Thiolysis coupled with reversed-phase HPLC-ESI-MS analysis
Reversed-phase HPLC-ESI-MS analysis was completed according to the method described by Li et al.[Citation15] and Wei et al.[Citation14] The proanthocyanidins in leaves of Huangjingui and Qilan cultivars (5 mg/mL in methanol, 50 µL) were placed in a vial and to this was added hydrochloric acid in methanol (3.3:96.7, v/v; 50 µL) and benzyl mercaptan in methanol (5:95, v/v; 100 µL). The solution was heated at 40°C for 30 min, and cooled to room temperature. The degradation products were injected into an Agilent 1200 system (Agilent, USA) interfaced to a QTRAP 3200 (Applied Biosystems, USA) with a Hypersil ODS column (4.6 mm × 250 mm, 5 µm; Elite, China). Two solvents, namely A = 0.5% (v/v) TFA in aqueous and B = CH3CN, were used. The gradient condition was: 0–45 min, 12–80% B (linear gradient); 45–50 min, 80–12% B (linear gradient). The column temperature was 25°C and the flow-rate was set at 1 mL/min. Detection was at a wavelength of 280 nm and the UV spectra were acquired between 200–600 nm. Degradation products were identified by chromatograms according to their relative retention times and LC-MS in the negative ion mode. For the MS analysis, voltage of capillary was set at 3000 V. The temperature of ion source and the desolvation was adjusted to 150 and 500°C, respectively. The spectra were scanned over a mass range of m/z 100–1500.
Antioxidant activity analyses
Three different assays were used for the evaluation of the antioxidant activities of the proanthocyanidins in leaves of Huangjingui and Qilan cultivars.
DPPH radical scavenging assay was conducted according to the method described by Brand-Williams et al.[Citation16] Briefly, 0.1 mL of various concentrations of samples was added to 3.9 mL of 25 mg/L DPPH radical solution in methanol. The mixture was shaken vigorously and allowed to stand at room temperature for 30 min. The absorbance at 517 nm was measured and synthetic antioxidant BHA was used as a positive control. The capability to scavenge the DPPH radical by the sample was calculated as follows: DPPH scavenging effect (%) = [(A1 – A2) /A1] × 100, where A1 is the absorbance of the control reaction, and A2 is the absorbance in the presence of the sample. Moreover, the concentration of the sample that could scavenge 50% of the DPPH radicals (IC50) was calculated.
ABTS radical scavenging assay was done according to the method described by Re et al.[Citation17] ABTS radical cation was generated by reacting 7 mM ABTS and 2.45 mM potassium persulfate after incubation at room temperature in dark for 16 h until reaching a stable oxidative state. On the day of analysis, the ABTS solution was diluted with 80% ethanol to an absorbance of 0.700 ± 0.050 at a wavelength of 734 nm. Various concentrations of samples (0.1 mL) of dissolved in 80% ethanol was added to ABTS solution (3.9 mL) and mixed thoroughly. The reactive mixture was allowed to stand at room temperature for 6 min and the absorbance was immediately recorded at 734 nm. The capability to scavenge the ABTS radical by the sample was calculated as follows: ABTS scavenging effect (%) = [(A1 – A2) /A1] × 100, where A1 is the absorbance of the control reaction, and A2 is the absorbance in the presence of the sample. Moreover, the concentration of the sample that could scavenge 50% of the ABTS radicals (IC50) was calculated. BHA was used as a positive control.
Ferric reducing antioxidant power (FRAP) assay was determined according to the method described by Benzie and Strain.[Citation18] Briefly, 3 mL of freshly prepared FRAP reagent was mixed with 0.1 mL of various concentrations of samples dissolved in methanol. The FRAP reagent was prepared from 300 mmol/L acetate buffer (pH 3.6), 20 mmol/L ferric chloride and 10 mmol/L TPTZ made up in 40 mmol/L hydrochloric acid. All the above three solutions were mixed together in the ratio of 25:2.5:2.5 (v/v/v). The absorbance of reaction mixture at 593 nm was measured spectrophotometrically after incubation at room temperature for 10 min. Moreover, the FRAP values, expressed in mmol ascorbic acid equivalents (AAE)/g dried samples, were derived from a standard curve. BHA was used as a positive control.
Enzyme inhibition assay
The enzyme inhibitory activities of the proanthocyanidins in leaves of Huangjingui and Qilan cultivars were determined by the method of Qiu et al.[Citation19] with a slight modification. In this investigation, 3,4-dihydroxyphenylalanine was used as a substrate for the enzyme activity assay. The reaction medium (3 mL) contained 0.5 mM 3,4-dihydroxyphenylalanine in 50 mM sodium phosphate buffer (pH 6.8). The final concentration of mushroom tyrosinase was 3.33 µg/mL. The enzyme activity was monitored by dopachrome formation at 475 nm accompanying the oxidation of the substrate. Absorption was recorded using a Genesys 10S spectrophotometer. The extent of inhibition by the addition of the sample was expressed as the percentage necessary for 50% inhibition (IC50) for enzyme activity assay. Controls, without inhibitor but containing 3.3% water, were routinely carried out. All measurements were carried out at 30°C.
Statistical analysis
Data were expressed as mean ± standard deviation (n = 3). One-way analysis of variance (ANOVA) and Student-Newman-Keuls test were used to examine the difference between samples (p < 0.05). The correlation coefficients among the different antioxidant assays were carried out using linear regression analysis. All statistical analyses were done with SPSS 16.0 for windows.
Results and discussion
MALDI-TOF-MS analysis of the proanthocyanidins
MALDI-TOF-MS is considered a preferred mass spectral method for the analysis of highly polydisperse and heterogeneous proanthocyanidins.[Citation9,Citation11,Citation14,Citation20,Citation21] The positive-ion MALDI-TOF mass spectra of the proanthocyanidins in leaves of A: Huangjingui and B: Qilan cultivars, recorded as CS+ adducts in the reflectron mode, are shown in . The mass of spectra showed a series of peaks at m/z 999, 1287, 1575, 1863, etc. The distance between the major peaks was 288 Da, which coincides with the mass of a (epi)catechin unit. Prolongation of the proanthocyanidins was due to addition of (epi)catechin monomers, extending up to octamers for Huangjingui cultivar and to nonamers for Qilan cultivar, respectively. Another repeated pattern within each main set of peaks was signals separated by Δ16 Da difference ( inset). These masses could be produced by the heterogeneity of monomer flavan-3-ol subunits. The subset of masses Δ16 Da lower indicated the polymer chains containing monomers with only one hydroxyl group at the aromatic ring B. The subset of masses Δ16 Da higher could be explained by heteropolymers of repeating flavan-3-ol units containing an additional hydroxyl group at the position 5’ of the B-ring as described by Reed et al.[Citation20] The proanthocyanidins in leaves of two tea cultivars were built up of procyanidins, propelargonidins, and prodelphinidins, all with the predominance of procyanidins.
Figure 2. MALDI-TOF positive reflectron mode mass spectra of the proanthocyanidins in leaves of A: Huangjingui and B: Qilan cultivars.
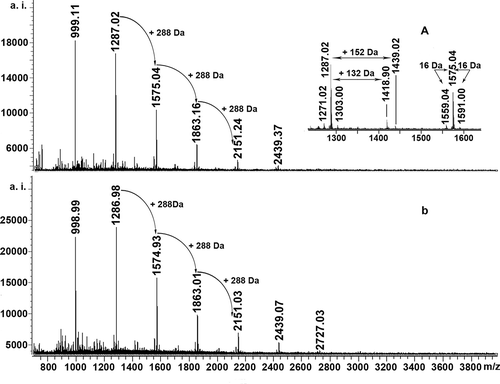
In addition, the series of peaks with more 132 Da and 152 Da were also detected in positive reflectron mode spectra of two tea cultivars ( inset). According to previous studies, the former might be quasimolecular ions [M + 2Cs – H]+ generated by simultaneous attachment of two Cs+ and loss of a proton,[Citation22] while the latter might be corresponding to the addition of one galloyl group at the heterocyclic C-ring.[Citation15,Citation20] Similar molecular forms and ionization patterns for Acacia confusa leaf proanthocyanidins were also reported by Wei et al.[Citation23]
Thiolysis-HPLC-ESI-MS analysis of the proanthocyanidins
Thiolysis coupled with reversed-phase HPLC-ESI-MS is commonly used to determine the nature and proportion of constituent units of proanthocyanidins in plants and fruits.[Citation14,Citation15] The typical chromatograms of thiolytic products of the proanthocyanidins in leaves of A: Huangjingui and B: Qilan cultivars are shown in . Eleven peaks corresponding to either terminal units or extension units were detected. Peaks 1, 2, 3, 4, and 6 were identified to be epigallocatechin, catechin, epicatechin, epigallocatechin-3-O-gallate and epicatechin-3-O-gallate by comparing the retention time and mass spectra with those of authentic standards, respectively. Peak 5 exhibited mass spectrum at m/z 273 ([M – H]–). It can be azfelechin or (epi)azfelechin, respectively. Peaks 7, 8, 9, 10, and 11 gave rise to m/z 427, 579, 411, 563, and 395, respectively, in negative ion mode. They were identified as (epi)gallocatechin benzylthioether, (epi)gallocatechin-3-O-gallate benzylthioether, (epi)catechin benzylthioether, (epi)catechin-3-O-gallate benzylthioether, and (epi)-afzelechin benzylthioether, respectively. Due to lack of authentic standards, the stereochemistry of these compounds could not be confirmed based on mass spectra.
Figure 3. Reversed-phase HPLC chromatograms of thiolytic products of the proanthocyanidins in leaves of A: Huangjingui and B: Qilan cultivars. Peaks 12 and 13 represent excessive benzyl mercaptan and thiolytic by-product, respectively.
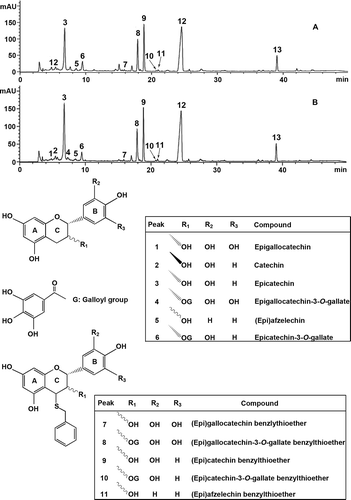
The results after thiolytic degradation suggested that the two tea cultivars exhibited similar proanthocyanidin terminal and extension unit flavan-3-ol profiles. The terminal units of the proanthocyanidins mainly consisted of epicatechin. The extension units of the proanthocyanidins were predominantly constituted by (epi)catechin and (epi)gallocatechin-3-O-gallate, with a small amount of (epi)-gallocatechin, (epi)catechin-3-O-gallate, and (epi)afzelechin. The proanthocyanidins in leaves of Huangjingui and Qilan cultivars consisted of a mixture of propelargonidins, procyanidins, and prodelphinidins, which was in agreement with the findings obtained by MALDI-TOF-MS analysis.
Antioxidant activities of the proanthocyanidins
In order to give a comprehensive evaluation of antioxidant activities of the proanthocyanidins in leaves of Huangjingui and Qilan cultivars, three different in vitro assays including DPPH radical scavenging, ABTS radical scavenging, and FRAP were carried out in the present study. DPPH and ABTS assays are simple and quick methods that have been extensively used to evaluate radical scavenging activities of pure compounds as well as plant extracts.[Citation24,Citation25] and shows DPPH and ABTS radical scavenging activities of the proanthocyanidins in leaves of two tea cultivars at different concentrations. The results demonstrated that these proanthocyanidins could scavenge DPPH and ABTS radicals in a concentration-dependent manner. In order to quantify the antioxidant activity further, the IC50 values were calculated and results are shown in . The IC50 values of the proanthocyanidins in leaves of Huangjingui and Qilan cultivars, as well as BHA, for the DPPH assay were 87.36 ± 0.83, 97.33 ± 0.61, and 117.57 ± 1.43 µg/mL, and for the ABTS assay were 43.57 ± 0.25, 55.01 ± 0.22, and 97.43 ± 0.49 µg/mL, respectively. A lower IC50 value is associated with a higher radical scavenging activity. Apparently, Huangjingui and Qilan cultivars proanthocyanidins exhibited higher radical scavenging activity than that of BHA, as measured by DPPH and ABTS assays.
Table 1. Antioxidant activities of the proanthocyanidins in leaves of Huangjingui and Qilan cultivars using the DPPH radical scavenging, ABTS radical scavenging and the FRAP assays.
Figure 4. Antioxidant activity of the proanthocyanidins in leaves of Huangjingui and Qilan cultivars: (A) DPPH radical scavenging activity; (B) ABTS radical scavenging activity; (C) Ferric reducing antioxidant power (FRAP).
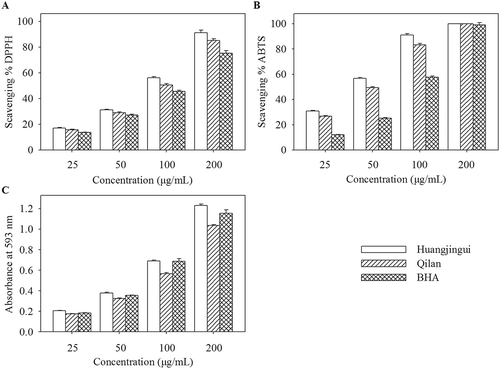
FRAP is another widely used method for the measurement of the antioxidants. The FRAP assay is based on the redox reaction of ferric ion in the presence of a reducer.[Citation26] Several researchers have confirmed that the antioxidant activity of plants is closely associated with their reducing power.[Citation27] A higher absorbance corresponds to a higher ferric reducing power. The proanthocyanidins in leaves of two tea cultivars exhibited increased ferric reducing power with the increasing concentration (). The FRAP values, expressed in AAE, are presented in . It was found that the FRAP values of the proanthocyanidins in leaves of Huangjingui and Qilan cultivars were 4.83 ± 0.03 and 3.97 ± 0.02 mmol AAE/g, respectively. Similar to the results obtained from the DPPH and ABTS radical scavenging assays, Huangjingui cultivar proanthocyanidins showed significantly higher ferric ion-reducing activities than that of Qilan cultivar. Furthermore, the correlation coefficients (R2) for DPPH-ABTS, DPPH-FRAP, and ABTS-FRAP established by liner regression analysis were 0.913, 0.826, and 0.979, respectively. The good liner correlations (R2 ≥ 0.826, p < 0.01) among all the methods suggested that all antioxidant activity assays were reliable and interchangeable for antioxidant activities of the proanthocyanidins in leaves of two studied tea cultivars. These results were in agreement with other reports in the literature.[Citation9,Citation28]
Inhibitory effects of the proanthocyanidins on mushroom tyrosinase activity
The inhibitory effects of the proanthocyanidins in leaves of two tea cultivars on oxidation of 3,4-dihydroxyphenylalanine catalyzed by mushroom tyrosinase were investigated. As shown in -, the proanthocyanidins significantly inhibited the activity of mushroom tyrosinase in a concentration-dependent manner. The IC50 values of the proanthocyanidins in leaves of Huangjingui and Qilan cultivars were determined to be 54.8 ± 1.27 and 20.0 ± 0.89 µg/mL, respectively. A lower IC50 value reflects greater tyrosinase inhibitory activity. Wang et al.[Citation29] reported that the methanolic extract from sorghum distillery residue exhibited tyrosinase inhibitory activity with an IC50 value of 580.0 µg/mL. Li et al.[Citation30] found that aminoethylisothiourea (AET) gave an inhibitory effect against the tyrosinase with an IC50 value of 574.1 µg/mL. The results suggested that the obtained proanthocyanidins in the present study might be developed as potent tyrosinase inhibitors.
Figure 5. (1) Inhibitory activity, (2) inhibitiory mechanism, and (3) inhibitiory type and constants of the proanthocyanidins in leaves of A: Huangjingui and B: Qilan cultivars against mushroom tyrosinase. The concentrations of the proanthocyanidins for curves 0-3 were 0, 6.66, 33.3, 66.6 µg/mL for Huangjingui cultivar, and 0, 13.3, 53.3, 80.0 µg/mL for Qilan cultivar, respectively.
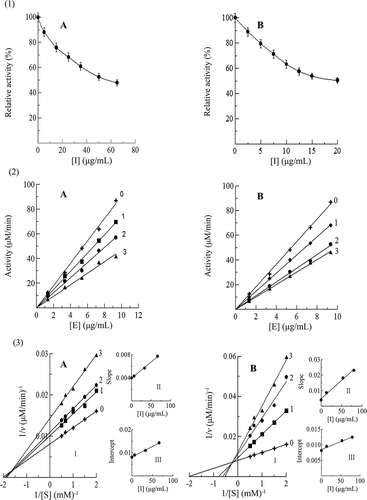
The inhibitory mechanisms of the proanthocyanidins in leaves of two tea cultivars on mushroom tyrosinase activity were also studied. As observed in -, the plots of the remaining enzyme activity versus the concentrations of enzyme gave a family of straight lines with different concentrations of the proanthocyanidins, which all passed through the origin. Increasing the inhibitor concentration resulted in a descending of the slopes of the lines, indicating that the presence of proanthocyanidins did not bring down the amount of enzyme but just lead to a decrease in the enzyme activity. The inhibitory mechanisms of the proanthocyanidins in leaves of Huangjingui and Qilan cultivars on the activity of mushroom tyrosinase belonged to be reversible.
The inhibitory kinetic behavior of the oxidation of 3,4-dihydroxyphenylalanine, catalyzed by mushroom tyrosinase at different concentrations of the proanthocyanidins in leaves of two tea cultivars, was then studied. From the double-reciprocal plots of 1/v versus 1/[S] at different concentrations of the proanthocyanidins, a family of straight lines crossed at the second quadrant were obtained (-). It indicated that the obtained proanthocyanidins in the present study were competive-uncompetive mixed-type inhibitors. The results revealed that the proanthocyanidins bound with free enzyme as well as enzyme-substrate complexes. The equilibrium constants for inhibitor binding with the free enzyme (KI) and with enzyme-substrate complex (KIS) were obtained from the plots of the slope or the vertical intercept versus the inhibitor concentration, respectively (-3II, III). The values of KI and KIS for the proanthocyanidins in leaves of Huangjingui and Qilan cultivars were determined to be 69.76 and 90.83, 20.27, and 165.96 µg/mL, respectively. The values of KIS were 1.3 and 8.2 times of KI for the proanthocyanidins in leaves of two tea cultivars, indicating that the affinity of the inhibitor for free enzyme was stronger than that for the enzyme-substrate complexes. Tight binding of the inhibitor to the enzyme or enzyme-substrate may be responsible for the low value.
Conclusions
The spectral data showed that the proanthocyanidins in leaves of Huangjingui and Qilan cultivars were mainly composed of procyanidins, with a small amount of propelargonidins and prodelphinidins. The proanthocyanidins in the studied tea cultivars exhibited strong antioxidant activities which is comparable to the synthetic antioxidant BHA. Moreover, the obtained proanthocyanidins also had excellent tyrosinase inhibitory activities, and were found to be a competitive–uncompetitive mixed-type inhibitor. Overall, these results suggested that the proanthocyanidins in leaves of two tea cultivars were promising to be developed as natural antioxidants and tyrosinase inhibitors for pharmaceutical and cosmetic applications.
Funding
This work was supported by grants from National Natural Science Foundation of China (81402309), Foundation of Henan Educational Committee (14B180013), Henan Planning Project of Science and Technology (142102310120, 132102310118), and the Yangtze Youth Fund of Yangtze University (2015cqn60, 2015cqn59).
Additional information
Funding
References
- Khan, N.; Mukhtar, H. Tea Polyphenols for Health Promotion. Life Sciences 2007, 81(7), 519–533.
- Mondal, T.K.; Bhattacharya, A.; Laxmikumaran, M.; Singh Ahuja, P. Recent Advances of Tea (Camellia Sinensis) Biotechnology. Plant Cell, Tissue, and Organ Culture 2004, 76(3), 195–254.
- Hollman, P.C.H.; Feskens, E.J.M.; Katan, M.B. Tea Flavonols in Cardiovascular Disease and Cancer Epidemiology. Proceedings of the Society for Experimental Biology and Medicine 1999, 220(4), 198–202.
- Cabrera, C.; Artacho, R.; Gimenez, R. Beneficial Effects of Green Tea—A Review. Journal of the American College of Nutrition 2006, 25(2), 79–99.
- Komes, D.; Horzic, D.; Belscak, A.; Ganic, K.K.; Vulic, I. Green Tea Preparation and Its Influence on the Content of Bioactive Compounds. Food Research International 2010, 43(1), 167–176.
- Ho, H.Y.; Cheng, M.L.; Weng, S.F.; Leu, Y.L.; Chiu, D.T.Y. Antiviral Effect of Epigallocatechin Gallate on Enterovirus 71. Journal of Agricultural and Food Chemistry 2009, 57(14), 6140–6147.
- Neto, C.C.; Krueger, C.G.; Lamoureaux, T.L.; Kondo, M.; Vaisberg, A.J.; Hurta, R.A.R.; Curtis, S.; Matchett, M.D.; Yeung, H.; Sweeney, M.I. MALDI-TOF MS Characterization of Proanthocyanidins from Cranberry Fruit (Vaccinium Macrocarpon) That Inhibit Tumor Cell Growth and Matrix Metalloproteinase Expression in Vitro. Journal of the Science of Food and Agriculture 2006, 86(1), 18–25.
- Fu, C.; Wang, H.; Ng, W.; Song, L.; Huang, D. Antioxidant Activity and Proanthocyanidin Profile of Selliguea Feei Rhizomes. Molecules 2013, 18(4), 4282–4292.
- Wei, S.D.; Chen, H.; Yan, T.; Lin, Y.M.; Zhou, H.C. Identification of Antioxidant Components and Fatty Acid Profiles of the Leaves and Fruits from Averrhoa Carambola. LWT–Food Science and Technology 2014, 55(1), 278–285.
- Chen, X.X.; Liang, G.; Chai, W.M.; Feng, H.L.; Zhou, H.T.; Shi, Y.; Chen, Q.X. Antioxidant and Antityrosinase Proanthocyanidins from Polyalthia Longifolia Leaves. Journal of Bioscience and Bioengineering 2014, 118(5), 583–587.
- Zhou, H.C.; Lin, Y.M.; Wei, S.D.; Tam, N.F.Y. Structural Diversity and Antioxidant Activity of Condensed Tannins Fractionated from Mangosteen Pericarp. Food Chemistry 2011, 129(4), 1710–1720.
- Robertson, A. The Chemistry and Biochemistry of Tea Production—The Non Volatiles. In Tea Cultivation to Consumption; Wilson, K.C.; Clifford, M.N.; Eds.; Chapman and Hall: London, 1992; 555 pp.
- Ho, C.T.; Chen, C.W.; Wanasundara, U.N.; Shahidi, F. Natural Antioxidants from Tea in Natural Antioxidants. AOCS Press: Champaign, IL, 1997.
- Wei, S.D.; Lin, Y.M.; Liao, M.M.; Zhou, H.C.; Li, Y.Y. Characterization and Antioxidative Properties of Condensed Tannins from the Mangrove Plant Aegiceras Corniculatum. Journal of Applied Polymer Science 2012, 124(3), 2463–2472.
- Li, C.M.; Leverence, R.; Trombley, J.D.; Xu, S.; Yang, J.; Tian, Y.; Reed, J.D.; Hagerman, A.E. High Molecular Weight Persimmon (Diospyros Kaki L.) Proanthocyanidin: A Highly Galloylated, A-Linked Tannin with an Unusual Flavonol Terminal Unit, Myricetin. Journal of Agricultural and Food Chemistry 2010, 58(16), 9033–9042.
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a Free Radical Method to Evaluate Antioxidant Activity. LWT–Food Science and Technology 1995, 28(1), 25–30.
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant Activity Applying an Improved ABTS Radical Cation Decolorization Assay. Free Radical Biology and Medicine 1999, 26(9–10), 1231–1237.
- Benzie, I.F.F.; Strain, J.J. The Ferric Reducing Ability of Plasma (FRAP) as a Measure of Antioxidant Power: The FRAP Assay. Analytical Biochemistry 1996, 239(1), 70–76.
- Qiu, L.; Chen, Q.H.; Zhuang, J.X.; Zhong, X.; Zhou, J.J.; Guo, Y.J.; Chen, Q.X. Inhibitory Effects of α-Cyano-4-Hydroxycinnamic Acid on the Activity of Mushroom Tyrosinase. Food Chemistry 2009, 112(3), 609–613.
- Reed, J.D.; Krueger, C.G.; Vestling, M.M. MALDI-TOF Mass Spectrometry of Oligomeric Food Polyphenols. Phytochemistry 2005, 66(18), 2248–2263.
- Ucar, M.B.; Ucar, G.; Pizzi, A.; Gonultas, O. Characterization of Pinus Brutia Bark Tannin by MALDI-TOF MS and 13C NMR. Industrial Crops and Products 2013, 49, 697–704.
- Xiang, P.; Lin, Y.M.; Lin, P.; Xiang, C.; Yang, Z.; Lu, Z. Effect of Cationization Reagents on the Matrix-Assisted Laser Desorption/Ionization Time-of-Flight Mass Spectrum of Chinese Gallotannins. Journal of Applied Polymer Science 2007, 105(2), 859–864.
- Wei, S.D.; Zhou, H.C.; Lin, Y.M.; Liao, M.M.; Chai, W.M. MALDI-TOF MS Analysis of Condensed Tannins with Potent Antioxidant Activity from the Leaf, Stem Bark and Root Bark of Acacia Confusa. Molecules 2010, 15(6), 4369–4381.
- Delfanian, M.; Kenari, R.E.; Sahari, M.A. Antioxidant Activity of Loquat (Eriobotrya Japonica Lindl.) Fruit Peel and Pulp Extracts in Stabilization of Soybean Oil During Storage Conditions. International Journal of Food Properties 2015, 18(12), 2813–2824.
- Zhou, X.L.; Zhou, M.; Liu, Y.; Ye, Q.; Gu, J.; Luo, G.Y. Isolation and Identification of Antioxidant Compounds from Gynura Bicolor Stems and Leaves. International Journal of Food Properties 2016, 19(1), 233–241.
- Loo, A.Y.; Jain, K.; Darah, I. Antioxidant and Radical Scavenging Activities of the Pyroligneous Acid from a Mangrove Plant, Rhizophora Apiculata. Food Chemistry 2007, 104(1), 300–307.
- Zhu, F.; Cai, Y.Z.; Sun, M.; Ke, J.; Lu, D.; Corke, H. Comparison of Major Phenolic Constituents and in Vitro Antioxidant Activity of Diverse Kudingcha Genotypes from Ilex Kudingcha, Ilex Cornuta, and Ligustrum Robustum. Journal of Agricultural and Food Chemistry 2009, 57(14), 6082–6089.
- Li, H.; Wang, X.; Li, Y.; Li, P.; Wang, H. Polyphenolic Compounds and Antioxidant Properties of Selected China Wines. Food Chemistry 2009, 112(2), 454–460.
- Wang, C.Y.; Ng, C.C.; Lin, H.T.; Shyu, Y.T. Free Radical-Scavenging and Tyrosinase-Inhibiting Activities of Extracts from Sorghum Distillery Residue. Journal of Bioscience and Bioengineering 2011, 111(5), 554–556.
- Li, S.B.; Nie, H.L.; Zhang, H.T.; Xue, Y.; Zhu, L.M. Kinetic Evaluation of Aminoethylisothiourea on Mushroom Tyrosinase Activity. Applied Biochemistry and Biotechnology 2010, 162(3), 641–653.
